Status of intestinal parasitic infections among rural and urban populations, southwestern Iran
Molouk Beiromvand, Esmat Panabad, Abdollah Rafiei ✉
1Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
2Department of Parasitology, School of Medicine, Ahvaz Jundishapur University of MedicalSciences, Ahvaz, Iran
Keywords:Intestinal parasitic infections Risk factors Iran
ABSTRACT Objective: To evaluate the prevalence and risk factors of intestinal parasitic infections in the urban and rural areas of Shushtar County, southwest Iran.Methods: A total of 1 008 fecal samples were analyzed by direct smear examination, formalinether concentration, and Ziehl-Neelsen and trichrome staining; furthermore, PCR was used to distinguish Trichostrongylus and hookworm species based on 28S rRNA gene.Results: Totally, 16.0% cases tested positive, either with a pathogenic or a non-pathogenic parasite. Protozoa were detected in 14.0%, helminths in 1.0%, protozoa and helminth coinfections were detected in 0.3%, and co-infections of two protozoa were detected in 0.7%of cases. The most common protozoa and helminths were Giardia duodenalis (7.7%) and Trichostrongylus spp. (0.5%), respectively. Among five microscopy Trichostrongylus positive cases, Trichostrongylus culbriformis was successfully identified in three isolates by sequencing.In the rural areas, the prevalence of parasitic infection was higher (9.8%) than that in the urban areas (6.2%). A significant association was found between educational level, type of drinking water, animals contact, hand-washing, and clinical symptoms.Conclusions: This study indicates that intestinal parasitic infections remain as a public health priority in Shushtar County. It seems that drinking water and environmental sanitation are the main risk factors of parasitic infections in rural areas.
1. Introduction
Intestinal parasitic infections, particularly soil-transmitted helminths, are considered neglected tropical diseases, and are attributed as the cause of morbidity and mortality in endemic areas[1]. Owing to the prevalence of 50% in developed countries and 95% prevalence in developing countries, it is estimated that about 450 million illnesses are caused by intestinal parasites[2].Ascaris lumbricoides, Ancylostoma duodenale, Necator americanus,Trichuris trichiura, Strongyloides stercoralis (S. stercoralis), Taenia saginata, and Taenia solium are the most common helminthic enteric parasites in humans[3]. It is known that children are more at risk of infection in comparison to adults, especially children who are under 5 years of age[4]. In sub-Saharan Africa, Cryptosporidium spp., Entamoeba histolytica and Giardia duodenalis (G. duodenalis),after Rotavirus, are the second biggest cause of diarrheal deaths in children under 5 years of age[3]. Annually, 200 million acute diarrhea caused by giardiasis and 50 million invasive amoebiasis are reported worldwide[3,5]. Various factors are associated with the prevalence of intestinal parasites, including safe drinking water, environmental sanitation, educational level, and socioeconomic status[2]. Although enteric parasites are usually transmitted directly by the ingestion of contaminated water, food, or soil, having close contact to infected animals or person-to-person contact are also possible routes of transmission[3]. Waterborne protozoan diseases are one of the main causes of four billion cases of diarrhea and the death of 1.6 million people per year, which has a worldwide distribution[6]. Furthermore,food-borne parasitic diseases are among major public health and socioeconomic problems. According to World Health Organization,7% of the food-borne diseases are caused by parasites[7]. Despite the efforts to improve health status, intestinal parasitic diseases still remains a global public health problem, especially in developing countries[4].
In Iran, like in other developing countries, intestinal parasitic infections are considered one of the major public health problems[8].A recently published systematic review and meta-analysis indicated that the prevalence of protozoa, helminths, and nonpathogenic parasites in Iran was 16.90%, 9.48%, and 18.50%, respectively[9].Giardiasis, the most common protozoan disease in Iran, has been reported 1% to 60% in different groups of people population[10].Given the increasing public awareness and the improving the medical services, particularly in urban regions, it is expected that there will be a significant reduction of intestinal parasitic diseases among the general population. However, in some rural areas of Iran, the lack of adequate safe drinking water, poor sanitation, close contact with domestic animals, and inadequate health services represent a potential threat to public health.
Several studies have been performed on the prevalence of intestinal parasitic infections in Iran[11-13]; however, limited research has been conducted to evaluate the prevalence and the associated risk factors of the infections in the urban and rural areas of Khuzestan Province,southwestern Iran[14].
The current study aimed to assess the prevalence of intestinal parasites in the rural and urban inhabitants of Shushtar County,southwestern Iran, using parasitological methods. This study also aimed to investigate the in fluence of risk factors, such as age, gender,residency, educational status, occupation, close contact with animals,and drinking water sources, on the intestinal parasitic diseases.
2. Materials and methods
2.1. Ethics approval and consent to participate
The ethics committee of the Ahvaz Jundishapur University of Medical Sciences reviewed and approved the protocol (approval number: 2017-229). The participants were first informed about the study. Then, informed consent was obtained from them.
2.2. Study area
Shushtar County is one of the northern counties of Khuzestan Province,southwestern Iran. The county is located at 32°02’ N 48°51’ E and is at an elevation of 65 meters above sea level. It has a population of 192 028[15] and an area of 2 436 km2, which is divided into 3 urban and 6 rural districts. The climate is hot and semi-arid, with an annual precipitation of 600 mm.
2.3. Sample collection
This cross-sectional study was performed from May to September 2017 among 11 villages and 3 districts of Shushtar County. Rural and urban participants were selected by simple random sampling.Population and geographical coordinates of the studied villages were as follows, respectively: Gavmishabad 5 688 (32°00’N, 48°50’E),Shahid Gholipour 11 757 (32°6’N, 48°47’E), Sardarabad 4 834(32°01’N, 48°47’E), Shahid Sherafat 10 151 (32°05’N, 48°45’E), Pir Daloo 2 300 (32°03’N, 48°52’E), Sheikh Shamsuddin 899 (32°06’N, 48°72’E), Konar Pir 1 096 (31°59’N, 48°50’E),Ferdows 420 (31°56’N, 48°55’E), Maleki 1 002 (32°01’N, 48°47’E), Gholamrezaee 359 (31°59’N, 48°57’E), and Kharmankhak 459 (31°54’N, 48°58’E). The sample size was determined based on a previous study carried out in Khuzestan Province[16].In Shushtar rural areas, each village contains a Health House,where auxiliary health workers known as “Behvarz” provide health education and basic health care programs. A labeled stool container and a questionnaire containing items about the sociodemographic characteristics as well as clinical data were provided to each participant. The next day, fecal samples were collected and transported on ice to the Ahvaz Jundishapur University of Medical Sciences for further analyses.
2.4. Microscopic examination, formalin-ether concentration,and Ziehl-Neelsen and Trichrome staining
All the fecal samples were initially evaluated macroscopically for helminths and stool consistency; then, direct smears were made from non-concentrated specimens with saline and lugol-iodine stain. The slides were microscopically examined at 100× and 400×magnifications for the presence of intestinal parasites.
The formalin-ether concentration technique was performed on all the fecal samples. Two grams of each fecal sample were briefly mixed with 7 mL of formalin and 3 mL of ether. After that, the samples were centrifuged at 2 000×g for 5 min[17]. Smears were prepared from the fecal pellets were stained with lugol-iodine solution; then, they were examined by a light microscope.
Ziehl-Neelsen and trichrome staining were used for Cryptosporidium spp. and Entamoeba histolytica/dispar identification, respectively[18,19].
2.5. DNA extraction and molecular examination
DNA isolation of nematodes eggs was performed on the approximately 200 µL of the fecal sediment using the QIAamp DNA stool mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Prior to extraction, samples were washed three times with PBS buffer. Then seven freeze-thaw cycles were performed by placing the tubes in liquid nitrogen and subsequently, thawing in a water bath at 100 ℃. DNA extracts were stored at -20 ℃ until molecular analysis was performed.
PCR was conducted for the amplification of a fragment of 485 bp and 380 bp of the ITS1, 5.8S and ITS2 regions, using the primer pairs RTHW1F (5’-GATGAGCATTGCWTGAATGCCG-3’) and RTHW1R(5’-GCAAGTRCCGTTCGACAAACAG-3’), in order to detect Necator americanus and Ancylostoma spp., respectively. The PCR was performed in a 25 µL volume reaction using 12.5 µL of the Taq DNA Polymerase 2×Master Mix RED (Ampliqon-Biomol, Hamburg, Germany),12.5 pmol of each primer, 5 µL H2O, and 5 µL DNA template. The amplification was performed under the following conditions: 5 min at 95 ℃ initial denaturation; 64 ℃ for 1 min, 72 ℃ for 2 min followed by 50 cycles of 30 s at 94 ℃, 30 s at 64 ℃, and 30 s at 72 ℃; and final extension at 72 ℃ for 7 min[20,21]. PCR amplification was performed targeting the ITS-1, 5.8S, and ITS-2 regions of the 28S rRNA gene for the identification of Trichostrongylus spp. and hookworms,using jhTsp (5’-TTATGTGCCACAAATGAAGA-3’) and NC2 (5’-TTAGTTTCTTTTCCTCCGCT-3’) primer pairs[22]. The PCR was conducted in the condition previously described[23]. PCR amplicons of 482 bp were electrophoresed on 2% agarose gel stained with ethidium bromide.
The PCR products were sequenced in both directions at the Bioneer Co. (Daejeon, South Korea). The obtained sequences were edited and aligned in MEGA 7.0 using ClustalW, and the neighbor-joining analysis was performed for the phylogenetic analysis.
2.6. Statistical analysis
Data analysis was performed using the SPSS 22 software (SPSS Inc., Chicago, IL, USA) and the Chi-squared test.
3. Results
3.1. Prevalence of intestinal parasites based on microscopic examination
A total of 1 008 individuals (436 rural and 572 urban) participated in the study from 11 villages and 3 districts of Shushtar County,southwestern Iran. Out of these participants, 531 (52.7%) were female and 477 (47.3%) were male. The age range was 1-80 years and the mean age was 26.31 years (Table 1). The overall prevalence of intestinal parasites was 16.0% with G. duodenalis (7.7%), which was the most common parasite in comparison to hookworms and S.stercoralis (0.1%) infections. Blastocystis hominis (B. hominis) was the most frequently observed protozoan, after G. duodenalis, with the prevalence of 5.6%; the most frequently observed helminths were Hymenolepis nana (H. nana) and Trichostrongylus spp., with the prevalence of 0.6% and 0.5%, respectively. The prevalence of parasitic infection was significantly higher (P=0.001) in the rural areas (9.8%) than that in the urban areas (6.2%). Of the 78 G. duodenalis cases, 55 (70.5%) were from the rural areas and 23 (29.5%) were from the urban areas. Among the 13 helminthic positive participants, 8 (61.5%) were rural inhabitants and 5 (38.5%)were urban residents.

Table 1. Sociodemographic parameters of the rural and urban residents of Shushtar County, southwestern Iran [n (%)].
Mono infection was found in 151(15.0%) and co-infections in 10(1.0%) of the participants (Table 2). Of the 161 positive samples,protozoa were detected in 141 (14.0%) samples, helminths in 10(1.0%) samples, protozoa and helminths co-infections in three(0.3%) samples, and co-infections of two protozoa in seven samples(0.7%) (Table 2).

Table 2. Proportion of infected cases with single infection or co-infections among the rural and urban residents of Shushtar County, southwestern Iran[n (%)].
3.2. Risk factors of intestinal parasitic infections
There was no significant correlation between gender and intestinal parasites (P=0.063). However, the males (18.2%) had a higher infection rate than the females (13.9%). With regard to the age group, the most frequently infected participants were found in 10-20 years age group (23.6%), followed by the age group under 10 years(15.5%). No significant correlation was found between age and infection. While 29.5% of the infected participants were illiterate,a significant association was not found between intestinal parasitic infections and educational status (P=0.07). Among the participants who used tap water, 11.7% were infected with intestinal parasites,while the participants with a history of consumption of unprocessed well or river water showed higher infection rates of 22.3% and 25.0%, respectively. Among families with 4-6 members or more,36.5% of the participants tested positive for intestinal parasites.There was a significant difference found between intestinal parasitic infections and having contact with animals at home, hand-washing,clinical symptoms, and stool consistency (P<0.05). More details were shown in Table 3.
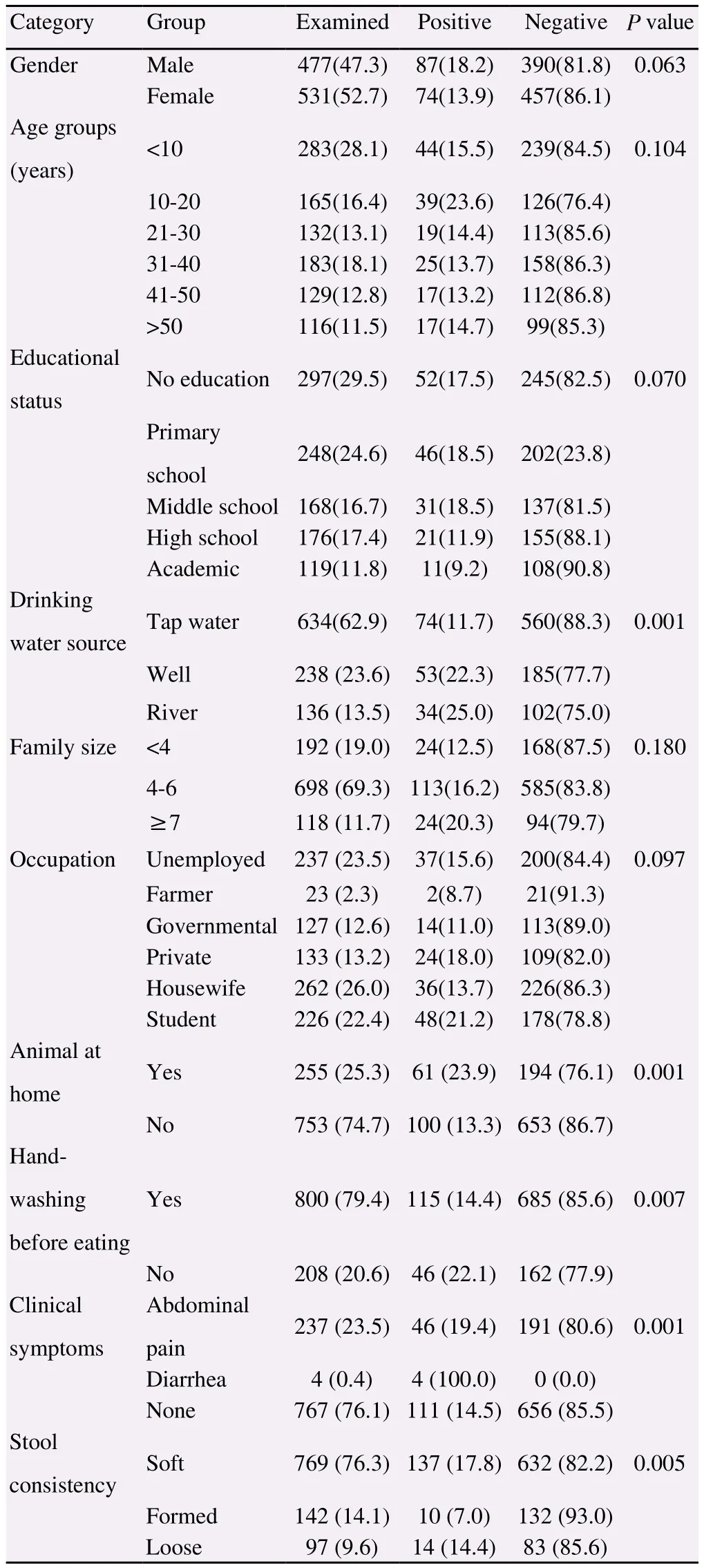
Table 3. Prevalence of intestinal parasitic infections and associated risk factors among the rural and urban residents of Shushtar County,southwestern Iran [n (%)].
Our findings revealed that, the highest infection rate (13.0%)was observed in Gholipour village and the lowest was found in Gavmishabad, Konar Pir, and Ferdows (1.2%) (Table 4).
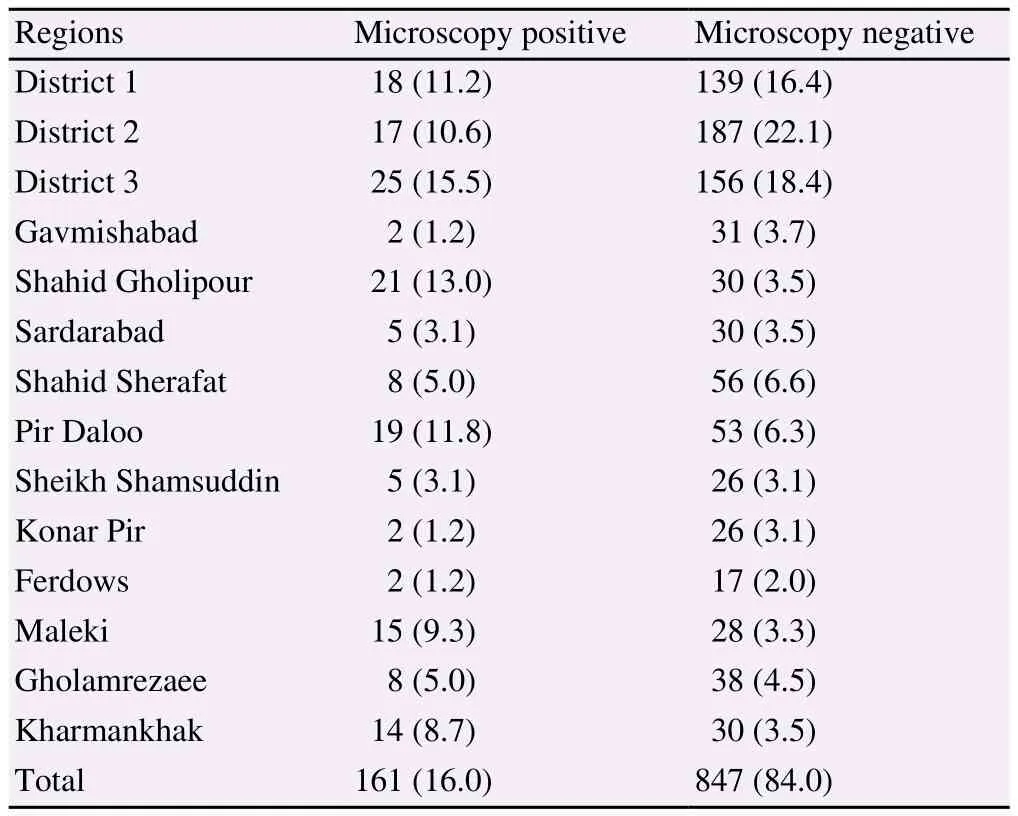
Table 4. Prevalence of intestinal parasitic infections by region among the rural and urban residents of Shushtar County, southwestern Iran [n (%)].
3.3. Molecular identification of nematodes
Out of the five microscopy positive samples for Trichostrongylus spp., four samples were amplified successfully at the 28S rRNA gene. Alignment analysis of the 28S rRNA sequences identified Trichostrongylus colubriformis (T. colubriformis) in three isolates.Sequencing results showed 100% identity with KF989495 obtained reference sequences from the GenBank (Figure 1). The remaining sample was not typeable. The only hookworm microscopy positive sample was not positive by PCR.
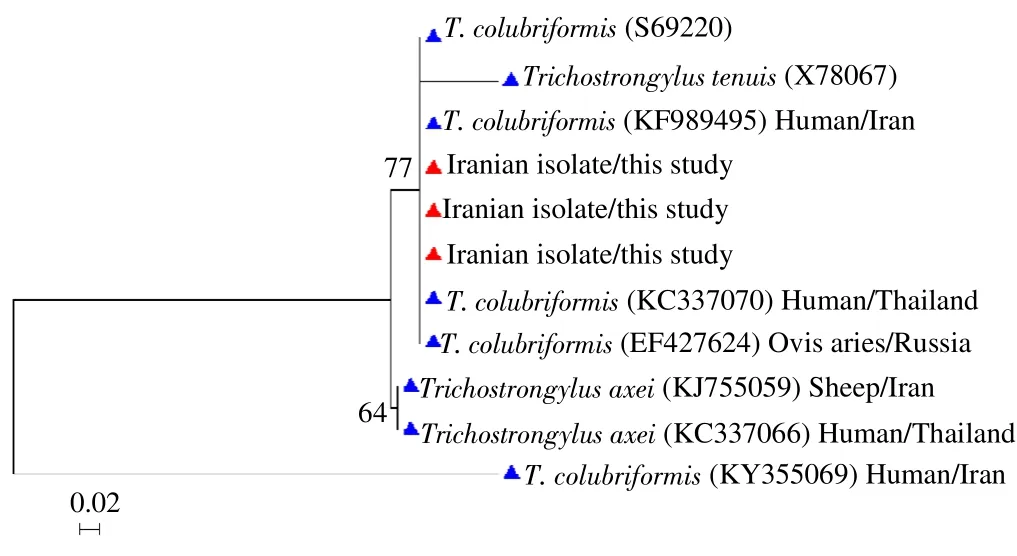
Figure 1. Phylogenetic tree for T. colubriformis based on 28S rRNA gene sequences.
4. Discussion
While intestinal parasitic infections were observed in 16.0% of the participants, 134 (13.3%) out of these participants were pathogenic.In a study conducted from 2005-2007 on 1 494 Khuzestan nomads,the prevalence of intestinal parasitic infections was reported to be 25.36%[14]. Further study in Khuzestan Province indicated an infection prevalence of 13.35%[16]. Although our results are similar to the results of the study conducted by Khoshnood et al.[16], unlike their study, which only focused on the number of participants attending the medical and health centers, we evaluated rural and urban inhabitants.
G. duodenalis and H. nana were the most frequent protozoan and helminth, respectively. The findings were in agreement with previous local studies, in which G. duodenalis and H. nana were reported as the most common pathogenic protozoan and helminth[14,16],respectively. Furthermore, the recorded data from the other parts of Iran indicated that G. duodenalis is more prevalent than other pathogenic intestinal parasites[24-26].
A significant finding in our study was the higher prevalence of G. duodenalis (12.6%) in the rural inhabitants in comparison to the urban inhabitants (4.3%). Poor sanitation, close contact with animals,lack of safe drinking water, limited awareness, and de ficient toilet facilities are associated with a higher infection of G. duodenalis in rural regions. Among the G. duodenalis positive samples, the highest frequency was found in consumers of river water (16.2%), while the lowest frequency (4.2%) was found in tap water consumers. G.duodenalis and Cryptosporidium spp. are among the most frequent waterborne causes of infectious diseases worldwide[6]. Despite the higher possibility of waterborne outbreaks caused by both protozoa in developing countries, suf ficient data is not available with regard to waterborne parasitic outbreaks[6]. In most Iranian rural areas and certain urban regions, safe water is not readily available. According to a survey carried out by Rafiei et al., 9.1% of the examined water samples from Ahvaz County were infected with G. duodenalis[27]. In a cross-sectional epidemiological study on children in Pakistan, G.duodenalis was reported 2.75% and 9.50% by direct microscopy and ELISA, respectively[28]. In a study conducted by Yilmaz et al. (2017)in Duhok and Erbil cities, Northern Iraq, G. duodenalis was found 9.5% in Duhok and 5.7% in Erbil by direct wet smear and iodine staining[29].
Our results showed that 6.0% (61/1 008) of the participants, who kept animals at home, were infected with intestinal parasites.Out of the participants who did not keep animals at home, 9.9%(100/1 008) were infected. Domestic animals, such as dogs, may play an important role in the transmission of zoonotic parasites owing to close contact with household members and through the contamination of soil with parasitic agents[30]. Children are at greater risk of zoonotic infections due to having more contact with domestic animals and contaminated soil during their playtime[31]. The obtained data indicated that 4.3% of the infected participants were younger than 10 years. As most of the streets are unpaved, therefore,people, particularly children, can be infected with protozoa and soiltransmitted helminths in the region. The observed prevalence of 1.3% for helminthic infections was higher in comparison to 0.10%,0.50%, 0.25%, and 0.15% prevalence reported from the southern,western, northern, and central parts of Iran[32-35]. Trichostrongylus culbriformis is the only detected geohelmiths infection with 0.5%prevalence. Iran, particularly the northern parts, is an endemic area for human trichostrongylosis[36]. Our findings are in line with previous studies from Iran[36-38]. In Khuzestan Province, there is no data on human trichostrongylosis; however, the only conducted study showed that T. culbriformis is the most common species in animals[39].
The results indicated that higher infections of 5.2% and 4.5%belonged to illiterate and primary school groups, respectively; lower infection rate, on the other hand, was observed in the participants holding a university degree. The high prevalence of parasitic infection is closely related to the level of education[4]. People at academic level are likely to be more aware of parasitic diseases. In this study, the correlation between family size and infection was not significant; however, the prevalence of intestinal parasites in the participants with a family size of 4-6 was 11.2%. The results agree with the findings from the north of Iran[40]. Thus, poor sanitation in large families might be the cause of higher infection among them.
Regarding occupation, a significant correlation was not observed.The highest prevalence was, however, found among the students(4.7%). This finding could be due to the greater exposure of the students to risk factors, such as domestic animals and contaminated soil. The males were more infected than the females; however, a significant association was not found. Similar results were reported by Sadeghi et al. from the northern parts of Iran and Arani et al. from Tehran[34,41]. In this study, of the 800 participants who were used to washing their hands before eating, 115 (14.37%) were infected.Moreover, of the 208 participants who occasionally practiced handwashing, 46 (22.1%) were infected. Hand-washing is an important factor that can interfere with the transmission of parasites[42].
In spite of the fact that 16.0% of the participants were infected with pathogenic and non-pathogenic parasites, most of the cases were asymptomatic, and only a small percentage of the infected participants complained of diarrhea or abdominal pains. Given that 68.9% of the positive cases were asymptomatic and unaware of their infections, anthroponotic transmission may play an important role in the transmission of intestinal parasites in the region. In this study,a number of participants (6.2%) showed co-infections of intestinal parasites. Our findings are in agreement with previous studies from Iran, which have reported infections with multiple parasites[43-45].
A striking finding in our study is the prevalence of intestinal parasites observed in District 3 of Shushtar County as well as the villages of Gollipour, Pirdallo, Maleki, and Kharmankhak (8.7%).In the villages with higher infection rate, the lack of wastewater management is probably the main cause behind higher prevalence,in spite of the fact that the possible contamination of surface and underground water might be associated with the transmission of intestinal parasitic diseases.
People in the rural regions were more infected than those living in the urban areas. The higher prevalence rate of protozoa infections,particularly G. duodenalis, is indicative of the public health problem in the region, especially for rural young children under 10 years of age. It seems that Trichostrongylus infection should have a zoonotic resource within the region. Our results also revealed that the lack of safe drinking water and waste water management can be the cause of further infections in certain rural regions. Considering that the majority of the infected cases were asymptotic, prevention and control programs should be implemented in the region. We believe that these findings could be useful in informing local health authorities to provide better strategies, such as providing safe drinking water, management of wastewater, and health education to promote public awareness on parasitic diseases.
Conflict of interest statement
We declare that we have no conflict of interest.
Foundation project
This project was supported by the Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Grant No. OG 96106).
 Asian Pacific Journal of Tropical Medicine2019年3期
Asian Pacific Journal of Tropical Medicine2019年3期
- Asian Pacific Journal of Tropical Medicine的其它文章
- SARS and its treatment strategies
- Phyllanthus acidus (L.) Skeels and Rhinacanthus nasutus (L.) Kurz leaf extracts suppress melanogenesis in normal human epidermal melanocytes and reconstitutive skin culture
- Antihydatic and immunomodulatory effects of Algerian propolis ethanolic extract: In vitro and in vivo study
- Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities
- Isolation and structural elucidation of antifungal compounds from Curcuma amada
- X-linked Toll-like receptor 7 polymorphism associated with susceptibility to Chikungunya Fever
