有机负荷对连续生物制氢反应器中氢气的影响
郝 鑫,敖梓鼎,John Dominick Kapumbe(卡不贝尔),季丹丹,张伊帆,李永峰
(东北林业大学林业学院,哈尔滨 150040)
I.Introduction
With the extensive use of fossil fuels such as coal and oil, the earth has seen an increase in serious pollution of the global environment. This pollution has been posing a threat to human survival. Additionally, fossil fuels are non-renewable energy source. Therefore, development and application of alternative source of energy, hydrogen has attracted extensive attention. It is also one of the most promising clean energies of the future energy structure and it will play an important role in the field of energy for its excellent and useful performance.
It is of great strategic significance that hydrogen energy is developed in China. As a matter of fact, it plays a real important role in developing energies for bio-hydrogen production technology[11]. Continuous-flow stirred tank reactor (CSTR) with mechanical mixing and high mass efficiency can boost hydrogen production efficiency. Additionally, this method that requires a relatively simple device and technology can significantly reduce the cost of bio-hydrogen production, resulting in the realization of the industrialized production of hydrogen gas[1,2,3]. This article studies the effect of organic load on an anaerobic bio-hydrogen system, which provides a theoretical basis for achieving the rapid degradation of organic wastewater and the production of hydrogen energy.
II Material and methods
A.Experimentalfacility
This experiment used continuous-flow stirred tank reactor (CSTR) with an effective volume of 7.0 L and a total volume of 19.4 L. The reactor with agitator realized that microorganisms can be completely mixed with intake water by mixing (at speeds of 120 r·min-1). There is a gas-liquid-solid three-phase separator inside the reactor and it has an integrated structure of reaction and sedimentation zones. Locked with water through the shaft, the reactor was under anaerobic conditions. The temperature was automatically controlled at the level of (35±1)℃ with resistance wire around the reactor wall, which guaranteed high activity of micro-organisms. The structure of continuous-flow mixing reactor is shown in Figure 1.

1.Molasses Solution 2.Feed 3.Reactor 4.Blender 5.Water Lock 6.Gas Flow MeterFig.1 Schematic diagram of CSTR
B.AnalyticalMethods
The biogas composition including hydrogen and carbon dioxide was measured by using a gas chromatograph (GC, 6809N Network GC System, Agilent Technologies, Waldron, Germany) equipped with a thermal conductivity detector (TCD). The column (2 m*5 mm) was filled with porapak Q (50-80 meshes), Nitrogen was used as carrier gas with a flow rate of 40 mL/min and Methane was not detected in the biogas.
CODs of the samples were measured according to Standard Methods[4]. The pH and ORP were measured by pH meter (PHS-25). A wet gas meter (LML-1) was utilized to measure biogas yield.
Volatile fatty acids (VFA) and ethanol in liquid samples were measured by using a gas chromatograph (GC, 6890N Network GC System, Agilent Technologies, Waldbrown, Germany) equipped with a flame ionization detector (FID). The column (Zm) was packed with supporter of GDX-103 (60-80 meshes). The temperatures of the injection part, the oven, and the detector were adjusted to 220℃, 190℃, and 220℃, respectively. The carrier gas was nitrogen at a flow rate of 30mL/min also.
C.Sludgeacclimationandruncontrolledparameters
The experiment used inoculation sludge from secondary sedimentation tanks of a certain sewage treatment plant in Harbin. The debris and particulate matter could be removed by being precipitated, washed, and filtered. Used brown sugar with a COD of 10 000 mg/L and added an amount of N and P-fertilizer, COD, N, and P had a mass ratio of 1 000∶5∶1, which ensured that the sludge micro-organisms met N and P nutrient needs during the growth. The sludge was cultivated with intermittent aeration for 20 days. During the process, aeration was stopped for 1 hour, rested, while the upper liquid was extracted and clean water was added on a daily basis. The mature sludge presented brown, granular and settled well after domestication.
The well domesticated sludge was turned into the hydrogen production reactor. Under the conditions of hydrogen retention time (HRT) 6 hours, temperature (35±1)℃, intake pH 7.0±0.1, organic load 12 kg/m3-d, suspended solid (SS) 12.93 g/L, Volatile suspended solids (VSS) 8.46 g/L, VSS/SS( biological activity ) 0.65, the reactor started with a continuous flow way. After about 30 days, it reached a stable state. At this point, the gas production was up to 2.0 L/d, hydrogen accounted for about 41%, pH of wastewater was around 5.0, ORP was -420 mV. The average concentrations of fermentation products (including small molecular fatty acids and alcohols) were: ethanol 280 mg/L, acetic acid 63 mg/L, propionic acid 66 mg/L, butyrate acid 87.5 mg/L and valeric acid 6 mg/L. Among them, ethanol and acetic acid accounted for 68.26%, which meant becoming the ethanol-type fermentation.
After stability, anaerobic activated sludge fermentation system kept the hydraulic retention time unchanged (HRT=6 h) in operation process. By increasing organic load and adjusting intake pH, we studied its impact on CSTR reactor for hydrogen production capacity. The running process in which organic load increased from 12 kg/m3-d to 32 kg/m3-d was divided into six stages, each stage increased 4 kg/m3-d and response (run) time was 6 days.
III. Results and analysis
A.ChangesofBiogas,hydrogenproductionrates
As is shown in Figure 2, in the process of the intake organic load increased from 12 kg/m3-d to 28 kg/m3-d gradually.The gas production rate witnessed a trend of sustained growth and the increasing rate of hydrogen production changed marginally. The hydrogen content varied from 22.9% to 50.4%. At each stage, the total gas production rates sometimes slightly dropped after the upgrade of the organic load, but hydrogen production rate remained stable without obvious changes. It was possibly because microorganisms other than the hydrogen-producing bacteria lacked the tolerant capacity of load impact and their activity declined, which caused a reduction in carbon dioxide and other gases[10]. In the next day after the organic load rose to 32 kg/m3-d, biogas production rate reached the highest point at 18.6 L/d and hydrogen production rate peaked at 6.4 L/d, hydrogen content was 34.4% at this moment. Compared with initial organic load 12 kg/m3-d, the biogas and hydrogen production rates increased 89% and 87%, respectively. Although in the following few days, all the three items mentioned above experienced a slight drop, there were still relatively high levels of gas production rates and hydrogen content. This phenomenon may be due to hydrogen-producing fermentation microorganisms need a process of adaptation to high organic load. High load impact within a short time coupled with the increasing yield of liquid fermentation of volatile acids made the biological activity inhibited and organic catabolic rate dip slightly. As can be seen from the graph, the microorganisms rapidly adapted to the environment of high organic load in the next two days and the gas production rates rebounded. Worth noticing is, it was low pH that caused the decline of gas production rates when organic load stood at 20 kg/m3-d. However, hydrogen production rate barely changed, which showed hydrogen-producing bacteria had the tolerant capacity of pH to a certain extent[8].
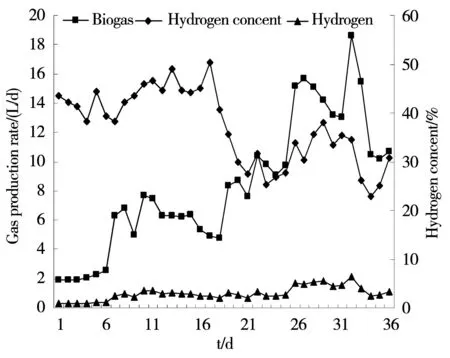
Fig.2 Changes of biogas and hydrogen gas in operation process of the reactor
B.ChangesofpH
pH is an essential ecological element which could affect different hydrogen-producing fermentations[6,9]. The activity of enzymes which participate in metabolic processes will be directly affected by variations of pH. Under different pH conditions, different kinds of bacteria grow at distinguishing speeds, which influence changes of microbial populations and communities in the biological hydrogen-producing reactor ultimately, thereby change the number and position of dominant populations and cause changes of fermentation types. Most fermentative hydrogen production is operated under acidic conditions which could inhibit the activity of methanogenic bacteria and help degradation of the organic load to be maintained in hydrogen-producing stage. Figure 3 reflects water pH fluctuation of entire process in the reactor. Numerous studies showed that the optimum pH was between 5.0 and 6.0. In this study, when the organic load amounted to 12 kg/m3-d -16 kg/m3-d, the outlet pH could be regulated to approximately 5.0 by adding a certain amount of NaOH to the intake. When the organic load increased from 20 kg/m3-d to 28 kg/m3-d, stopped adding NaOH and the intake pH plummeted to around 5.85. The sudden decrease of pH affected microbial enzyme activity which gave rise to a downward trend of the metabolic rates and outlet pH declined to roughly 4.3 as well. Micro-organisms gently adapted to the intake pH changes, metabolic rate rocketed and outlet pH reached a plateau at about 4.6 when the organic loading arrived at 24 kg/m3-d.

Fig.3 Changes of pH in operation process of the reactor
C.Changesofoxidation-reductionpotential(ORP)
ORP is also an ecological factor of overriding importance. In the hydrogen fermentation process, low oxidation-reduction potential is a necessary condition for the growth of hydrogen-producing fermentation microorganisms[7]. This is because only the low ORP environment is suitable for anaerobic microorganisms’ survival requirements. Some of them including Coenzyme I, Ferredoxin and Flavoprotein, etc-the dehydrogenase system require low ORP environment to maintain activity. During the entire operating process, the system ORP changed between -430 mV and -328 mV, as shown in figure 4. ORP was mainly implicated by the changes of pH and we can discern from figure 3 and figure 4, ORP and pH were negatively correlated. When the pH lowered, the hydrogen-producing fermentation microbial activity was inhibited; changes of hydrogen-producing fermentation environment in system caused an increase in ORP. During the 1 to 12 days, ORP had no evident fluctuation, peaking at -328 mV on the eighteenth day and then decreased steadily, leveled off at -390 mV, eventually.
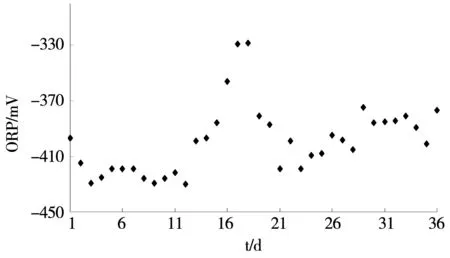
Fig.4 Changes of ORP in operation process of the reactor
D.Changesofliquidterminalproducts
Figure 5 reflects changes of liquid terminal products by the anaerobic activated sludge ferment produce hydrogen system in the whole operation process. With the continual increase of the organic load of intake water, the total content of liquid terminal fermentation products kept increasing, from 506.7 mg/L of the initial organic loading 12 kg/m3-d to 1 376.2 mg/L of organic load 32 kg/m3-d. Ethanol increased more obviously than other liquid terminal products when the volatile acids fluctuated. From the 15thto 30thday, the activity of acidogenic fermentation microorganisms was controlled by the low pH. At the same time, the content of ethanol and acetic acid decreased apparently, dropping from 603.8 mg/L and 283.5 mg/L to 327.0 mg/L and 134.7 mg/L, respectively. When the organic loading increased from 28 kg/m3-d to 32 kg/m3-d, the dominant butyrate gave priority to volatile acid. At this point, both the ethanol-type fermentation and butyric acid type fermentation existed, but the ethanol-type played the dominant role[12]. As the intake organic load increasing from 12 kg/m3-d to 32 kg/m3-d in the whole reaction system, the total content of liquid terminal fermentation products in the various stages represented 506.7、730.4、860.4、975.5、1 274.6、1 376.2 mg/L while the content of ethanol and acetic acid were 346.8、499.4、646.1、757.7、935.4、1 012.447 mg/L,which respectively accounted for 68.4%、68.4%、75.1%、77.7%、73.4%、73.6% of the total. From the above data, we came to the conclusion: although the conditions such as water pH, organic load, ORP and so on changed in various degrees, the system remained ethanol-type fermentation all the time.
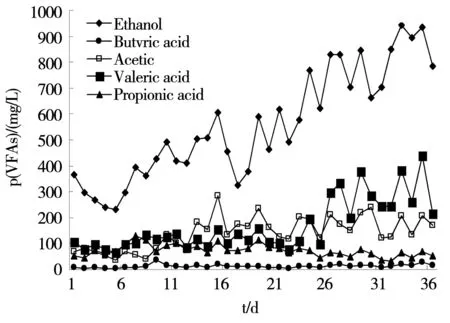
Fig.5 Changes of concentration of fermentation liquid products in operation process
E.ChangesofCODdegradationrate
Figure 6 showed changes of intake and outlet COD concentration and COD degradation rate in the whole system. The variation trend of intake COD concentration was almost the same with the outlet; COD degradation rate changed between 34.8% and 57.2%. In the traditional way of anaerobic wastewater treatment, COD was dislodged mainly by the way that the methanogens change the intermediate products into methane. While in single-phase CSTR reactor, it was hydrogen-producing acidogenic bacteria that played a dominant role; COD was degraded by hydrolysis and aerogenesi, and was converted into organic acid of liquid terminal products. Therefore, the degradation rate cannot be high[5].
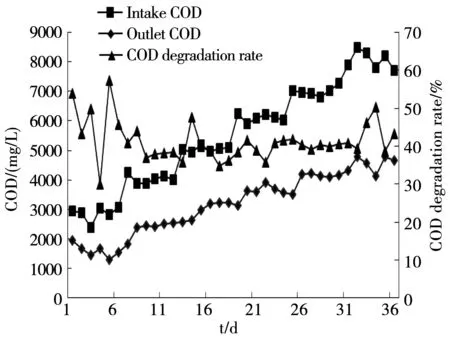
Fig.6 Changes of COD degradation rate in operation process of the reactor
IV.Conclusions
From this study, we draw a conclusion that it is better to control the continuous stirred tank reactor (CSTR) with an intake of pH 7.0±0.1, an oxidation-reduction potential (ORP) of -420 mV, a temperature of (35±1)℃ and a hydraulic retention time (HRT) of 6 hours. Under these conditions, while intake of organic load increased from 12 kg/m3-d to 32 kg/m3-d, the hydrogen production capacity of anaerobic activated sludge continued to rise. When the organic load stood at 32 kg/m3-d, the maximum biogas and hydrogen production rate reached 18.6 L/d and 6.4 L/d. When compared with organic load 12 kg/m3-d, they improved 89% and 87%, respectively.
Hydrogen-producing bacteria have better tolerant capacity of high organic load on a brown sugar effluent. They can quickly adapt to the changing environment of high organic load in a short time, and then reach a steady production of hydrogen. In this test, when the organic load was 20 kg/m3-d, as not adjusting the intake pH, anaerobic activated sludge microorganisms were unable to adapt to environmental changes caused by low pH. As a result, its activity was stifled, biogas and hydrogen production rates all declined. At this moment, ORP reached the highest point at-328mV.Therefore, as intake COD concentration increasing, it’s better to add a certain amount of NaOH into the reactor to regulate pH and ensure that hydrogen fermentation of micro-organisms have higher activity. Meanwhile, stability of ethanol-type fermentation is maintained and a higher rate of stable hydrogen production is realized.

