Polymorphism of hypoxia-inducible factor-1α gene in pediatric acute respiratory distress syndrome
Sema Yilmaz, Aysegul Kuskucu, Ozden Ozgur Horoz, Oznur Suakar, Nergiz Imamova,Gizem Gongor, Dincer Yildizdas
1Kutahya Health Science University, Faculty of Medicine, Pediatric Hematology/Oncology, Evliya Celebi Yerleskesi, Tavsanlı yolu 10. km, Kutahya, Turkey
2Yeditepe University Faculty of Medicine, Medical Genetics, Acıbadem Mah. Bağ Sok. No: 8 İstek Vakfı Binası Kadıköy Istanbul, Turkey
3Cukurova University Faculty of Medicine, Department of Pediatric Intensive Care Unit. Çukurova Üniversitesi Tıp Fakültesi Balcalı Hastanesi 01330 Balcalı,Adana, Turkey
Keywords:Acute respiratory distress syndrome Child Hif-1α Polymorphism
ABSTRACT Objective: To examine if hypoxia-inducible factor-1α (Hif-1α) polymorphisms are associated with pediatric acute respiratory distress syndrome (PARDS). Methods: Twenty two patients with PARDS and 11 non-PARDS controls were examined in pediatric intensive care unit in Cukurova University Balcali Hospital. Blood polymorphism was used to assess the Hif-1α C1772T and G1790A polymorphisms of Hif-1α gene, and differences in genotypes between the 2 groups were compared. Results: Hif-1α C1772T polymorphism was observed only in one case of PARDS group but non-PARDS group didn't show any C1772T polymorphism. Particularly, the difference in number of cases with Hif-1α G1790A polymorphism was not significant between PARDS and non-PARDS groups. In addition,Hif-1α G1790A polymorphism was significantly related to the distribution of lung opacities in children with PARDS (P<0.05). Conclusions: Our results indicate that Hif-1α G1790A polymorphism is related to an increased susceptibility to pulmonary for PARDS children. The detection of G1790A polymorphism could help pediatricians to predict the extensity of PARDS early in lung tissue.
1. Introduction
Pediatric acute respiratory distress syndrome (PARDS) is diagnosed by the presence acute-onset and progressive hypoxic condition caused by direct damage to the lung (e.g., pneumonia and gastric aspiration) or indirect damage (e.g., sepsis, trauma and pancreatitis)[1]. Hallmarks of ARDS include hypoxemia and decreased lung compliance, increased work of breathing, and impaired gas exchange. PARDS admissions account for 1%-4% of all pediatric intensive care unit (PICU). Mortality is often accompanied by multiple organ failure[2-4]. Hypoxic conditions induce some adaptations for the maintenance of oxygen delivery to tissues.Hypoxia inducible factor-1 (Hif-1) is a transcription factor that acts as principal regulator of oxygen homeostasis, playing a fundamental role in the physiological response to hypoxia. Hif-1 is composed of two subunits: Hif-1α and Hif-1β. Hif-1β is a constitutive core protein, whereas Hif-1α mediates adaptive responses to reduced oxygen availability by regulating gene expression[5]. Activation of Hif-1α is a major driver of acute inflammation[6]. The human Hif-1α gene, located at chromosome 14q21-24, codes for a 3 919 bp cDNA and produces an 826 amino acid protein. Single nucleotide polymorphisms in coding regions can drive amino acid changes and affect both structure and biological activity of the translated protein. The most common investigation of Hif-1α polymorphisms is 1772 C/T (Pro582Ser, rs11549465) and 1790 G/A(Ala588Thr, G1790A, rs11549467), which lead to proline-to-serine and alanine-to-threonine amino acid replacements, respectively.Under hypoxic and normoxic conditions, these polymorphisms can cause to high transcriptional activity of Hif-1α[7].
The relationship between Hif-1α polymorphisms and malignancies is presented by many investigations. Previous studies had shown that 1772 C/T and 1790 G/A polymorphisms of Hif-1α contribute tumor vasculature resulted in tumor progression[8]. Therefore Hif-1α polymorphisms was linked to various types of cancers[9,10]. Recently,it was found that genetic polymorphisms of Hif-1α have also positive association with chronic obstructive pulmonary disease[11].The estimation of the association of Hif-1α polymorphism with acute hypoxia was noted in adults[12]. However, the role of Hif-1α 1772 C/T and 1790 G/A polymorphisms has not been well-studied in children with ARDS. Therefore, we hypothesized that the Hif-1α polymorphism may take a role in PARDS cases.
2. Materials and methods
2.1. Ethical statement
This study was approved by the Ethics Committee numbered with 42/4-2015 in Cukurova University, and written informed permissions were obtained from each patient’s family.
2.2. Study subjects
Total of 22 children (aged 1 month to 18 years old) diagnosed with PARDS and 11 of non-PARDS patients were recorded in our study in between April 2016 and April 2017. Patients were fulfilled the PALICC definition for PARDS and intubated. Non-PARDS cases were selected from neuromuscular, intoxication and postoperation surgery cases. The PARDS definition included: 1) hypoxemia with oxygenation index ≥4 or oxygenation saturation index ≥5; 2) new radiological lung infiltrates; 3) occurred within 7 d of a known clinical insult; and 4) not explained by cardiac failure or fluid overload[2]. Children with congenital cyanotic heart disease, chronic lung disease and left ventricular dysfunction are also allowed as long as they fulfilled the criteria above and the acute deterioration in oxygenation and pulmonary infiltrates cannot be explained by their pre-existing diseases. The exclusion criteria were perinatal lung disease, to be on noninvasive ventilation, premature neonates with corrected age less than 35 weeks, neonates being cared for in the neonatal ICU and neonates deemed to have perinatal lung disease. Age, gender, weight, height, length of stay and last status of all patients were recorded. Sequential pediatric logistic organ dysfunction (PELOD) and pediatric risk of mortality (PRISMⅢ)scores were used for assessing progressive organ dysfunction[13,14].Two mL of venous blood was acquired from each child in both PARDS and non-PARDS groups and then transferred to an ethylenediamintetraacetic acid bottle for laboratory test and PCR.
2.3. Isolation of DNA in peripheral blood
The test was performed according to product instructions. Total DNA of peripheral blood was obtained by a DNA isolation kit(QİAamp DNA Mini Kit, Qiagen, Germany). The polymerase chain reaction (PCR) was carried out in a 25 μL reaction volume containing 2 μL of extracted DNA, 2 μL of forward primer (10 pmol/mL), 2 μL of reverse primer (10 pmol/mL), 12.5 μL of Master mixture, 1.5 μL Coral Load, 1.5 μL MgCl2, 2.5 μL Q solution and 3 μL of deionized water.
The PCR primer sets were as follows Hif-1α, rs11549465(C1772T) (95 bp) F:AGTTACGTTCCTTCGATCAGTTG; R:CTGCTGGAATACTGTAACTGTGCT and Hif-1α, rs11549467(G1790A) (67 bp) F:CAGTTACGTTCCTTCGATCAGTTG; R:GACTTGCGCTTTCAGGGC.
2.4. PCR
PCR kits were obtained from Qiagen, Germany. denaturation at 95 ℃ for 15 min, followed by 40 cycles of denaturation at 94 ℃for 20 s, annealing at 62 ℃ for 30 s, and extension at 72 ℃ for 20 s; followed by a final extension at 72 ℃ for 5 min. Each well was loaded with 4 μL of amplified products and depended to 40 min of electrophoresis under 120 V performed with 2 % agarose gel involving 2 mg/mL ethidium bromide in a 0.5 × TAE buffer solution.The amplification bands were observed under ultraviolet light and photographed to check if the PCR was accomplished.
2.5. Detection of gene polymorphisms
A pyrosequencing method was designed to determine genetic polymorphisms of Hif-1α. Sequencing primer order of Hif-1α, rs11549465 (C1772T) and rs11549467 (G1790A)were GCGGAACTGCTTTCTAA and TGCGCTTTCAGGGCT,respectively. Pyrosequencing was carried out in a PyroMark Q24(Qiagen, Germany) and the data were analyzed by using sequence analysis software.
2.6. Statistical analyses
SPSS 20.0 (SPSS Inc., Chicago, IL, USA) was applied for all data analysis. The results of the study were presented as mean ± standard deviation (SD). The two groups were compared by independent samples t-test, while comparisons among multiple groups were analyzed by analysis of Mann-Whitney U test. The level of significance was set at P<0.05.
3. Results
Twenty two patients with PARDS (9 girls, 13 boys) and 11 non-PARDS, control group (5 girls, 6 boys) were recorded in the study.The mean age in PARDS and non-PARDS group were (88.8±76.8)and (102.5±76.3) months, respectively (Table 1). There was no significant difference in age, gender, weight and height between two groups.
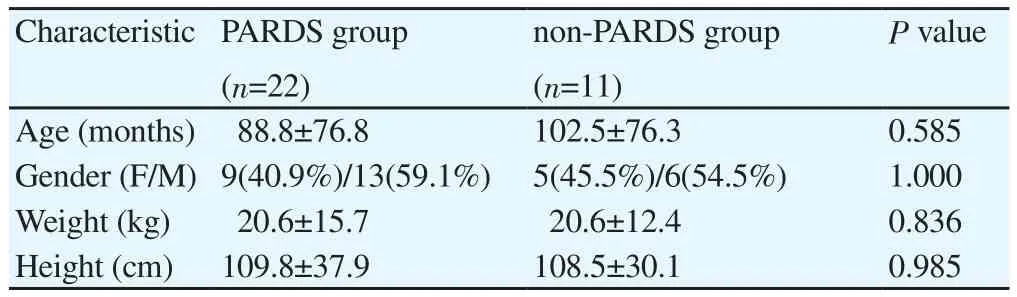
Table 1 Demographic features of patients.
Pneumonia (13 of 22 patients, 59.1%) was the most common pulmonary reason of PARDS, followed by aspiration [3(13.6%)],while sepsis [5(22.7%)] and trauma [1(4.5%)] were the extrapulmonary causes. Three PARDS patients had mild degree of hypoxemia (13.6%), 9 (40.9%) moderate and 10 (45.5%) severe hypoxemia.
PRISM Ⅲ and PELOD scores were 12.0±8.5 and 20.7±11.1 in PARDS group, respectively; while the scores were 3.7±3.3 and 3.9±6.4 in non-PARDS group. The mean length of stay in hospital was (23.4±15.9) d, the hospitalization in PICU was (20.5±14.0) d in PARDS patients. Non-PARDS children stayed (11.0±8.8) and(10.2±7.1) d in hospital and PICU, respectively. The comparision between PARDS and non-PARDS group showed significantly difference in PRISM Ⅲ, PELOD scores, the duration of staying in hospital and PICU (P<0.05). The survival rate was 90.9% (20/22)and 100.0% (11/11) in PARDS and non-PARDS group, respectively.In addition, there was statistically significant difference in BUN,creatinin, AST, procalcitonin (PCT) and PCO2between two group(P<0.05) (Table 2).
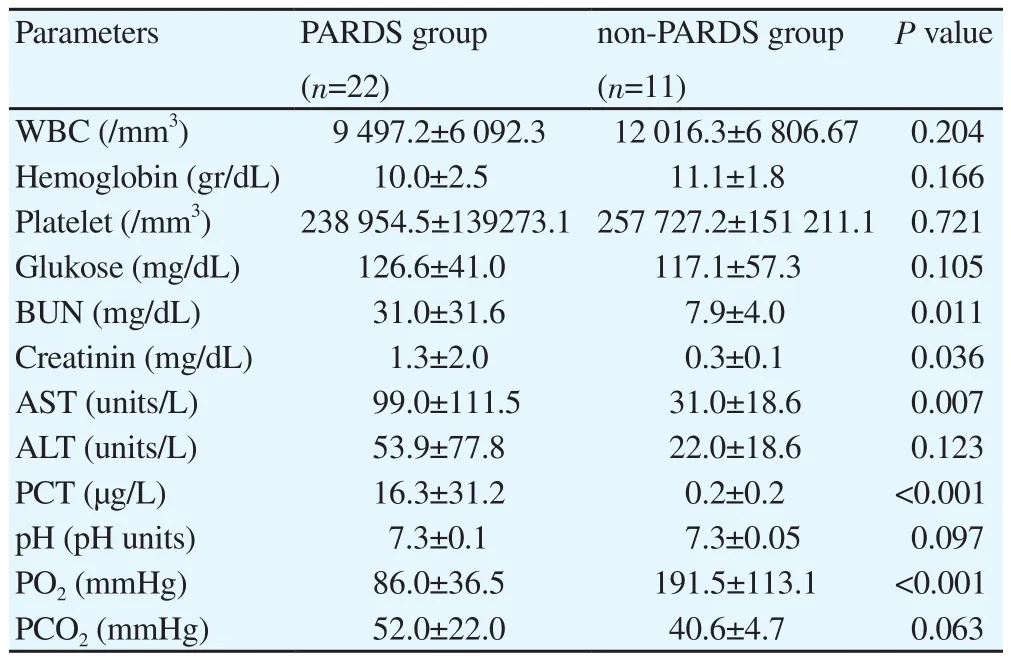
Table 2 Laboratory results.
One PARDS patient had (4.5%) Hif-1α rs11549465(C1772T), while 7 (31.8%) showed rs11549467 (G1790A) gene polymorphisms. While there was no Hif-1α rs11549465 (C1772T)gene polymorphism in non-PARDS group, only one case (9.1%)had Hif-1α rs11549467 (G1790A) gene polymorphism. No statistically significant difference was observed in case number of Hif-1α rs11549465 (C1772T) and rs11549467 (G1790A) gene polymorphisms between PARDS and non-PARDS groups.
For patients with rs11549467 (G1790A), there was no significant difference in degree of hypoxemia or reason of PARDS. But there was significant difference in case number for different infiltration of lung area (Table 3).
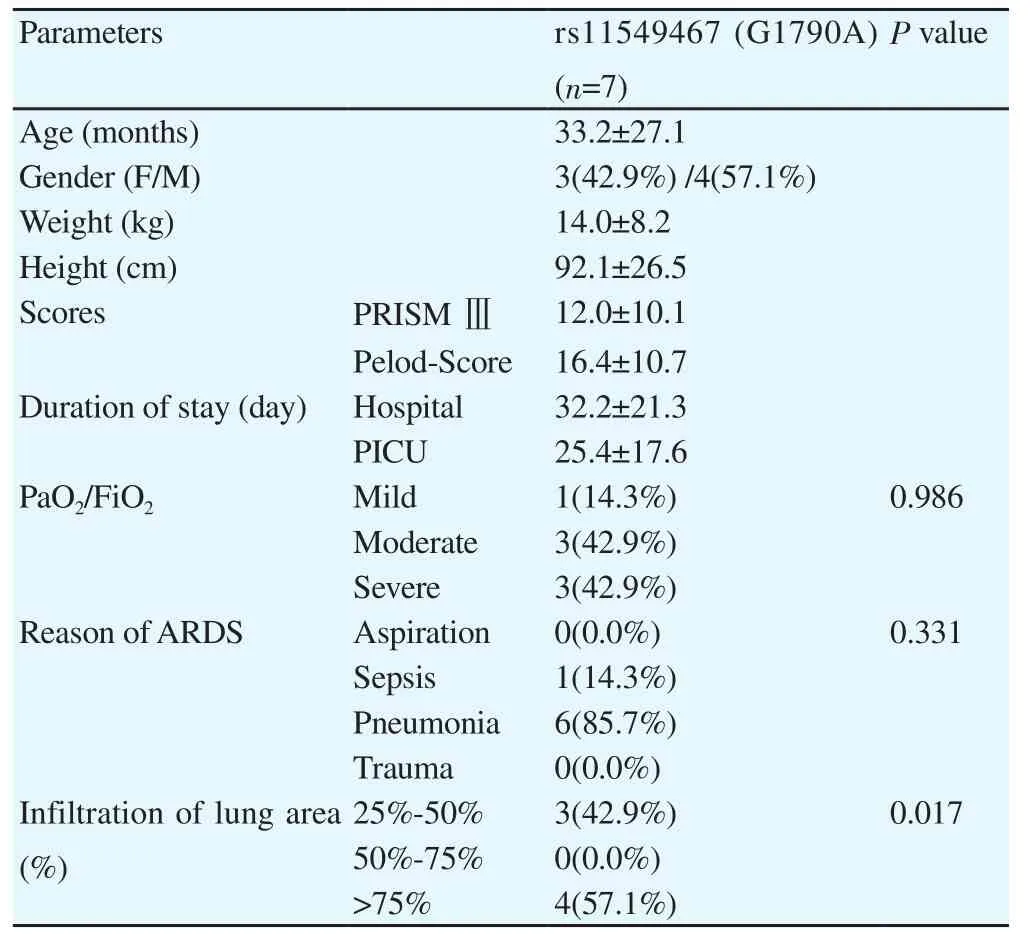
Table 3 Features of PARDS patients with rs11549467 (G1790A).
4. Discussion
Although outcomes for PARDS have improved over the past decade, mortality of PICU remain up to 30%. The genetic investigation of ARDS has been increasingly well described in adult populations, but data from PARDS patients are not sufficient.Hif-1α gene polymorphism is the most popular research in adults with chronic obstructive pulmonary diseases[15,16]. Many studies related to the role of Hif-1α in cancer showed that overexpression of Hif-1α has a prominent association with tumor development and metastasis[17]. Molecular basis of ARDS may conduce to not only identify outcomes, but also create targeted therapies. In the light of these approaches, we wanted to investigate Hif-1α gene polymorphism that could be a biomarker for understanding molecular phenotypes of PARDS.
An acute hypoxic exposure in cells induces the expression of Hif-1α[18]. A stable Hif-1α protein is critical for cells to adapt,thrive and survive in a hypoxic environment. Experimental studies showed that exposure of Hif-1α-deficient fibroblasts to chronic hypoxia results in cell death due to excessive levels of reactive oxygen species[19]. Hif-1α rs11549465 (C1772T) and rs11549467(G1790A) gene polymorphisms could influence acute responses to hypoxia[17]. In our research, we investigated if Hif-1α polymorphisms are related to acute respiratory distress syndrome in children. PCR-restriction fragment length polymorphism was performed to evaluate the C1772T and G1790A polymorphisms of Hif-1α gene, and diversity in genotypes were contrasted with between the two groups.The significant difference was not found between PARDS cases and controls in the distribution of the Hif-1α rs11549465 (C1772T) gene polymorphism. Meanwhile, it was noted that Hif-1α, rs11549467(G1790A) gene polymorphism had increased risk in patients with chronic obstructive pulmonary disease[16]. Our study showed that the distribution of the rs11549467 (G1790A) gene polymorphism was significantly different between PARDS and non-PARDS patients.However, to our knowledge, no study has reported any association between the Hif-1α gene polymorphism and PARDS. Although Hif-1α, rs11549467 (G1790A) gene polymorphism was shown in chronic obstructive pulmonary disease, G1790A polymorphisms should be taken into account in ARDS.
Hif-1α, C1772T polymorphism alters Hif-1α activity in normoxic, as well as in hypoxic conditions. An altered Hif-1 activity during normoxia could influence very acute responses to hypoxia[12]. Hif-1α gene polymorphism has been widely studied in a broadspectrum of diseases associated with hypoxia and neovascularization, especially in cancer types[10]. In one study, a meaningful relationship was observed between Hif-1α G1790A polymorphism and lung cancer risk. Liao et al reported that Hif-1α C1772T polymorphism was found in cases with lung cancer[11]. In addition, Hif-1α, rs11549467 (G1790A) gene polymorphisms was noted in patients with ovarian, cervical, endometrial and colorectal cancer, as well as coronary artery disease[17]. Hif-1α, rs11549467(G1790A) gene polymorphisms should be considered for cancer risk.
Although Hif-1α C1772T and G1790A polymorphisms were not related to reasons of PARDS, our results indicated that the extension and distribution of lung opacities was related to Hif-1α G1790A polymorphism. Therefore, regarding of Hif-1α G1790A polymorphism, the severity of lung infiltration could be predicted early. Furthermore, considering the relationship between Hif-1α G1790A polymorphism and lung malignancy risk, lung damage might be included for following up of PARDS cases closely during adulthood.
5. Conclusion
Our study has limitations. Firstly, we included twenty two PARDS cases, and the number of case could be a little few. Secondly, we followed up PARDS cases in a short duration, and some systemic variances may be disregarded. Thirdly, the assessment of the widening and diffusion of pulmonary opacities in PARDS patients had been limited to only chest X-rays. Lung imaging techniques(ultrasound, computed tomography, positron emission tomography or magnetic resonance imaging) could not be obtained in the ICU because of the possible adverse events when patients were carried to the radiology unit. In addition, the major limitation of present investigation was that we could not analyse the other types of polymorphism of Hif-1α.
The data presented in our study may provide clear evidence that Hif-1α, rs11549467 (G1790A) polymorphisms, which could play a critical role in PARDS. Therefore considering the finding of relationship between infiltration area of lung and Hif-1α, rs11549467(G1790A) gene polymorphisms, early prediction of prognosis should be provided. Future investigations are required to validate present findings and should be conducted to assay the kind of Hif-1α gene polymorphism-dependent prognosis and treatment modalities in children with ARDS.
Conflict of interest statement
The authors report no conflict of interest.
 Journal of Acute Disease2019年2期
Journal of Acute Disease2019年2期
- Journal of Acute Disease的其它文章
- Tardive phenomenon presenting as isolated dysarthria: A rare entity mimicking stroke
- Shoshin syndrome: report of a treatable disaster
- Long-term diabetes-related severe complications among individuals with T2DM in Jazan, Saudi Arabia
- Characterization and frequency of antibiotic resistance related to membrane porin and efflux pump genes among Acinetobacter baumannii strains obtained from burn patients in Tehran, Iran
- The red cell distribution width to platelet ratio predicts 30-day mortality of acute pulmonary embolism patients
- Distribution of extended-spectrum β-lactamase genes in antibioticresistant strains of Pseudomonas aeruginosa obtained from burn patients in Ahvaz, Iran
