Characterization and frequency of antibiotic resistance related to membrane porin and efflux pump genes among Acinetobacter baumannii strains obtained from burn patients in Tehran, Iran
Majid Noori, Behzad Mohsenzadeh, Aghil Bahramian, Fatemeh Shahi, Habibollah Mirzaei,Saeed Khoshnood✉
1Department of Infectious Diseases, Faculty of Medicine, Army University of Medical Sciences, Tehran, Iran
2Golestan Hospital, Army University of Medical Sciences, Tehran, Iran
3Department of Microbiology, school of Medicine, Shahid Beheshti University of Medical sciences, Tehran, Iran
4Department of Microbiology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
5Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
6Hepatitis Research Center, Lorestan University of Medical Sciences, Khorramabad, IR, Iran
Keywords:Acinetobacter baumannii Multidrug resistance Burn Efflux pump genes
ABSTRACT Objective: To explore the characterization and frequency of antibiotic resistance related to membrane porin and efflux pump genes among Acinetobacter baumannii (A. baumannii)strains obtained from burn patients in Tehran, Iran. Methods: In this cross-sectional descriptive study, 100 strains of A. baumannii isolated from burn patients visiting teaching hospitals of Tehran were collected from January 2016 to November 2017. After A. baumannii strains were confirmed, antimicrobial susceptibility testing was done via Kirby-Bauer disc diffusion method according to the Clinical and Laboratory Standards Institute guidelines. PCR amplification was performed for detection of β-lactamase adeR, OprD, adeS genes among A. baumannii strains. Results: All isolates (100%) were resistant to ceftazidime, cefotaxime, cefepime,ciprofloxacin, and piperacillin, and most isolates indicated high resistance (95%-97%) to meropenem, imipenem, gentamicin, ceftriaxone, trimethoprim-sulfamethoxazole, piperacillintazobactam, amikacin, and tetracycline. The most effective antibiotic against A. baumannii isolates was colistin (97% sensitivity), followed by tigecycline. The frequency of OprD, adeS,and adeR genes were 98%, 91%, and 77%, respectively. Conclusions: This study shows that the majority of A. baumannii isolates are highly resistant to the antibiotics most commonly used in burn patients. Also, high distribution of OprD and adeRS genes may be responsible for the observed resistances among A. baumannii isolates that demonstrate the possible role of both efflux pumps in simultaneous of carbapenemase production during antibiotic resistance.
1. Introduction
Bacterial infections continue to be a main cause of mortality among burn patients in spite of good treatment and effective prophylaxis.Often treatment is complex and difficult by the expanding of antibiotic resistant bacteria. In spite of significant progresses in infection control practices and burn wound care, infection remains the leading cause of death in burn patients[1,2].
Burn injuries are one of the most severe and devastating forms of trauma. Seventy-five percent of all deaths in patients with severe burns over 40% are due to infection. These patients have a vital need for specialized care to decrease mortality effects[3]. In burn patients, immune system responses and normal human skin function are impaired. Burn wounds provide appropriate environment for the growth of different types of bacteria and factors such as degree burn and age, can affect these infections[4,5].
Acinetobacter baumannii (A. baumannii) is one of the most important bacteria in hospital-acquired infections, mainly in burn wards and the intensive care units. This opportunistic bacterium can be easily isolated from soil, hospital facilities, and water. A. baumannii as opportunistic bacteria is resistant to many types of antibiotics and responsible for numerous infections, including surgical wounds,meningitis, ventilator-associated pneumonia, urinary tract infection and bacteriemia[6,7]. A. baumannii is resistant to UV and types of detergents and common chemical disinfectants. This nosocomial pathogen can survive in dry environments of hospital and is isolated from clinical equipment, curtain and bed[8].
Emergence of resistance to multiple antibacterial drugs in A.baumannii has become an important public health issue. Multi-drug resistance (MDR) is labeled because of their resistance to more than one antibacterial drug. Infection with MDR can lead to prolonged antibacterial treatment[9]. Resistance mechanisms in A. baumannii have largely been reported in various studies, which are associated with β-lactam anti¬biotics resistance including producing enzymes,over-expression of efflux pumps, mutation in membrane porin, and chang¬ing in conformation of penicillin-binding proteins[10].
The expression of AdeABC efflux pump is tightly regulated by the two-component system, which contains a sensor kinase AdeS and a response regulator AdeR, encoded by the adeRS operon transcribed in the opposite direction of AdeABC operon[11]. The aim of this study was to explore the characterization and frequency of antibiotic resistance related to membrane porin and efflux pump genes among A. baumannii strains obtained from burn patients in Tehran, Iran.
2. Materials and methods
2.1. Study design, data and specimen collection
The present study was carried out by using 100 strains of A.baumannii isolated from burn patients visiting Golestan and Besat hospitals located in Tehran, Iran, from January 2016 to November 2017. The study was conducted in accordance with the Declaration of Helsinki. The clinical specimens including Urine (15%), sputum(8%), blood (20%), catheter (22%), and wound (35%) were obtained from the patients by using sterile swabs, and then were cultured on Mac Conkey and blood agars, and also on Nutrient media (Merck,Germany) to confirm the Gram-negative bacteria via Gram stain microscopy analysis. Furthermore, by applying standard biochemical and microbiological examinations (e.g. citrate, oxidase, and moving tests, and also growth at 42 ℃), the 100 isolates were determined and confirmed to be included in the current study. Then, species identification was performed by the polymerase chain reaction(PCR) amplification of the intrinsic blaOXA-51-like carbapenemase gene.
2.2. Antimicrobial susceptibility testing
Antimicrobial susceptibility assay was conducted via Kirby-Bauer disc diffusion technique in which A. baumannii ATCC19606 and Escherichia coli ATCC 25922/Pseudomonas aeruginosa ATCC 27853 were used as the control strain and negative controls, respectively.Moreover, zone of inhibition was measured according to the Clinical and Laboratory Standards Institute recommendations 2017. To determine the susceptibilities, the following antibiotics were used:piperacillin-tazobactam, ceftriaxone, ceftazidime, cefotaxme,cefepime, ciprofloxacin, imipenem, meropenem, gentamicin,tigecycline, amikacin, tetracycline, trimethoprim-sulfamethoxazole,piperacillin, and colistin.
2.3. DNA extraction and PCR analysis
The DNA Extraction Kit provided by Bioneer, Republic of Korea was used in genomic DNA extraction, based on the previous studies[5]. PCR amplification (using a master mix provided by Bioneer Company, Korea) was conducted to detect the β-lactamase adeR, OprD, adeS genes among A. baumannii strains via primers pairs indicated in Table 1. The amplification was performed under the following conditions in 35 cycles: 3 min at 95 ℃, 1 s at 52-55 ℃,45 s at 72 ℃, and final 5 min at 72 ℃, respectively for denaturation,annealing, extension, and final extension. In additionally, PCR purification and sequencing were done by PCR purification kit(Bioneer Co., Korea and the Bioneer Company, respectively).Ultimately, analysis of the nucleotide sequences was carried out via Chromas 1.45 software and BLAST.

Table 1 Primers used for detection of OprD, adeS, and adeR genes among A.baumannii isolates.
2.3. Statistical analysis
The data were entered and analyzed using the SPSSTMsoftware,version 22.0 (IBM Corporation, Armonk, NY, USA) and Microsoft Excel 2016 (Microsoft Corporation, USA) statistical software.
3. Results
A total of 100 A. baumannii isolates were collected from different sources. Sixty-three (63%) patients were male and 37 (37%) were female and mean age of them was (30±1) years, with a range of 2 to 65 years. The effect of antimicrobial agents studied in the present work against A. baumannii isolates is listed in Table 2. As it has been demonstrated, all isolates were found to be 100% resistance to ceftazidime, cefotaxime, cefepime, ciprofloxacin, and piperacillin,and most isolates indicated high resistance to meropenem,imipenem, gentamicin, ceftriaxone, trimethoprim-sulfamethoxazole,piperacillin-tazobactam, amikacin, and tetracycline, showing a high resistance rate to these agents among A. baumannii strains isolated from burn patients examined in this study. On the other hand, the most effective agent against A. baumannii isolates was colistin.A total of 97% of the strains were fully sensitive to colistin, and conversely only 3% of the isolates were found to be intermediately resistant to the drug. In this way, colistin was followed by tigecycline, as the second effective agent used in this investigation.As Table 2 showing, 58% and 11% of the strains were found to be susceptible and intermediately resistant to tigecycline, respectively;and only 31% of the isolates were fully resistant to the drug.Furthermore, the rate of MDR among strains tested in this study was high, and 91 isolates (91%) had MDR.
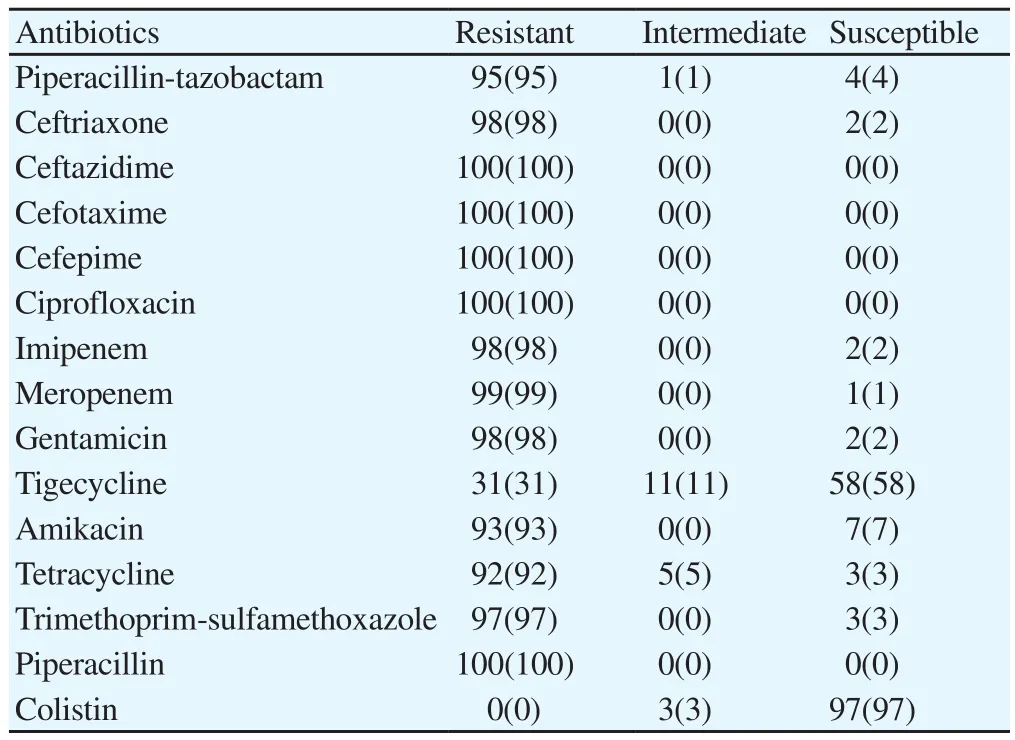
Table 2 Antibiogram patterns of A. baumannii isolates against different antibiotics[n(%)].
The PCR findings demonstrate a wide distribution of OprD, adeS,and adeR genes among A. baumannii isolated tested in this study.The frequencies of OprD, adS, and other genes among A. baumannii strains were shown to be 98%, 91%, and 77%, respectively, that were correlated with high distribution of resistance to studied antimicrobial agents among the strains.
4. Discussion
Burn wound surface is considered as an appropriate environment for colonization and dissemination of microbial pathogens that may arise either endogenously or exogenously from the patient’s body skin or contaminated external surfaces, respectively; and may render burn patients more susceptible to colonization of opportunistic pathogens[12]. Currently, A. baumannii is known as one of the most common opportunistic bacterial pathogens in hospital-associated infections, in particular, among those patients hospitalized in burn intensive care unit[13].
The significant mortality rate of burn patients with MDR A.baumannii infections has been emerged as a challenge, globally,which demands more investigations on this subject for better management of these patients[14]. In the present work, it was shown that 91% of the A. baumannii strains isolated from burn patients were MDR isolates. This finding is in accord with the results reported by previous study conducted by Vakili and colleagues which demonstrated that 95% of A. baumannii isolates were MDR isolates[15], and another study by Yang et al.[10] which showed that 61.4% of isolates were MDR.
In the current study we found that all of the strains were fully(100%) resistant to ceftazidime, cefotaxime, cefepime, ciprofloxacin,and piperacillin. Vakili et al.[15], Shakibaie et al.[16], Ghasemian et al.[17], and Khoshnood et al.[5] reported the same results for ciprofloxacin, piperacillin, cefepime, and ceftazidime/ ciprofloxacin/cefepime. These studies revealed that 100% of isolates were fully resistant to these antibiotics.
Furthermore, colistin and tigecycline were the most effective antimicrobial agents used in the current study against A. baumannii isolates, of which 97% and 58% were fully susceptible to these agents, respectively. Ghasemian et al.[17] indicated that 84% of strains were sensitive to colistin in their study. In addition, Rastegar-Lari et al.[18] reported that 100% strains isolated from burn patients were sensitive to colistin.
In our work, the distribution of OprD, adeS, and adeR genes among A. baumannii strains was 98%, 91%, and 77%, respectively.Similarly, study of Asadolah-Malayeri et al.[19] from Iran showed that 100% of A. baumannii isolates were resistant to ceftriaxone,ciprofloxacin, cefotaxime, ceftazidime, meropenem, cefepime,piperacillin, co-trimoxazole and piperacillin/tazobactam, and the prevalence of adeR and adeS genes among the A. baumannii isolates was 95% and 60%, respectively.
In a study from China, it was shown that 80% of A. baumannii isolates resistant to imipenem, carried adeR and adeS genes[20],which is in line with the results reported in our study. We found the frequency of the mentioned genes among the isolates that were 98%resistant to imipenem were 77% and 99%, respectively. Rajamohan et al.[21] reported that the frequency of adeS and adeR genes was 55% among A. baumannii isolates tested in their study. Rastegar-Lari et al.[19], found that the rates of resistance to ciprofloxacin,ceftazidime, cefepime, and imipenem were 88%, 88%, 74%, and 72%, respectively, and reported that 36% of 50 clinical A. baumannii isolates carried adeR and adeS genes, simultaneously.
Regarding to OprD gene, a study from Iraq investigated the frequency of the gene among 44 isolates of A. baumannii, and found that 100% of the isolates were positive for OprD gene, which is similar to the present study[19]. The most frequent mechanisms for antibiotic resistance include enzymatic inactivation of the drugs via bacterial enzymes, reduced permeability or active efflux of the drugs by upregulation of multidrug RND efflux pumps or modifications of outer membrane protein, respectively[20].
Previous studies have shown that in compared to the wild-type parent, A. baumannii with mutated OprD gene did not demonstrate differences in the resistance to imipenem, meropenem, ceftazidime,and ciprofloxacin suggesting that the A. baumannii OprD gene is not responsible for resistance to these antibiotics[21]. Similarly,in the present work, we found a high distribution of OprD gene in parallel with high resistance to mentioned drugs among the isolates,supporting this notion that OprD gene is not responsible for this resistance. Studies demonstrated that AdeABC confers resistance via extruding various antibiotics such as aminoglycosides and β-lactams. So that, reduced susceptibility to cefepime, cefpirome, and cefotaxime has been correlated with overexpression of AdeABC.
On the other hand, it was shown that AdeRS mediate constitutive expression of AdeABC in a transcriptional activator manner[22].Hence, based on AdeRS findings from this study, it can be concluded that the high distribution of adeRS genes may be responsible for the observed resistances among these isolates in an AdeABC manner. The existence of adeR, adeS genes in more than 70% of A.baumannii isolates demonstrate the possible role of both efflux pump in simultaneous of carbapenemase production during antibiotic resistance of A. baumannii isolates. So, new strategies are required in order to stop the vertical or horizontal exchanges of the efflux pump and OXA-23 genes from the resistant A. baumannii isolates to sensitive strains.
Conflict of interest statement
The authors report no conflict of interest.
 Journal of Acute Disease2019年2期
Journal of Acute Disease2019年2期
- Journal of Acute Disease的其它文章
- Tardive phenomenon presenting as isolated dysarthria: A rare entity mimicking stroke
- Shoshin syndrome: report of a treatable disaster
- Long-term diabetes-related severe complications among individuals with T2DM in Jazan, Saudi Arabia
- Polymorphism of hypoxia-inducible factor-1α gene in pediatric acute respiratory distress syndrome
- The red cell distribution width to platelet ratio predicts 30-day mortality of acute pulmonary embolism patients
- Distribution of extended-spectrum β-lactamase genes in antibioticresistant strains of Pseudomonas aeruginosa obtained from burn patients in Ahvaz, Iran
