Early treadmill exercise increases macrophage migration inhibitory factor expression after cerebral ischemia/reperfusion
Min Cheol Chang, Chae Ri Park, Seung Hwa Rhie, Woo Hyun Shim, , Dae Yul Kim,
1 Department of Rehabilitation Medicine, College of Medicine, Yeungnam University, Daegu, Republic of Korea
2 Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
3 Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
4 Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
AbstractThe neuroprotective function of macrophage migration inhibitory factor (MIF) in ischemic stroke was rarely evaluated. This study aimed to investigate the effects of early treadmill exercise on recovery from ischemic stroke and to determine whether these effects are associated with the expression levels of MIF and brain-derived neurotrophic factor (BDNF) in the ischemic area. A total of 40 male Sprague-Dawley rats were randomly assigned to the ischemia and exercise group [middle cerebral artery occlusion (MCAO)-Ex,n = 10), ischemia and sedentary group (MCAO-St, n = 10), sham-surgery and exercise group (Sham-Ex, n= 10), or sham-surgery and sedentary group (Sham-St, n = 10). The MCAO-Ex and MCAO-St groups were subjected to MCAO for 60 minutes, whereas the Sham-Ex and Sham-St groups were subjected to an identical operation without MCAO. Rats in the MCAO-Ex and Sham-Ex groups then ran on a treadmill for 30 minutes once a day for 5 consecutive days. After reperfusion, the hanging time tested by the wire hang test was longer and the relative fractional anisotropy determined by MRI was higher in the peri-infarct region of the MCAO-Ex group compared with the MCAO-St group. The expression levels of MIF and BDNF in the peri-infarct region were upregulated in the MCAO-Ex group. Increased MIF and BDNF levels were positively correlated with relative fractional anisotropy changes in the peri-infarct region. There was no significant difference in the levels of MIF and BDNF in the peri-infarct region between the Sham-Ex and Sham-St groups. Our study demonstrated that early exercise (initiated 48 hours after the MCAO) could improve motor and neuronal recovery after ischemic stroke. Furthermore, the increased levels of MIF and BDNF in the peri-infarct region (penumbra) may be one of the mechanisms of enhanced neurological function recovery. All experiments were approved by the Institutional Animal Care and Use Committee in Asan Medical Center in South Korea (2016-12-126).
Key Words: ischemic stroke; early exercise; macrophage migration inhibitory factor; brain-derived neurotrophic factor; motor recovery; neural regeneration
Introduction
Ischemic stroke results from the occlusion of the blood vessels that supply blood to the brain. It accounts for around 80% of strokes, which is a leading cause of physical disability (Dennis et al., 1993; Jang and Chang, 2013). The early commencement of exercise after stroke is recommended in many clinical practice guidelines to improve the recovery from physical disability (Adams et al., 1994; Bernhardt et al.,2008; Billinger et al., 2014). However, although several studies have reported the positive effects of exercise after acute ischemia, these effects have not been clearly elucidated.
Ischemic regions can be divided into the ischemic core and the penumbra, according to the pattern of neuronal damage.Neurons in the ischemic core are rarely salvageable due to their immediate necrosis. In contrast, the ischemic insult of neurons in the penumbra is relatively mild (Lindely et al.,1993; Yoo et al., 2009). Therefore, the penumbra has potential for post-ischemic recovery and is considered to be the target of therapeutic intervention (Lindely et al., 1993; Yoo et al.,2009). Furthermore, after the acute stage of ischemic injury,additional damage in the ischemic area can occur through the activation of the inflammatory response and apoptotic pathways (Broughton et al., 2009; Kawabori and Yenari, 2015;Vidale et al., 2017). Accordingly, protecting neurons in the ischemic area from in flammation and apoptosis is a potential strategy to improve post-ischemic stroke recovery.
Macrophage migration inhibitory factor (MIF) was first identified as an in flammatory cytokine with a focus on the potential protective function of MIF coming later (Bernhagen et al., 1993; Calandra and Roger, 2003). Several studies have reported that MIF has a protective function during ischemic events (Amaral et al., 2007; Miller et al., 2008;Koga et al., 2011; Zhang et al., 2014). However, the majority of these studies involved intestinal ischemia and myocardial infarction. Therefore, little is known about the neuroprotective function of MIF in ischemic stroke.
In the current study, we investigated the neuroprotective effects of early treadmill exercise on the damaged brain and whether these effects are associated with MIF expression in the ischemic area in a rat model of ischemic stroke. Additionally, we evaluated the changes in the level of brain-derived neurotrophic factor (BDNF), which is one of the crucial factors in neurogenesis (Chen et al., 2016), and analyzed the correlation between the expression of BDNF and the effects of early treadmill exercise.
Materials and Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee in Asan Medical Center in South Korea (2016-12-126). A total of 40 male Sprague-Dawley rats (body weight 250—280 g, aged 8 weeks) were randomly assigned to four groups using a random table method: an ischemia and exercise group [middle cerebral artery occlusion (MCAO)-Ex, n = 10)], an ischemia and sedentary group(MCAO-St, n = 10), a sham-surgery and exercise group(Sham-Ex, n =10), and a sham-surgery and sedentary group(Sham-St, n =10). The rats were habituated under a 12-hour light/dark cycle (lights on at 07:00 a.m.) at 22 ± 1°C with ad libitum access to food and water.
Middle cerebral artery occlusion
Focal cerebral ischemia was induced in the rats through transient occlusion of the right middle cerebral artery (MCA)using the intraluminal filament technique as described by Sasaki et al. (2009). In brief, the animals received inhalational anesthesia with 5% isoflurane (JW Pharm, Seoul, South Korea), and temporalis temperatures were monitored and maintained at 37 ± 0.5°C using a circulating heating pad. A midline incision was made on the ventral neck, and the right external, internal, and common carotid arteries (ECA, ICA,and CCA) were exposed. A silicon-coated mono filament suture was introduced through the ECA, passed into the ICA,and advanced to the root of the right MCA. After 1 hour of MCAO, the filament was withdrawn, and reperfusion was observed. The sham-surgery rats were subjected to an identical operation without MCAO. In the sham-surgery rats,the filament was not inserted into the MCA. The rats were sacrificed 7 days after MCAO, and the impaired cortical areas were extracted for subsequent biochemical analysis.
Exercise training
A motor-driven treadmill (ZH-PT Treadmill; Huaibei Zhenghua Bio Equipment Co., Ltd., Huaibei, Anhui Province,China) was used for exercise training. Before the MCAO procedure, rats in the exercise groups were trained to run on the treadmill for 10 min/day over a 3-day accommodation period. Forty-eight hours after the MCAO procedure, rats in the MCAO-Ex and Sham-Ex groups were trained on the treadmill (0° slope) for 5 consecutive days (15 m/min, 30 min/day). Rats in the MCAO-St and Sham-St groups did not engage in any exercise.
Behavioral test
The wire hang and Garcia tests were performed at 24 hours and 7 days after reperfusion to evaluate the motor strength and neurological functions of the animals, respectively.These tests were performed by a rater who was blinded to the treatment. For the wire hang test (Oliván S et al., 2015),a wire mesh grid (15 cm × 25 cm) was used. The rats were placed on the wire mesh grid 40 cm above a foam cushion.Then, the mesh was inverted 180°, and the rats were forced to grasp the wire using their four limbs. The hanging time was recorded as the duration the rats remained hanging before falling on the cushion. This test was repeated 5 times with an interval of 3 minutes between trials. The cut-off time was set at 60 seconds. The mean latency to fall in the five trials was determined. The Garcia test for evaluating neurological functions consisted of 6 subtests: spontaneous activity,symmetry of movements, symmetry of forelimbs, climbing wall of wire cage, reaction to touch on either side of trunk,and response to vibrissae touch (Garcia et al. 1995). The total scores ranged from 3 to 18, and higher scores corresponded to better functions. Scores were measured three times by a rater, and the mean score was used for analysis.
Imaging analysis
For all four groups, magnetic resonance imaging (MRI) was performed at 24 hours and 7 days after ischemia/reperfusion modeling (Figure 1). MRI was conducted using a 9.4 T/160 mm animal MRI system (Agilent Technologies, Santa Clara, CA, USA). A 72 mm birdcage volume coil was used for excitation, and a four-channel phased array surface coil served as the receiving coil. All animals were anesthetized through the spontaneous inhalation of 2.0—2.5% iso flurane(JW Pharm) in a 1:2 mixture of O2:N2O using a mask. Respiration was monitored, and the rats were kept normothermic at 37.5 ± 0.5°C using an air heater system.
T2-weighted images (T2WIs) were acquired with a fast spin-echo sequence [time to repeat (TR) = 4000 ms, k-zero= 3, echo spacing = 10.98 ms, 32 segments, echo train length= 8, effective time to echo (TE) = 32.95 ms, average = 1, matrix = 256 × 256, field of view (FOV) = 30 mm × 30 mm, and slice thickness = 1.0 mm, no gap). Diffusion-weighted images (DWIs) were obtained using a 4-shot spin-echo-based echo planar imaging sequence with TR/TE = 3750/46.22 ms, 96 × 96 matrix, and an encoding scheme of 30 gradient directions with a b-value of 1000 s/mm2. The number and orientation of slices and FOV in the DWIs were the same as in the T2WIs.
The degree of ischemic injury was evaluated by measuring the fractional anisotropy (FA) values on the FA map derived from diffusion MRI data. All regions of interest (ROIs) were drawn free-hand using Analysis of Functional NeuroImages software (AFNI, http://afni.nimh.nih.gov/afni/) based on the FA map. Four ROIs were drawn for the brain of each rat (Figure 1). The ROIs were drawn on the ischemic core(ROI 1) and the peri-infarct region (penumbra; ROI 2) of the ipsilesional hemisphere as well as on the corresponding regions of the contralesional hemisphere (ROIs 3 and 4).The Diffusion Toolkit (http://trackvis.org/dtk/) was used to calculate voxel-wise values for FA. All FA values in each ROI were obtained individually to generate group differences. Then, relative FA (rFA) values were calculated as a ratio,i.e., ipsilesional FA value/contralesional value. In the case of the sham groups, the area corresponding to the ROIs of the MCAO groups was used for the analysis of the rFA.
Immunohistochemical analysis
At 7 days after reperfusion, the rats were anesthetized with 5% isoflurane and perfused transcardially with 100 mL phosphate-buffered saline (PBS) and 100 mL of 4% paraformaldehyde in 0.1 M PBS. The brains were harvested, fixed in 4% paraformaldehyde, dehydrated in a graded ethanol series, and embedded in paraffin. Then, 4 μm-thick sections were cut from each block. These sections were deparaffinized in xylene, rehydrated in a graded series of ethanol, washed with deionized water, and subjected to microwave in citrate buffer for 10 minutes. Endogenous peroxidase activity was blocked using hydrogen peroxide for 15 minutes to reduce nonspecific immunopositivity. Primary antibodies against MIF (1:100, incubated overnight at 4°C; Abcam, Cambridge, MA, USA) and BDNF (1:100, incubated overnight at 4°C; Abcam) were detected with the HRP/DAB kit (Dako EnVision System; Dako, Glostrup, Denmark). Secondary antibodies include goat anti-rabbit immunoglobulin (1:200 dilution for 1 hour at room temperature; Thermo Fisher Scientific, Waltham, MA, USA). Immunostained sections were digitized using a 40× objective (DFC290; Leica, Heerbrugg,Germany) with the Leica Application Suite (version 3.3.0;Leica).
In the MCAO-Ex and MCAO-St groups, five views of each of the ischemic core, peri-infarct region, and corpus callosum were captured in grayscale. In the Sham-Ex and Sham-St groups, the areas that corresponded to the areas in the MCAO groups were captured. Optical densities were measured in five rectangles of 10,000 μm2by MCID analysis (evaluation version 7.0; Imaging Research Inc.,Ontario, Canada), and the measured values were averaged.The optical density of the corpus callosum was used for normalization.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 software (IBM, Armonk, NY, USA). The rFA values and the results of the behavioral tests and immunohistochemical analysis for the exercise and sedentary groups were compared using the independent samples t-test. The correlation between the rFA and immunohistochemical results was evaluated by Pearson's correlation analysis. The statistical significance was set at P < 0.05.
Results
Infarction formation after focal cerebral ischemic stroke in rats
Rats that underwent MCAO surgery (n = 20) showed acute tissue damage in most of the MCA territory in the right hemisphere, which was confirmed by both T2WIs and DWIs.
Changes in motor strength and neurological functions after treadmill exercise
At 24 hours after reperfusion, there was no significant difference in the wire hang test results between the MCAO-Ex and MCAO-St groups (P = 0.181). However, at 7 days after reperfusion, the hanging time in the MCAO-Ex group was significantly longer than that in the MCAO-St group (P =0.042; Figure 2). On the other hand, there was no significant difference in the hanging time between the Sham-Ex and Sham-St groups at 24 hours and 7 days after reperfusion (24 hours: P = 0.430, 7 days: P = 0.129; Figure 2).
Figure 2 Results of the wire hang test of rats.
At both 24 hours and 7 days after reperfusion, there was no significant difference in the results of the Garcia test for evaluating neurological functions between the MCAOEx and MCAO-St groups (24 hours: P = 0.330, 7 days: P =0.180). Similarly, there was no significant difference in the results between the Sham-Ex and Sham-St groups (24 hours:P = 0.500, 7 days: P = 0.170).
Changes in the relative fractional anisotropy after treadmill exercise
At 24 hours after reperfusion, the rFA values were not significantly different between the peri-infarct region of the MCAO-Ex and MCAO-St groups (P = 0.332). However, at 7 days after reperfusion, the rFA values were significantly higher in the MCAO-Ex group than in the MCAO-St group(P = 0.006). On the other hand, there was no significant difference between the Sham-Ex and Sham-St groups at 24 hours and 7 days after reperfusion (24 hours: P = 0.886, 7 days: P = 0.860; Figure 3).
The rFA values were not significantly different between the ischemic core of the MCAO-Ex and MCAO-St groups or between the Sham-Ex and Sham-St groups (MCAO-Ex vs.MCAO-St - 24 hours: P = 0.784, 7 days: P = 0.856; Sham-Ex vs. Sham-St - 24 hours: P = 0.454, 7 days: P = 0.812; Figure 3).
Changes in the MIF and BDNF expression levels following treadmill exercise
The expression of MIF at 7 days after reperfusion in both the peri-infarct region and the ischemic core was significantly higher in the MCAO-Ex group than in the MCAO-St group(peri-infarct region: P < 0.001, ischemic core: P < 0.001;Figure 4). The area corresponding to the peri-infarct region showed no significant difference in the MIF expression level between the Sham-Ex and Sham-St groups (P = 0.103; Figure 4). However, in the area corresponding to the ischemic core, the MIF expression level was significantly higher in the Sham-Ex group than in the Sham-St group (P = 0.039;Figure 4).
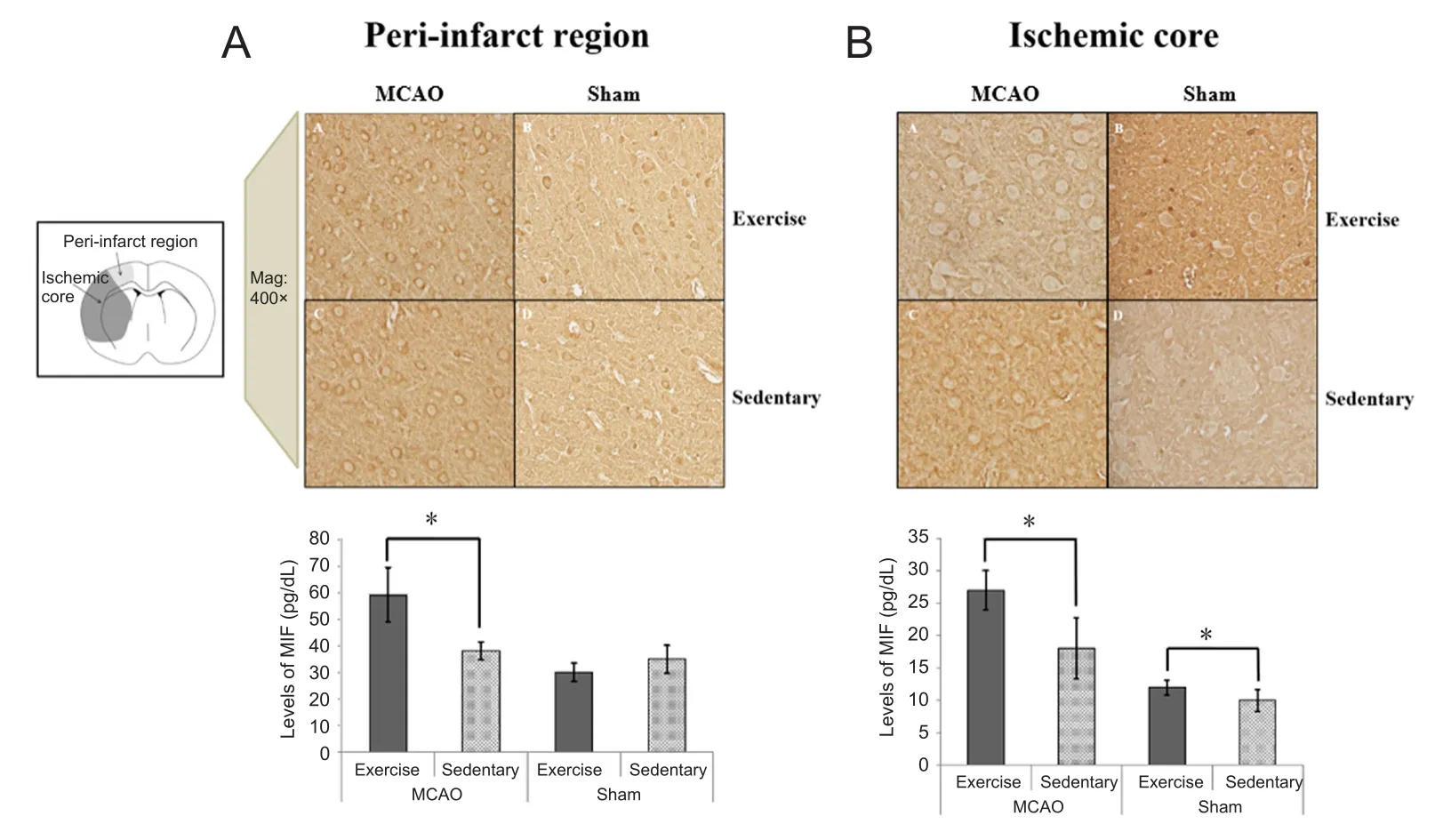
Figure 4 Immunohistochemical findings of MIF in the periinfarct region (A) and ischemic core (B) in rats at 7 days after reperfusion.
In the peri-infarct region, the average expression level of BDNF was significantly higher in the MCAO-Ex group than in the MCAO-St group (P < 0.001). However, there was no significant difference in the BDNF level between the Sham-Ex and Sham-St groups (P = 0.320; Figure 5). In the ischemic core, the level of BDNF was not significantly different between the MCAO-Ex and MCAO-St groups (P =0.079; Figure 5). Additionally, there was no difference in the BDNF level between the Sham-Ex and Sham-St groups (P =0.965; Figure 5).
Correlation between the relative fractional anisotropy and expression levels of MIF and BDNF
The rFA values in the peri-infarct region of the MCAO groups (MCAO-Ex and MCAO-St) were significantly positively correlated with the expression levels of MIF (P = 0.037,r = 0.661) and BDNF (P = 0.033, r = 0.673) in the peri-infarct region (Figure 6). The expression level of MIF was significantly partially correlated with rFA after controlling BDNF level (P = 0.041, r = 0.495). The expression level of BDNF was significantly correlated with rFA even through the level of MIF was controlled (P = 0.043, r = 0.456).
Discussion
In the current study using a rat model of ischemic stroke, we found that the recovery of motor strength was enhanced, and the rFA in the peri-infarct region (penumbra) was increased following early treadmill exercise. Immunohistochemical results revealed that the expression of MIF was upregulated in the ischemic core and the peri-infarct region of rats that performed early treadmill exercise. Additionally, the level of BDNF in the peri-infarct region was also increased after early treadmill exercise. The increased expression levels of MIF and BDNF in the peri-infarct region were associated with changes in the rFA in the peri-infarct region.
Previous studies have demonstrated that early exercise after ischemic stroke can enhance motor recovery (Stoller et al., 2012; Coleman et al., 2017; Yang et al., 2017). Coleman et al. (2017) reported that initiation of rehabilitation within the first 2 weeks of stroke is beneficial to improve recovery of impaired motor function. Yang et al. (2017) found that 2-week exercise initiating at 24 hours after MCAO promoted the motor and spatial memory recovery in rats with MCAO.In agreement with the results of these studies, our study also showed that motor strength improvement was greater in ischemic rats that exercised early than in those that did not exercise. However, there was no significant difference in the hanging time between two groups.
When interpreting our results regarding the Garcia test,we should consider the fact that our result might have statistical significance without clinical significance. Additionally,we found that the rFA in the peri-infarct region was higher after early exercise in rats that were subjected to MCAO.The FA represents the degree of diffusion directionality of white matter microstructures, such as the axons, myelin,and microtubules (Assaf and Pasternak, 2008; Neil, 2008). A high FA value indicates that the density and directionality of the fiber bundles and myelination are increased (Assaf and Pasternak, 2008; Neil, 2008). Therefore, our results suggest that early treadmill exercise could protect the peri-infarct region (i.e., penumbra) from additional damage after ischemic stroke and promote neuronal recovery. Several possible mechanisms have been proposed to explain the positive effects of early exercise. Previous studies have found that early exercise promotes angiogenesis, increases microvessel density, and improves cerebral blood flow (Zhang et al., 2013;Morland et al., 2017). These effects are known to reduce brain infarct volume (Zhang et al., 2013; Morland et al.,2017). Additionally, Zhang et al. (2012) demonstrated that early exercise after ischemic stroke could improve functional outcomes through inhibiting acute neuroinflammatory response in the ischemic area.
In our study, we found that the expression levels of MIF and BDNF were increased following exercise. The role of MIF during ischemic stroke remains controversial; it has been proposed to be protective through the suppression of apoptosis or to be harmful through the promotion of in flammation. The redox activity of MIF may be attributed to its Cys-Xaa-Xaa-Cys group, associated with the suppression of oxidative stress-induced apoptosis (Nguyen et al., 2003a, b). In addition, Zhang et al. (2014) found that MIF protected neurons from oxidative stress and apoptosis in rats with MCA territory infarction. In their study, the disruption of the MIF gene in MIF-knockout rats induced neuronal loss and increased the infarct volume. In contrast,Inácio et al. (2011b) reported that MIF could promote the response of macrophages and microglia, which may induce neuronal death and aggravate neurologic deficits after ischemic strokes. However, another study by the same group indicated that MIF was not involved in the promotion of the in flammatory process during the 7 days after ischemic stroke (Inácio et al., 2011a). On the other hand, BDNF is a neurotrophic factor found in the brain, and the upregulation of BDNF can enhance neural repair processes (Chen et al., 2016). In our study, the expression levels of MIF and BDNF were higher in the peri-infarct region of rats that were subjected to MCAO, and the increased expression levels in the peri-infarct region were correlated with the rFA values in the peri-infarct region of the rats. We hypothesized that the expression levels of MIF and BDNF were increased through early treadmill exercise, resulting in the inhibition of apoptosis and the promotion of neural repair processes in the peri-infarct region. Interestingly, MIF expression in rats in the sham groups was also increased after treadmill exercise. This result suggests that exercise can be beneficial for the brain health of people who do not have any brain disorders.
Conclusion and limitations
Our study demonstrated that early exercise could improve motor and neuronal recovery after ischemic stroke. Additionally, the expression levels of MIF and BDNF in the peri-infarct region were higher in groups that exercised for 5 consecutive days after 48 hours of MCAO, and their levels were correlated with neuronal recovery in the peri-infarct region. Our study suggests that elevated MIF and BDNF levels, through early exercise, could protect neurons against damage after acute ischemic stroke or promote the recovery of damaged neurons. Therefore, strategies that enhance the expression of MIF or BDNF within the therapeutic level could be beneficial for patients with ischemic stroke. Our study has several limitations. First, the sample size was relatively small. Second, only young male rats (8 weeks) were recruited. Third, the infarct size was not measured. Fourth, we did not evaluate the long-term effects of early exercise after ischemic stroke. Additionally, further mechanistic studies are required to identify the detailed mechanisms that are responsible for the improved recovery observed after ischemic stroke through early exercise.
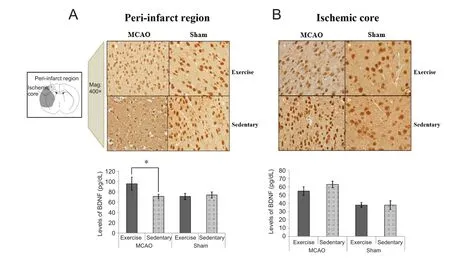
Figure 5 Immunohistochemical findings of BDNF in the periinfarct region (A) and ischemic core (B) in rats at 7 days after reperfusion.
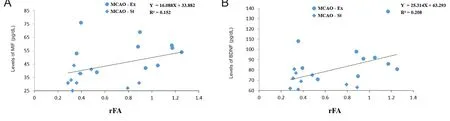
Figure 6 Correlation between the rFA and immunohistochemical results in the peri-infarct region.
Author contributions:Data analysis and manuscript writing: MCC;experiment performance and data analysis: CRP, SHR, and WHS;study design and manuscript writing: DYK. All authors approved the final version of this paper.
Conflicts of interest:None declared.
Financial support:This study was supported by the Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education, No. 2016R1A2B4012772(to DYK). The funding body played no role in the manuscript conception and design, in the critical revision of manuscript for intellectual content, and in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Institutional review board statement:All experiments were approved by the Institutional Animal Care and Use Committee in Asan Medical Center in South Korea (2016-12-126) and conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Jonathan M. Borkum, University of Maine,USA.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Transcriptional dysregulation in neurodegenerative diseases: who tipped the balance of Yin Yang 1 in the brain?
- Redistribution of nerve strain enables end-to-end repair under tension without inhibiting nerve regeneration
- Choroid plexus tumor necrosis factor receptor 1:a new neuroin flammatory piece of the complex Alzheimer's disease puzzle
- Magnesium: pathophysiological mechanisms and potential therapeutic roles in intracerebral hemorrhage
- Bridging larger gaps in peripheral nerves using neural prosthetics and physical therapeutic agents
- Exogenous neural stem cell transplantation for cerebral ischemia

