Bridging larger gaps in peripheral nerves using neural prosthetics and physical therapeutic agents
Muhammad Sana Ullah Sahar , Matthew Barton, Geoffrey Douglas Tansley
1 School of Engineering and Built Environment, Griffith University, Gold Coast, Queensland, Australia
2 Clem Jones Centre for Neurobiology and Stem Cell Therapies, Griffith University, Gold Coast, Queensland, Australia
Abstract Peripheral nerve injuries are relatively common and can be caused by a variety of traumatic events such as motor vehicle accidents. They can lead to long-term disability, pain, and financial burden, and contribute to poor quality of life. In this review, we systematically analyze the contemporary literature on peripheral nerve gap management using nerve prostheses in conjunction with physical therapeutic agents. The use of nerve prostheses to assist nerve regeneration across large gaps (> 30 mm) has revolutionized neural surgery. The materials used for nerve prostheses have been greatly refined, making them suitable for repairing large nerve gaps. However, research on peripheral nerve gap management using nerve prostheses reports inconsistent functional outcomes, especially when prostheses are integrated with physical therapeutic agents, and thus warrants careful investigation. This review explores the effectiveness of nerve prostheses for bridging large nerve gaps and then addresses their use in combination with physical therapeutic agents.
Key Words: nerve repair; nerve conduits; nerve prosthesis; physical therapeutic agents; electric stimulation;nerve gap; axonal growth; nerve regeneration
Introduction
The basic structure of a peripheral nerve fiber comprises an axon, myelin sheath (if myelinated) and the endoneurium.Damage to any of these components, whether temporary or permanent, is likely to hamper nerve functionality. Among nerve lesions, complete transection is the most severe as it involves the total severing of the nerve fiber, causing a discontinuity of electrical transmission and thereby making the affected innervated region dysfunctional. This form of nerve trauma invariably warrants surgical intervention to bridge the gap between the resected ends; however, even with the aid of modern surgical techniques, the regenerative period may span months to years (Menorca et al., 2013).
Nerve prostheses are being widely employed in clinical settings to bridge neural gaps of various lengths. In addition to natural materials, they can be manufactured from a wide range of biocompatible synthetic materials. Nerve prosthesis materials, in conjunction with the use of physical therapeutic agents (PTAs), such as electrical stimulation, electromagnetic excitation, ultrasound, and laser therapy, play a vital role in successful nerve regeneration. However, much of the literature has only considered modest gap lengths (less than 10 mm) in rodents, which have a high nerve regeneration capacity. Thus, the true efficacy of nerve prostheses in humans is unknown. In this review, we first focus on the use of nerve prostheses (with different materials) for bridging large nerve gap defects (≥ 10 mm) and then focus particularly on nerve prostheses integrated with PTAs. The extracorporeal use of PTAs has recently gained much popularity in clinical practice. These therapeutic tools can accelerate peripheral nerve regeneration by mitigating the loss of muscle function and up-regulating growth factors at the injury site.
A systematic literature search was performed (Figure 1)which resulted in the collection of 616 studies from online databases related to peripheral nerve gap management using nerve prostheses and the use of PTAs to enhance nerve regeneration. The selection criterion (Figure 1) for the first half of this review was studies related to complete nerve transections with gap lengths ≥ 10 mm. The second half included studies considering complete nerve transections of any gap length, as there is a lack of literature on the use of nerve prostheses with integrated PTAs. All studies based on models of nerve crush injuries, as well as duplicate studies,were discarded; subsequently, a total of 68 studies were retained. Figure 2 provides the publication trend for studies on the use of nerve prostheses to repair large segmental defects in peripheral nerves and use of PTAs to assist nerve regeneration processes. It is apparent that research into the use of PTAs to ameliorate nerve repair has increased over the last two decades.
Scope of the manuscript
The scope of this manuscript is to highlight the variety of nerve prostheses that are suitable for nerve regeneration across large gaps and the use of PTAs to promote nerve regeneration. This review is significant as it provides insight into the selection of suitable biomaterials for engineering nerve prostheses for large gaps.
Nerve Prostheses
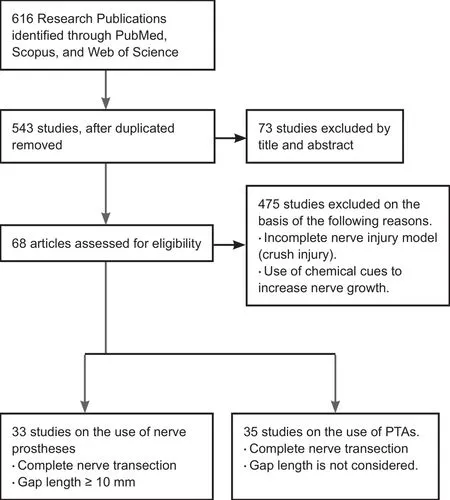
Figure 1 Literature search scheme and selection criteria to enroll studies in this review.
Nerve prostheses, also known as nerve guides, nerve cuffs,nerve protectors, nerve couplers, and nerve conduits, are surgical options for creating favorable environments for nerve fibers to regenerate in large nerve gap injuries. Nerve prostheses essentially provide scaffolding for regenerating nerve fibers without necessitating biological graft material(nerves, veins, etc.). The main concept of every nerve prosthesis is to facilitate continuity of the resected nerve ends by encasing them and allowing the proximal nerve stump to grow and orientate toward the distal nerve stump. These prostheses can be derived from natural sources such as veins, gut fibers, and skeletal muscles, or synthetically manufactured from a variety of biocompatible materials. Nerve repair using prostheses is employed when the end-to-end distance between the resected nerve ends is greater than 10 mm; although once gaps reach 30 mm, outcomes are generally poor (Babu et al., 2008).
Nerve prostheses are credited with providing several benefits such as enhancing nerve regeneration, mitigating scar tissue development, and preventing the leakage of intraneural fluid. However, due to the lack of any clinical standard, selecting the most suitable nerve prosthesis remains contentious and relies on individual surgical judgment. Nerve prostheses may be classified as degradable and non-degradable and include single or multi-internal channels, permeable membranes, and embedded electrodes for electrical stimulation.
Non-degradable prostheses
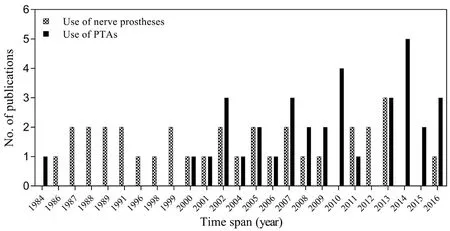
Figure 2 Trend of publications on peripheral nerve regeneration using nerve prostheses.
The use of non-degradable prostheses dates back to the 1980s. These prostheses are derived from natural resources or synthesized from a variety of polymers. Among non-degradable prostheses, silicone and latex are sourced from natural rubber and have demonstrated neurological recovery superior to autologous nerve repair (Ganga et al., 2012), while axons regenerated in a silicone prosthesis showed properties comparable to suture repair across a 10 mm gap two months postoperatively (Fields and Ellisman,1986). Similarly, a polyester prosthesis coated with laminin showed better nerve regeneration across a 10 mm gap in the sciatic nerve of a rat compared to a control (Yoshii et al.,1987). A polytetrafluoroethylene prosthesis was reported to be beneficial in bridging a gap of 29 mm in a human ulnar nerve, where the patient achieved excellent motor and sensory recovery (Stanec and Stanec, 1998). A three-year post-operative review revealed normal nerve tissue within the prosthesis. Furthermore, in a poly-hydroxyethyl-methacrylate prosthesis, axonal regeneration was comparable to autografting a 10 mm gap in rat sciatic nerves, but signs of calcification were noted (Belkas et al., 2005).
Unlike degradable materials, non-degradable materials are often non-permeable and, thus, completely isolate the repaired nerve from other tissue. Moreover, non-degradable material may induce in flammation due to a lack of biocompatibility and, hence, require removal once nerve regeneration is achieved. For instance, a silicone prosthesis caused fibrosis when allowed to remain in situ over a period of 6—12 months (Merle et al., 1989); unfortunately, this limits the effectiveness of such prostheses. To overcome this limitation,prosthetic materials and structural designs have been refined in a quest to achieve more effective prostheses. Along with many structural variations, permeability has been enhanced in silicone prostheses; this approach displayed better outcomes than impermeable prostheses (Jenq and Coggeshall,1987) because porous membranes allow prostheses to exchange material with the external environment and maintain a chemical milieu and ionic balance across the material.The use of degradable prostheses in repairing longer segmental defects has increased in popularity as they are, at least theoretically, superior to non-degradable prostheses because of their ability to self-degrade and reabsorb, thereby eliminating the need for surgical retrieval. These benefits,among others, have increased the quest for other natural materials that are suitable for manufacturing a variety of nerve prostheses to support nerve regeneration.
Degradable prostheses
Collagen, a structural protein, and chitosan, a linear polysaccharide, are both materials used to manufacture nerve prostheses and have recently attracted significant attention due to their biocompatibility, biodegradability, ease of man-ufacture, and unique physicochemical properties (Busilacchi et al., 2013; Haastert-Talini et al., 2013). Initially, both appeared to have only a limited benefit on nerve regeneration(Eppley and Del fino, 1988; Ruskin, 1991), probably due to the lack of advanced micromanufacturing technologies, but now their use appears indispensable. Collagen prostheses are known to promote nerve regeneration by increasing re-myelination and neurite extension in rats, and improving lost sensation in humans, compared to autografting (Archibald et al., 1991). On bridging a gap of 20 mm in a rat sciatic nerve, collagen prostheses had significantly higher numbers and diameters of regenerated myelinated axons compared to autografts (Yoshii et al., 2001). Moreover, a collagen prosthesis cross-linked with poly(N-isopropylacrylamide) produced a significant increase in neurite extension and the number of axons increased three-fold compared to using the prosthesis alone (Newman et al., 2006). Chitosan prostheses are credited with creating favorable physiological environments for nerve generation (Li et al., 1999; Tansey et al., 2011) and helping to bridge gaps more efficiently when cross-linked with dibasic sodium phosphate (Fregnan et al.,2016). In gap lengths of 30 mm (rat sciatic nerves), although regeneration was observed 12 weeks postoperatively, no axons reached the distal end (Yoshii et al., 2002). This suggests that chitosan and collagen prostheses are suited for repairs of less than 30 mm, as their ability to support nerve regeneration beyond this limit appears inadequate.
Poly ε-caprolactone and poly L-lactide-co-ε-caprolactone have the potential to facilitate nerve regeneration (Jin et al., 2012) and are credited with restoring lost sensation in humans; while in rodents they produced a good sciatic function index, increased the numbers of myelinated axons and sensory and motor cell bodies, and allowed progressive degradation of the prosthesis (Aldini et al., 1996). The effectiveness of poly ε-caprolactone prostheses was tested in gaps of 5, 15, and 45 mm in rats. Favorable nerve regeneration and functional recovery were observed for the 5 and 15 mm-sized defects six months post-repair, but due to poly ε-caprolactone's mechanical stiffness, the 45 mm defects failed to produce any functional recovery (Chiono et al.,2009). In humans, recovery of sensation in affected patients was observed to be comparable with the surgical standard autograft group (Bertleff et al., 2005).
Poly-glycolic acid (PGA) and poly-lactic acid are biodegradable, thermoplastic polymers with some degree of mechanical strength. Nerve prostheses made of these materials and/or their respective copolymers have demonstrated successful recovery of sensory loss in humans, increased the diameter of regenerated nerves in rabbits, and facilitated better functional repair in rats compared to suture repairs.Successful recovery of sensory loss in lingual nerve repairs with gaps of 50 mm was achieved using a PGA prosthesis(Seo et al., 2008); however, functional recovery was inconsistent. Moreover, PGA repairs were found to be functionally equivalent to monofascicular suture repairs (Marshall et al.,1989), although myelinated axons were larger than those repaired by autografts (Nakamura et al., 2004). In dogs, PGA prostheses established successful functional recovery of injured phrenic nerves (Yoshitani et al., 2007) and an 80 mm gap in a dog's peroneal nerves were bridged using a PGA prosthesis coated with collagen fibers (Matsumoto et al.,2000), which effectively restored the dog's walking pattern 12 months post-implantation.
Similarly, using a microporous poly-lactic acid prosthesis,magnetic resonance imaging showed that a 20 mm gap in a rabbit sciatic nerve was successfully bridged four months postoperatively. The diameter of the regenerated fibers continued to increase while the prosthesis degraded over 18 months (Hsu et al., 2011). However, on repairing 30 mm gaps in the ulnar nerves of monkeys, nerve regeneration was observed but there were no significant differences compared with the control group in terms of mean fiber diameter, amplitude and conduction velocity (Dellon and Mackinnon,1988). PGA prostheses, therefore, may be considered superior to nerve grafting for nerve gaps of up to 30 mm. However,collagen prostheses are reported to support regeneration better than PGA prostheses in terms of greater myelinated axon diameters and functional recovery (Waitayawinyu et al.,2007).
A variety of other biodegradable nerve prostheses has been reported to yield promising results. A poly-3-hydroxybutyrate nerve prosthesis supported nerve regeneration in a 40 mm gap in a rabbit common-peroneal model, with a signi ficantly greater number of fibers than in an autograft group(Young et al., 2002). Functional recovery upon repair of a 30 mm gap using a poly 3-hydroxybutyrate-co-3-hydroxyvalerate prosthesis was superior to nerve grafting, where the sciatic function indexes were 40.1 and 66.2, respectively(Biazar et al., 2013). A nerve prosthesis manufactured from freeze-dried alginate gel covered by PGA mesh was found to be helpful in improving both motor and sensory functional recovery across a 50 mm gap in cat sciatic nerves 13 weeks post-implantation and the prosthesis degraded completely with minimal inflammation (Suzuki et al., 1999). Figure 3 provides a summary of the research groups involved in the studies discussed above and highlights the gap lengths they have successfully repaired.
Nerve Prostheses with Physical Therapeutic Agents
It is evident that nerve prostheses are widely utilized to reconstruct large gap nerve defects; however, no clinically available prostheses are known to guarantee full functionality. Subsequently, this has made the reconstruction of larger nerve defects a significant clinical challenge. Many commercially available prostheses are unable to sustain nerve regeneration across defects longer than 30 mm because of limitations in their fabrication, short degradation times and imprecise mechanical properties. Advances in biomedical engineering and the discovery of PTAs have made noticeable progress in restoring the functionality of repaired peripheral nerves. The use of PTAs involves the systematic application of various sources of energy, which will now be explored.
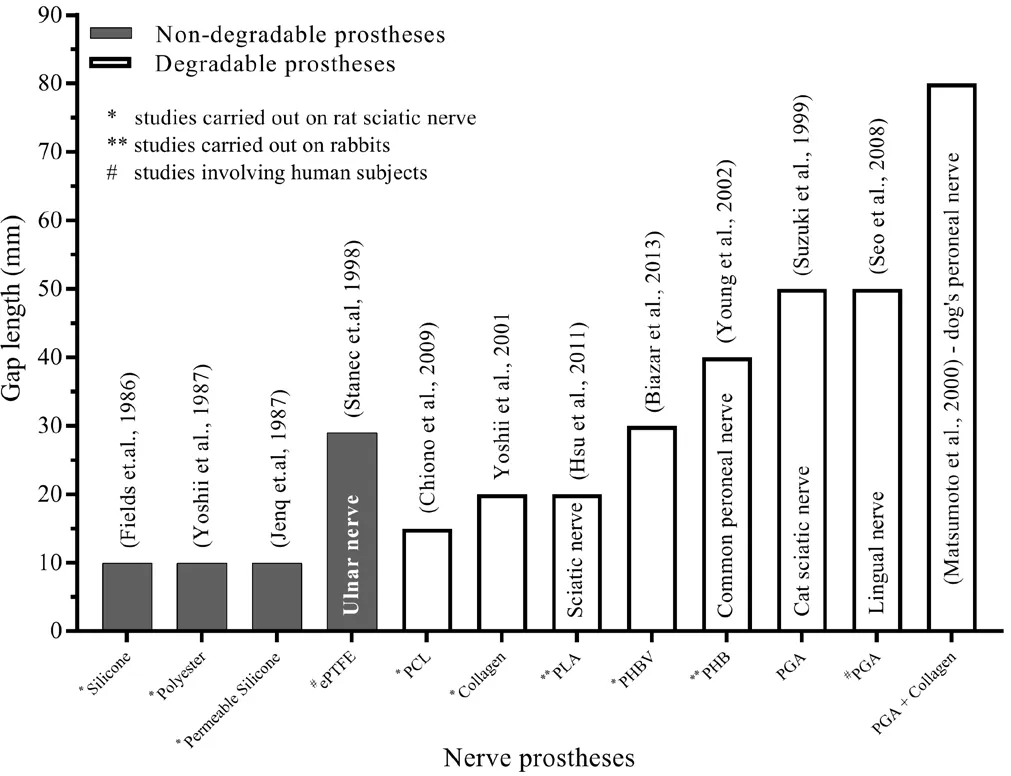
Figure 3 The use of various nerve prostheses in bridging a variety of gap lengths.
Electrical and magnetic stimulation
The topical use of controlled electric fields to excite nerve growth rates in order to increase the number of regenerating axons has been reported widely. The ethos behind the application of electrical stimulation (ES) is to promote functional recovery and reinnervation, even after delayed repair. The ES technique has many clinical applications and is being used in neuromuscular reinnervation and to accelerate wound healing (Michlovitz, 2005). It elevates neuronal cyclic adenosine monophosphate and, in turn, the expression of neurotrophic factors and other growth-associated genes, including cytoskeletal proteins (Gordon, 2016). Recent attempts have been made to engineer electrically conductive prostheses by embedding electrodes and carbon nanotubes in their structures to increase the regrowth rate of motor axon. Prostheses with embedded gold electrodes in thin polyamidefilms have been reported to maximize axonal sprouting (Lacour et al., 2008)while the addition of multiwalled carbon nanotubes in poly 2-hydroxyethyl methacrylate prostheses improved electrical conductivity up to 11-fold (Arslantunali et al., 2014). Studies have demonstrated that a weak electric field enhances neurite outgrowth both in vitro (Zhang et al., 2007; Nguyen et al., 2014) and in vivo (Zealear et al., 2002; Al-Majed et al.,2004). Electrical stimulation at 20 Hz for 1 hour was found to promote motor axonal regeneration in-vivo (Brushart et al., 2002). Interestingly, electrical stimulation (0.1 ms square pulse at 20 Hz) of a rat femoral nerve for 1 hour, even before repairing a gap of 2 mm using a polyethene prosthesis, resulted in accelerated functional recovery and increased the soma size of regenerated motoneurons, which indicates that they are projecting correctly (Ahlborn et al., 2007). However, the axonal thickness and myelination density of the regenerated axons remained inferior to those of controls. One hour of ES is reported to accelerate both axonal regeneration and preferential motor reinnervation in a rat femoral nerve transection model (Al-Majed et al., 2000). On using a silicone prosthesis to repair a 10 mm gap in a rat sciatic nerve, the application of ES (2 Hz at 1 mA) immediately after surgery and every other day for two weeks resulted in significantly larger evoked muscle action potential and amplitude of the reinnervated gastrocnemius muscle than that of the control group (Lin et al., 2015). Gaps of 15 mm in rat sciatic nerves with perforated chitosan prostheses displayed better electrophysiology and functional recovery than did the control group when electrically stimulated for 1 hour at 20 Hz shortly after repair (Huang et al., 2010). In a prosthesis made up of deacetyl chitin, ES(0.1 ms, 3 V, 20 Hz) for 1 hour resulted in superior nerve conduction velocity and an increased number of myelinated fibers (Zhang et al., 2014). In a comparative study, a daily 15-minute percutaneous application of low-frequency ES (1 mA at 2 Hz) from 1—6 weeks post-surgery resulted in a greater number of myelinated fibers, higher axon density, and a higher ratio of blood vessels compared to the control group(Lu et al., 2008). The best timing for ES is yet to be elucidated;however, a short delay of approximately eight days has shown promise (Yeh et al., 2010). In a repair performed 24 weeks after injury, nerve conduction velocity was greatly improved on using brief ES (3 V, 20 Hz, 20 minutes) applied prior to inserting the resected ends of a sciatic nerve into a prosthesis(Huang et al., 2013). In contrast, a delay of one month resulted in poorer outcomes due to tissue degeneration and distal stump fibrosis (Han et al., 2015).
Similar to ES, daily exposure to 400 electromagnetic pulses per second for 15 minutes resulted in a greater number of myelinated nerve fibers (Raji, 1984). A weak magnetic field (0.5 mT, 50 Hz) when applied for 4 hours/day starting at three weeks post-repair helped to attain a high functional recovery after 32 weeks (Bervar, 2005). Similar results were found using a weak electromagnetic field (0.3 mT, 2 Hz)while repairing resected sciatic nerves using a chitosan prosthesis (Mohammadi et al., 2014).
However, some studies have reported that electromagnetic and ES therapies have negligible and/or negative effects on nerve regeneration (Kelleher et al., 2006; Xia et al., 2007),while increased neuromuscular activity due to ES can reduce sprouting in partially denervated muscles and, thus, be detrimental (Tam et al., 2001). The use of ES after facial nerve repair made no statistical difference in terms of functional recovery between a control and treatment group (Mendez et al.,2016). Similarly, the use of a static electromagnetic field did not enhance regeneration of a resected median nerve inside a biodegradable polymer prosthesis in sheep (Kelleher et al.,2006). These disparate findings suggest that ES and electromagnetic therapy could have different effects, thus necessitating protocols to prevent detrimental stimulation (Chen, 2011).
Ultrasound therapy
Ultrasound therapy is believed to facilitate nerve regeneration after peripheral nerve injury by reducing pain, which appears dependent on its ability to limit the upregulation of the neurokinin-1 receptor, substance-P, tumor necrosis factor-α, and interleukin-6 around the injured nerve (Chen et al., 2015). The use of ultrasound therapy at an intensity of 1.6 μW/m2for 20 min/d for 12 days demonstrated an increased number and size of regenerated axons, as well as improved myelination in rat sciatic nerves compared to controls (Crisci and Ferreira, 2002). Similarly, using a prosthesis manufactured from poly(lactic-co-glycolic acid) to bridge a gap of 10 mm in a rat sciatic nerve, then using ultrasound therapy(1 MHz, 4 × 10-3W/mm2) for two minutes once per week for eight weeks revealed that axons reached the distal stump faster than those in the control group (Park et al., 2010).
Laser therapy
Laser therapy involves irradiating an area of tissue with a beam of low-energy photons to raise the temperature of damaged tissue, thereby increasing neuronal activity and promoting healing. It is reported to have a protective and immediate effect in maintaining the functional activity of injured nerves by decreasing scar tissue formation and signi ficantly increasing axonal growth (Rochkind et al., 2009). Similarly, one hour of optical treatment of an injured sciatic nerve,even applied prior to repair, can help to attain a significantly greater evoked electromyography response in rats (Ward et al., 2016). Low-level laser therapy (660 nm aluminum gallium indium phosphide laser at an energy density of 0.576 J/m2),focused on an injured nerve for 30 minutes immediately after surgery, resulted in successful regeneration across a 15 mm gap in a rat sciatic nerve repaired with a biodegradable nerve prosthesis (Shen et al., 2013a, b). Furthermore, transcutaneous laser therapy for nine consecutive days (5 min/day at an energy density of 96 μJ/m2) resulted in a significantly higher sciatic functional index and improved functional recovery 12 weeks after implantation. A significant increase in axonal growth in the peroneal nerve of a rabbit was also observed after application of laser therapy (980 nm wavelength, 2 W output power, 43 seconds exposure time, 800 × 10-6m2area and total energy of 65 J; Anders et al. (2014)). Contrary to this, the use of low-level laser therapy (aluminum gallium arsenide; 660 nm, 40 kJ/m2, 26.3 mW, beam area of 63 mm2) on a rat sciatic nerve did not aid functional recovery (dos Reis et al., 2009).
Table 1 shows a summary of works cited in the context of using PTAs for nerve regeneration. All studies reported here used complete nerve transection models with gaps of various sizes. Most of the studies support the idea that the excitation of nerves repaired using PTAs, applied soon after repair, is most helpful in increasing nerve regeneration rates; however, a few studies (marked with asterisks) reported inferior outcomes.
Conclusions
Peripheral nerve injuries remain a significant source of long-lasting morbidity, disability, and economic burden. Peripheral nerve gap management using nerve prostheses does not always produce reliable results in terms of nerve regeneration and functional recovery. It is noteworthy, however, that many studies demonstrate benefits (such as increased axon regeneration, myelination, and superior functional recovery)from using nerve prostheses to bridge large nerve gaps. Nevertheless, in most cases, the outcomes were not translated into clinical trials. This highlights the importance of adopting a hybrid approach where nerve prostheses are integrated with regeneration support such as PTAs to maximize functional recovery. The materials used for nerve prosthesis should be selected carefully, as should the internal orientation needed to support axonal growth, and their porosity is a crucial fac-tor to be controlled. Using PTAs is helpful in initiating the neuronal activity needed to increase nerve regeneration rates;therefore, it is recommended that the use of PTAs (electrical,magnetic or laser) should not be delayed beyond the formation of scar tissue and fibrosis at the nerve stumps.

Table 1 Studies using nerve prostheses in combination with PTAs
In this review, the use of nerve prostheses with adjunct technologies to repair gap defects in peripheral nerves was discussed. Several studies used PTAs to enhance peripheral nerve regeneration, an approach that shows potential for clinical translation. Given the complexity of nerve regeneration,further research is required to observe the limits and ideal parameters of PTAs in nerve regeneration and to explore the use of PTAs in maximizing functional outcomes. Optimal nerve regeneration cannot be achieved by a single element;both the structure and material of prostheses must support axonal growth, and the addition of PTAs should further accelerate this process. Care is necessary in applying PTAs, as inappropriate electrical, magnetic and/or laser stimulation can negatively affect axonal growth. Commercially available nerve prostheses integrated with the use of PTAs provide a promising avenue for bridging large nerve gaps, but these techniques remain in their infancy and research is still yielding unreliable results. New therapeutic agents that allow modulation of physical stimuli will be key to improving large gap nerve repairs.
Author contributions:Manuscript conception and design: MSUS; literature search and initial manuscript drafting: MSUS and MB; critical revision: MB and GDT; Final approval for publication: MSUS and GDT.
Conflicts of interest:We declare no conflicts of interest related to this manuscript.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:David Romeo-Guitart, Universitat Autònoma de Barcelona Facultat de Biociències, Spain.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Effects of Ginkgo biloba extract EGb761 on neural differentiation of stem cells offer new hope for neurological disease treatment
- Redistribution of nerve strain enables end-to-end repair under tension without inhibiting nerve regeneration
- Transcriptional dysregulation in neurodegenerative diseases: who tipped the balance of Yin Yang 1 in the brain?
- Magnesium: pathophysiological mechanisms and potential therapeutic roles in intracerebral hemorrhage
- Exogenous neural stem cell transplantation for cerebral ischemia
- Potential therapeutic molecular targets for bloodbrain barrier disruption after subarachnoid hemorrhage

