Exogenous neural stem cell transplantation for cerebral ischemia
Ling-Yi Liao, Benson Wui-Man Lau, Dalinda Isabel Sánchez-Vidaña, Qiang Gao, ,
1 Department of Rehabilitation Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan Province, China
2 Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hong Kong Special Administrative Region, China
Abstract Cerebral ischemic injury is the main manifestation of stroke, and its incidence in stroke patients is 70—80%.Although ischemic stroke can be treated with tissue-type plasminogen activator, its time window of effectiveness is narrow. Therefore, the incidence of paralysis, hypoesthesia, aphasia, dysphagia, and cognitive impairment caused by cerebral ischemia is high. Nerve tissue regeneration can promote the recovery of the aforementioned dysfunction. Neural stem cells can participate in the reconstruction of the damaged nervous system and promote the recovery of nervous function during self-repair of damaged brain tissue.Neural stem cell transplantation for ischemic stroke has been a hot topic for more than 10 years. This review discusses the treatment of ischemic stroke with neural stem cells, as well as the mechanisms of their involvement in stroke treatment.
Key Words: nerve regeneration; stem cell therapy; neural stem cells; cell transplantation; ischemic stroke;cerebral ischemia; neuroplasticity; functional recovery; neural regeneration
Introduction
Despite advances in scientific knowledge, cerebrovascular diseases are a major cause of death and disability worldwide.The prevalence and cost of stroke are expected to rise as the global population ages, and in less-developed countries, the incidence and mortality rates of stroke are increasing due to rapidly changing lifestyles and population structures (Kes et al., 2016). Ischemic stroke occurs following the obstruction of blood vessels supplying blood to the brain, and accounts for approximately 80% of all stroke events (Nentwich &Grimmnitz, 2016). Due to the limited regeneration capacity of the central nervous system, patients with brain damage resulting from ischemic stroke often suffer from lifelong disabilities (Mietto et al., 2015). In addition, current therapies do not always adequately improve disease outcomes, and may not be appropriate to use in all patients.
Cell therapy is emerging as a promising new treatment to enhance neurological recovery from ischemic stroke (Savitz et al., 2011). It has been demonstrated to have therapeutic potential in animal models of neurological disorders (Descloux et al., 2015). Several studies have assessed the therapeutic potential of different types of stem cells, such as neural stem cells (NSCs), mesenchymal stem cells, embryonic stem cells, and human induced-pluripotent-stem-cell-derived NSCs, as treatments for ischemic stroke. We conducted a search of Ovid Medline, PubMed, and Embase for all studies using stem cells in ischemic stroke patients or animal models before December 31, 2017. “Stem cells”, “stem cells therapy”, “neural stem cells”, “NSCs”, “stroke”, “ischemic stroke”, “cerebral ischemia” and “stem cell replacement”were used as key words for searching. Using these studies,we reviewed stem cell therapy in the treatment of cerebral ischemia.
Neuroplasticity and Functional Recovery from Stroke
In the early stage of brain injury, it is essential to deal with cellular oxidative stress as well as in flammation and edema in the penumbra. Ma et al. (2018) reported that pinocembrin pretreatment, shortly before tissue-type plasminogen activator infusion, protects blood-brain barrier function and improves neurological outcomes. However, if the time of ischemia is too long, which beyond the regular 4.5-hour tissue-type plasminogen activator time window, it may change the functional recovery within the penumbra of patients;however, the evidence is insufficient. In the post-acute injury stage, the repair process can be divided into three phases:(1) absorption of dead cells, cell repair, cell metabolism, and neural function recovery; (2) axonal growth and dendritic remodeling and activation; and (3) formation of new neural networks and restoration of function (Wieloch and Nikolich, 2006). The injury also triggers neurogenesis to a limited extent. It is also known that external interventions can accelerate repair processes (Nudo, 2011).
The biological mechanisms of recovery from brain injury are currently being explored. In the past decade, the field of neuroplasticity has opened up a new research direction for the treatment of brain injury, providing fundamental support for neurorehabilitation. Neuroplasticity occurs at different levels, from subtle changes in cells to massive changes in cortical remapping (Jang et al., 2017). The role of brain plasticity is widely recognized in rehabilitation medicine in the functional recovery of motor, sensory, and cognitive abilities. The brain plasticity theory proposes that the brain can recover from functional deficits, including paralysis,hypoesthesia, aphasia, aphagia, and cognitive impairment after brain damage. When an injury occurs in the brain, the damaged synapses and the cells surrounding dead neurons undergo a series of changes as a response to the injury, while also triggering adaptation to compensate for any functional deficits. Axonal sprouting, cell genesis, growth factors, brain activation, an enriched environment, and NSCs are all important factors in the functional recovery of an injured brain(Wieloch and Nikolich, 2006).
Overview of Neural Stem Cells
Traditional perspectives suggested that neurons in the central nervous system of mammals could only be produced in the embryonic stage or during a short period after birth. It was believed that adult mammalian neurons did not have the ability to self-renew, implying that neurons could not regenerate once damaged. However, in 1962, a study by Altman suggested the possibility of neuronal proliferation in adult rats. It was not until the late 1990s, however, that Eriksson et al. (1998) demonstrated that the human hippocampus retains its ability to generate new neurons throughout its life. NSCs can self-renew or differentiate into the different types of cells that make up the central nervous system(Clarke et al., 2000). The discovery of NSCs thus dispelled the misunderstanding that the mature adult central nervous system could not self-renew or self-repair. The discovery of NSCs also provided new ideas for the treatment of neurological diseases, such as traumatic brain injury and stroke(Decimo and, 2012).
NSCs are defined as pluripotent and self-renewing cells with the ability to generate all of the major cell types of the central nervous system in adult animals (Ludwig et al.,2018). NSCs can differentiate into neurons, astrocytes, and oligodendrocytes, which are the three main cell types in the central nervous system. Furthermore, NSCs are capable of self-maintenance and self-renewal. NSCs are also responsible for maintaining a certain number of cells in specific locations that can proliferate when needed. NSC proliferation takes places in two ways: symmetric division (Zhong et al.,2018a), which produces two stem cells or two progenitor cells, and asymmetric division, which produces one new stem cell and one progenitor cell (Zhong et al., 2018b). Progenitor cells only exist for a short period due to their continued migration and differentiation or death after proliferation. To maintain the stability of the stem cell pool and the replenishment of progenitor cells, the rate of NSC division is slow. Newborn cells can be continuously added to the neural networks in the restricted locations where NSCs are present(Weng and Lee, 2011).
The two major neurogenic regions of NSCs in the adult mammalian brain have been identified, and these are known as the subgranular zone, in the hippocampal dentate gyrus,and the subventricular zone, which is located along the lateral walls of the lateral ventricles (Zhao et al, 2008; Yao et al.,2012; Kempermann et al., 2015) (Figure 1). New granular neurons are generated in the subgranular zone, while new neuroblasts are continuously produced in the subventricular zone before migrating along the rostral migratory stream to the olfactory bulb, as well as to neocortical areas (Trujillo et al., 2009; De Filippis and Binda, 2012; Bellenchi et al., 2013).Neonatal neurons integrating into existing neural networks are essential for specific brain functions.

Figure 1 Neurogenic regions shown in a sagittal section of the rat brain.
The proliferation, migration, and differentiation of NSCs are strongly associated with their microenvironment, including the surrounding neural cells, stromal cells, and extracellular matrix. The molecular mechanisms that control the developmental processes and determine cell fate are complex, with multiple signaling pathways, transcription factors, and interactions involved (Trujillo et al., 2009).
There are two main mechanisms that regulate NSC differentiation: self-regulation and exogenous signal regulation.Self-regulation is mediated by transcription factors and other intrinsic factors within NSCs in the developmental phase. For example, when Notch signaling is activated, NSCs enter into a proliferation phase, thus increasing the number of NSCs. In addition, other transcription factors or pathways, such as the Wnt signaling pathway and the Sonic hedgehog pathway, are also involved in the self-regulation of NSCs (Faigle and Song,2013). Exogenous signal regulation refers to the regulation of NSCs by cytokines and other neurological factors in the NSC microenvironment. For example, it is widely recognized that epidermal (Cooke et al., 2011) and fibroblast (Sirko et al.,2010) growth factors play an important role in the proliferation, differentiation, and self-renewal of NSCs. Other factors,such as platelet-derived growth factor (Xu et al., 2013) and brain-derived neurotrophic factor (Bath et al., 2012), are also involved in NSC differentiation. The regulation of exogenous signaling is important for the development of NSCs; NSCs from the same origin can differentiate into different types of neurons according to microenvironmental conditions.
Neural Stem Cells in the Treatment of Cerebral Ischemia
Cerebral ischemia induces the proliferation and migration of endogenous NSCs and progenitor cells in the brain(Reynolds and Weiss, 1996; Garcia et al., 2004), and these play a neuroprotective role by regulating the endogenous injury responses (Lois and Alvarez-Buylla, 1994; Doetsch et al., 1999). The transplantation of exogenous NSCs can enhance the regulatory endogenous processes after stroke by stimulating axonal germination, neurogenesis, and angiogenesis (Carleton et al., 2003). Exogenous NSCs can also be directly integrated into the damaged neural network by their differentiation into neurons and glial cells (Sanai et al.,2004). Therefore, exogenous NSC transplantation could be considered a novel approach for the treatment of stroke.
Sources and classifications of exogenous neural stem cells
Adult neural stem cells and progenitor cells
Adult NSCs and progenitor cells are one type of bone-marrow-derived cells isolated from the central nervous system of adult mammals. These cells are an important autologous transplant tool in regenerative medicine, as they can secrete factors that protect cells in the ischemic brain, i.e., glial cell line-derived neurotrophic factor, and promote the migration of adult NSCs and progenitor cells to the ischemic areas, and can inhibit microglial infiltration and apoptosis (Kameda et al., 2007). A two-year follow-up study assessed the safety and feasibility of cotransplantation of neural stem and progenitor cells in patients with ischemic stroke. In this study, the most common side effect of stem cell transplantation in these six cases was low fever that usually lasted 2-4 days after each therapy. One patient exhibited minor dizziness. All side effects appeared within the first 2-24 hours of cell transplantation,and they resolved without special treatment. There was no evidence of neurological deterioration or neurological infection.Most importantly, no tumorigenesis was found at a 2-year follow-up. Results suggested that neurological function, disability level, and daily living abilities of the patients were improved(Qiao et al., 2014). These observations support the use of transplantation of combined neural stem/progenitor cells as a safe and feasible method of improving neurological function.
Human fetal neural stem/progenitor cells
Human fetal neural stem/progenitor cells are cells derived from human fetal forebrain tissues, mostly extracted from embryos at 8 weeks of gestation. They need to be expanded in vitro for approximately 24 weeks and are cultured for 4 days before transplanting into the injured area (Ishibashi et al., 2004). These cells have the potential to restore functions; for example, motor function improvements have been demonstrated in numerous animal models of Parkinson's disease (Morizane et al., 2008) and spinal cord injury (Tetzlaff et al., 2011; Fujimoto et al., 2012). However, a rejection of the donor neural cells is an inevitable concern, and this must be addressed for any such therapy (Liu et al., 2013).
Human fetal striatal neural stem cells
Human fetal striatal NSCs are derived from the human fetal striatum. After transplantation, these NSCs can stimulate striatal neurogenesis, migrate from the implant site, and differentiate into mature neurons (Darsalia et al., 2011). They express an early neuronal marker while in the proliferative state. Under the appropriate conditions, these cells efficiently differentiate into neurons, and after 4 weeks, approximately 50% of the cells are positive for βIII-tubulin. They also express the mature neuronal marker NeuN and neuronal subtype markers such as aminobutyric acid, calbindin,and dopamine and adenosine 3′,5′-monophosphate-regulated phosphoprotein, Mr 32 kDa (Monni et al., 2014). These cells are described as one of the most safe, effective, and highly resilient NSCs.
Induced pluripotent stem cells
Induced pluripotent stem cells represent a very promising candidate stem cell type that can be generated directly from adult cells. Since induced pluripotent stem cells can be derived directly from adult tissues, they not only bypass the need for embryos, but can be made in a patient-matched manner, which means that each individual could have their own pluripotent stem cell line. Induced in vitro, these cells have the ability to differentiate into motor neurons, dopaminergic neurons, glial cells, and other nerve cells. Induced pluripotent stem cells can be induced into NSCs in vitro and then transplanted into the brain through intracerebral injection (Jensen et al., 2013). While the iPSC technology has not yet advanced to a stage where therapeutic transplants have been deemed safe, induced pluripotent stem cells are readily being used in personalized drug discovery efforts and understanding the patient-specific basis of disease (Hockemeyer and Jaenisch, 2016).
Induction of immortalized neural stem cells from other types of stem cells
These types of cells include mesenchymal cells and desirable autologous cells, which have a wide range of resources. Most adult stem cells are lineage-restricted (multipotent) and are generally referred to by their tissue origin (mesenchymal stem cell, adipose-derived stem cell, endothelial stem cell,and dental pulp stem cell). Because these cells do not present ethical and immunological problems compared with other types of stem cells, they have recently become a hot topic.
Pathways in the transplantation of exogenous neural stem cells
Direct injection to the infarct
It was originally believed that greater effects would be gained if the transplantation of exogenous NSCs were close to the infarct area. However, direct injections of NSCs into the infarct cortex risk damaging the tissue after stroke. In some studies, NSCs have been injected into the infarct lumen,which separates the infarct and the infarcted area; the loose tissue provides a potential space for cell transplantation(Wang et al., 2011). However, cells injected into this cavity die immediately due to inflammation, lack of blood supply, and pro-apoptotic factors (Cameron et al., 1993). The necrotic core of the infarct tissue cannot provide the transplanted cells with the appropriate matrix and the necessary growth factors to help them regenerate and recombine the damaged tissue. Thus, although the infarct or damaged tissue is the target of nerve repair for stroke and other degenerative diseases in the central nervous system, the limited vascular and nutritional support in the target area, coupled with an increased in flammatory response, may explain the limited and varying effects in the treatment of diseases such as stroke and Parkinson's disease.
Diffusion through the blood system
NSCs can be delivered through blood vessels. There are two pathways to deliver NSCs: intravenous injection and intra-arterial injection. The femoral vein or tail vein are the most common routes for intravenous injection. This technique is advantageous in that it requires a simple operation that is low risk and induces fewer traumas than other methods, and so it is widely used in animal experiments. The internal carotid artery (Ishizaka et al., 2013) and common carotid artery (Doeppner et al., 2015) are frequently used for intra-arterial injection. NSCs can migrate a long distance from the vessels to the target sites. However, while migrating, interruption of migration and localized differentiation may take place. Therefore, the number of cells that enter the brain is very limited (Bacigaluppi et al., 2016). Intra-arterial injection is conducive to behavioral recovery (Ishizaka et al.,2013), but this method also has risks: there are high mortality rates (approximately 40%) (Li et al., 2010) and high blood flow reductions (up to 80%) (Walczak et al., 2008). Therefore, it needs to be emphasized that, despite the benefits of intra-arterial delivery of stem cells to the ischemic brain,there is a clear risk of vascular occlusion. Recently, a study reported that cell dose and infusion velocity contribute to complications encountered after intra-arterial cell transplantation (Cui et al., 2015). These variables should therefore be considered before planning efficacy studies in rats and, potentially, in stroke patients.
Diffusion through the cerebrospinal fluid
NSCs transplanted by lumbar puncture can circulate through the cerebrospinal fluid to the entire brain and spinal cord. This approach induces less damage compared to a direct injection or an operation. In addition, lumbar puncture transplantation of NSCs is suitable for a large infarct area or when there is severe damage. However, the NSCs could diffuse to a large area of the central nervous system through the cerebrospinal fluid, and have no specific target, some cells localize to areas outside the injury site. The results demonstrate the potential of delivering NSCs using the minimally invasive lumbar puncture method for the treatment of cerebral ischemia (Lepore et al., 2005; Seyed Jafari et al., 2011).
Procedure of exogenous neural stem cell transplantation Surgery
This method has been applied clinically in humans or animals for more than fifteen years. Before transplantation,NSCs should be expanded in vitro for 2—3 passages and be characterized using immunochemical techniques (Zhang et al., 2009). Targeted treatment should be performed in the infarct area, which is relatively stable after cerebral ischemia.A microinjector is used to administer the cell suspension at a speed of 0.5 mL/min. After the cells are administered, the needle is kept in place for 5 minutes and removed slowly(Zhang et al., 2011). The position of stereotaxic transplantation in the brain is accurate and allows for a targeted administration of NSCs, thus avoiding the spread of cells into other regions. However, because this procedure is invasive,brain tissue can easily be damaged.
Targeted NSC transplantation therapy for stroke has been a hot topic over the past ten years. A novel approach for NSC transplantation was recently developed. This involves the use of NSCs embedded in biocompatible matrices to enhance their survival and/or differentiation in stroke injury.Several studies have been conducted using a biopolymer as an NSC-carrying stent for transplantation, and have shown that hyaluronic acid affects NSCs in neural tissue development (Ballios et al., 2015). Therefore, hyaluronic acid has been proposed as a suitable biomaterial for NSCs in nerve tissue engineering. Ninety percent of the components of hyaluronic acid comprise water, suggesting that hyaluronic acid can provide a loose matrix to promote cell migration.Another study found that chondroitin sulfate glycosaminoglycan-based hydrogels were able to regulate NSC self-renewal and facilitate growth factor enrichment locally(Karumbaiah et al., 2015). Beyond graft stability, gene-centered therapy may be used to selectively alter the secretome of NSCs, in an effort to maximize their therapeutic potential by overexpressing brain-derived neurotrophic factors, such as brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, neurotrophin 3, and vascular endothelial growth factor (Bernstock et al., 2017).
Arteriovenous injection
The procedures that have been used to carry out intravenous injections include the injection of 1 × 106cells (dissolved in 200 μL of phosphate-buffered saline) into the femoral vein for 10 minutes, and the injection of 1 × 106neural precursor cells marked by green fluorescent protein (dissolved in 400 μL of 0.1 mM phosphate-buffered saline) into the tail vein (Bacigaluppi et al., 2009). In recent studies of transient middle cerebral artery occlusion in mice, transplanting the injected cells with silica-coated superparamagnetic iron oxide nanoparticles (SiO4@SPIONs) meant that cells could be efficiently guided toward the ischemic hemisphere using exterior magnetic fields. This method subsequently contributed to reduced brain atrophy volume and improved neurobehavioral outcomes in these mice. Because of the promising findings using the magnetic targeting technique, it has been proposed as an effective approach to improve cell homing in brain diseases. Furthermore, endothelial progenitor cells have been demonstrated to be a promising cell type for ischemic stroke therapy (Li et al., 2013).
In intra-arterial injection, NSCs (1 × 106neural precursor cells dissolved in 200 μL of phosphate-buffered saline) are injected into the distal common carotid artery within 10 minutes (Chua et al., 2011). It has been shown that cell size,cell dose, and infusion rate are strongly associated with reduced blood flow and increased incidence of adverse effects,and the study suggests that the IA delivery of MSCs can be performed safely and efficaciously at the maximum tolerated dose of 1 × 105delivered at 24 hours in rodent models of stroke (Yavagal et al., 2014). One study suggested that a cell-dose-related reduction in cerebral blood flow was noted,as well as an increase in embolic events, concomitant lesion size, and sensorimotor impairment. In addition, a low infusion velocity (0.5 mL/6 minutes) was associated with a high rate of complications (Cui et al., 2015). Thus, the current data suggest that intra-arterial injection of stem cells should be carried out with extreme care.
Studies have shown that mortality was high using the intra-arterial delivery route in mouse models of stroke, despite the fact that intra-arterial neural precursor cell transplantation is as effective as intravenous cell grafting. Therefore, the intravenous injection method is safer and more reliable than the intra-arterial injection method (Doeppner et al., 2015).
Lumbar puncture
NSCs can be injected into the cerebrospinal fluid to then diffuse into the central nervous system. Using the lumbar puncture method, after inducing ischemia, the anesthetized animal is fixed onto the operating table so that the trunk and hind limb are at right angles to each other. Then, a newborn lumbar puncture needle (24 G) enters the spinal canal at the level of L5—6 or L6—S1 (Bakshi et al., 2004). NSCs are labeled with PKH26, and 1 × 107cells are dissolved in 50 μL of phosphate-buffered saline and injected into the cerebrospinal fluid (Seyed Jafari et al., 2011). Transplantation of neural progenitor cells by direct injection to the ischemia in animal models causes damage to the brain parenchyma by the injecting needle, and limits cell migration. In contrast, the procedure to inject NSCs via lumbar puncture is fast, easy to perform, and inexpensive. Therefore, transplantation of NSCs into the cerebrospinal fluid by lumbar puncture is an attractive choice compared with other methods (Seyed Jafari et al., 2011; Tian et al., 2013). However, there are only a few studies in which the injection of NSCs into ischemic animals was performed by lumbar puncture because this method carries the risk of subdural hematoma.
Therapeutic effects of exogenous neural stem cells
Positive results
Transplantation of NSCs can enhance endogenous neurogenesis and is considered a promising strategy for the treatment of cerebral ischemia (Cheng et al., 2015; Bernstock et al., 2017). Preclinical studies have explored the feasibility of using NSCs to treat ischemic stroke, and have found that NSCs can survive and differentiate into neurons and astrocytes after transplantation, and that they can improve neurological recovery in rodent models of ischemia (Hou et al., 2017). Two clinical trials have shown that injecting NSCs from teratocarcinoma cell lines improved the European Stroke Scale motor score and the Everyday Memory test score of stroke patients; however, the sample size (4—7 patients per group) was too small to demonstrate any therapeutic effects (Kondziolka et al., 2000, 2005). Evidence from animal studies has shown that delayed transplantation of NSCs, at 3 days after cerebral ischemia, can have a neuroprotective role by inhibiting inflammation and focal glial scar formation, which suggests that NSCs may prolong the effective time window for the treatment of cerebral ischemia (Bacigaluppi et al., 2008). In a middle cerebral artery occlusion rat model, during acute ischemic injury (24 hours after ischemia), human embryonic NSCs that are directly transplanted into the infarcted cortical region using a microinjector can affect the proliferation of subventricular zone cells and the levels of angiogenesis in infarcted areas. Recent studies have also demonstrated that the intravenously transplanted human neural stem cell line HB1.F3 may provide neuroprotective effects in middle cerebral artery occlusion rat models by modulating early in flammatory events in cerebral ischemia (Watanabe et al., 2016). Thus, previous studies confirm that transplantation of NSCs could be an effective approach for treating cerebral ischemia. Recent reports of the transplantation of exogenous NSCs in treating cerebral ischemia are summarized in Table 1 (Chen et al., 2014;Huang et al., 2014; Liu et al., 2014; Abeysinghe et al., 2015;Cheng et al., 2015; Rosenblum et al., 2015; Song et al., 2015;Yao et al., 2015; Bacigaluppi et al., 2016; Hou et al., 2017;Zhu et al., 2017).
Apart from stem cell therapy, gene therapy has also proven to be effective in treating experimental stroke. However,it has some disadvantages that limit its clinical application,such as the difficulty of intracranial delivery of therapeutic genes, low efficacy in transfecting host cells, and the longterm expression of exogenous genes. However, stem-cellbased gene therapy combines gene and stem-cell therapies.In recent years, many studies have used stem cells that overexpress different neurotrophic factors, such as brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, vascular endothelial growth factor, SODI, or NT3, and have reported that the delivery of these genetically modified stem cells to animal models of ischemic stroke is both safe and effective (Chen et al., 2013; Bernstock et al., 2017). The use of stem cells that overexpress neurotrophic factors can improve the survival rate of grafted cells and induce their differentiation into neurons (Yamashita and Abe, 2016; Bernstock et al., 2017). Thus, these results suggest that stem-cellbased gene therapy may be a promising treatment for stroke.
Negative results
Transplantation of NSCs must be rigorously tested in animal models before progressing to clinical trials. Many studies have shown that a nourished microenvironment, created by the in flammatory response, enhances the migration and functional benefits of NSCs that are transplanted after stroke in rats (Reekmans et al., 2012). Transplanting NSCs in an early post-stroke phase, before the inflammatory response is established, provides the best recovery results. Hicks et al. (2008) found that over an extended survival time, a rich environment did not increase the survival or migration oftransplanted NSCs, and most cells (up to 99%) died within 2 months of transplantation. Poor subventricular zone cell survival was associated with an increased in flammatory response (that is, more activated microglia). Many surviving cells expressed astrocytic phenotypes, while functional recovery did not improve. Therefore, it is necessary to further optimize post-stroke transplantation of NSCs in the subventricular zone, to enhance long-term cell survival and thus maximize functional benefits (Hicks et al., 2008). Future studies are needed to clarify the roles of specific components of the in flammatory response on stem cell survival and migration, and to investigate whether attenuating inflammation can improve stem cell survival. Simultaneously, NSC transplantation only resulted in a temporary reduction in brain damage because the number of cells decreased gradually during the 3-month observation period (Hicks et al.,2008). Although the absolute number of exogenous NSCs was still significantly higher than before, these remaining cells were unlikely to be integrated into the resident neural network and did not promote nerve recovery. Similarly,contralateral cell transplantation had no therapeutic effect in post-ischemic brain injury. Although a small number of cells did migrate to ischemic lesions, the number of NSCs that reached the ischemic striatum was small and insufficient to cause a therapeutic effect (Doeppner et al., 2015).
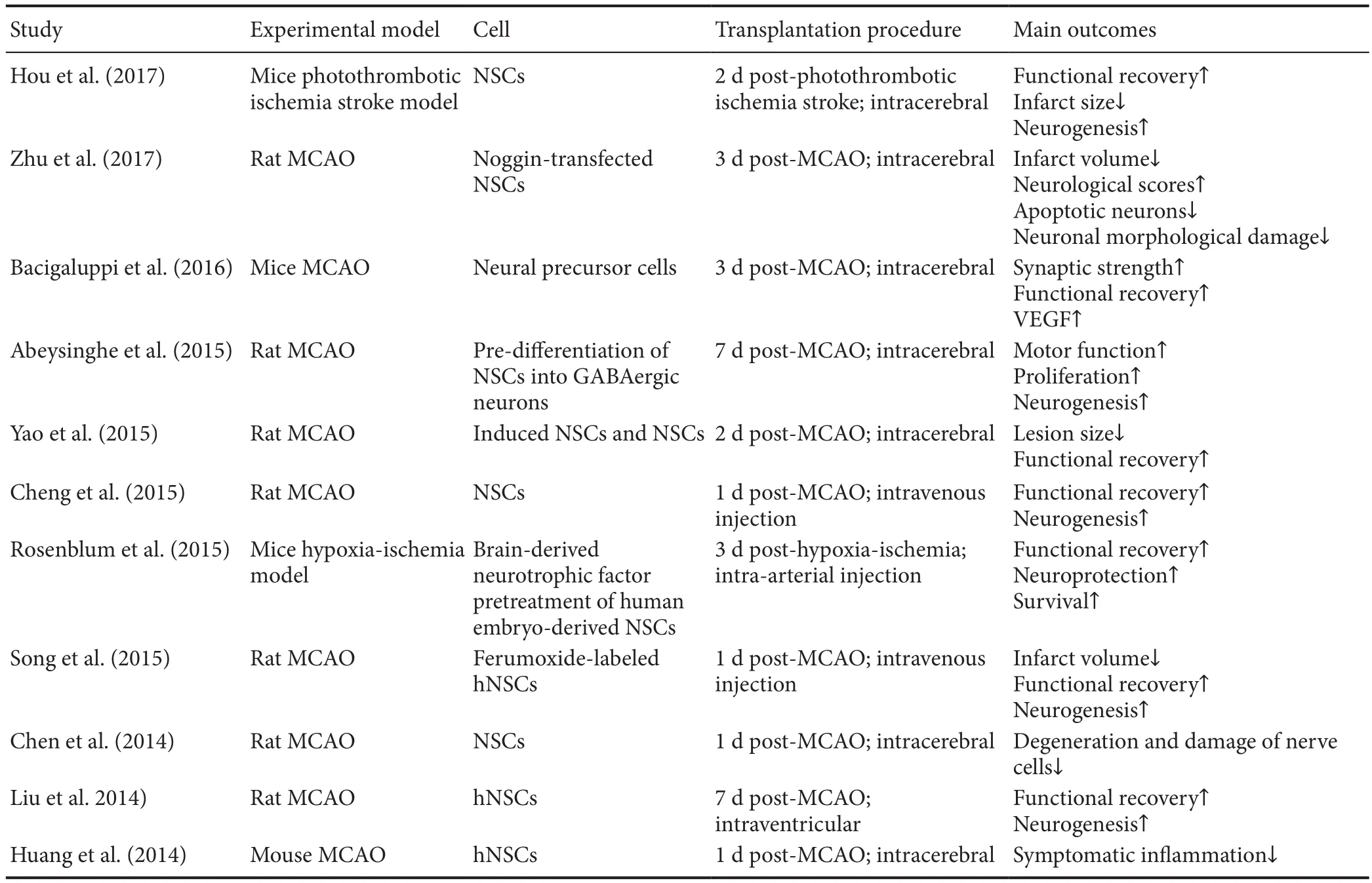
Table 1 Transplantation of exogenous neural stem cells in the treatment of cerebral ischemia
Adverse reactions and side effects
Transplanted NSCs have the potential to secrete angiogenic and immunoregulatory factors into the body (Barbash et al.,2003). Although most clinical studies have shown promising results, some side effects have been encountered in some trials. For example, a trial involving the transplantation of fetal porcine NSCs into five patients was stopped due to the presence of side effects in two patients; one had temporary worsening of motor deficits 3 weeks after transplantation,and another developed seizures 1 week after transplantation(Savitz et al., 2005). However, another study showed that,when a cell suspension consisting of cells from immature nervous and hemopoietic tissues was subarachnoidally transplanted into 10 stroke patients, no patients demonstrated any serious complications of cell therapy over more than 6 months of observation (Rabinovich et al., 2005). In addition, a clinical trial initiated by a UK company reported that all patients with disabling ischemic stroke who underwent NSC treatment had no transplant-related adverse events. This clinical trial found that the patients' sensorimotor function was improved after transplanting NSCs for 6 to 12 weeks (Mack, 2011), suggesting that NSC transplantation may be safe in patients with ischemic stroke. However, none of these clinical trials had a sufficient sample size and all lacked the inclusion of strict controls; therefore, more clin-ical evidence is needed to ensure that NSC transplantation is safe and reliable. Conclusions regarding the safety and efficacy of stem cell therapy in stroke patients can only be achieved using larger sample sizes and appropriately controlled studies.
Although studies have shown that intra-arterial injection is as effective as intravenous injection, many studies have shown that intra-arterial injection leads to an increase in test animal mortality (Chua et al., 2011; Karlupia et al., 2014).Therefore, the safety of this approach still needs further study.
Problems and Perspectives
The use of transplanted exogenous NSCs in the treatment of cerebral ischemia has been shown to have promising results in preclinical studies, but several controversies have arisen from this therapeutic approach. First, NSCs have the potential for unlimited proliferation, which may lead to the generation of potential tumors. Second, the controlled handling of exogenous NSCs for transplantation, to regulate differentiation, and to achieve the desired therapeutic effects remains to be explored. Furthermore, the effective time window of exogenous NSC transplantation, the optimal number of cells, and the identification of transplant pathways may all affect the clinical application of transplanted exogenous NSCs in the treatment of cerebral ischemia. Nonetheless,transplantation of exogenous NSCs is expected to become an important method for treating cerebral ischemia in the future.
Author contributions:Conception and design of the work: QG; data analysis and manuscript writing: LYL; data analysis with constructive discussions: BWML and DISV. All authors approved the final version of the paper.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work is supported by the National Natural Science Foundation of China, No. 0040205401797 (to QG); the General Research Fund of China, No. 15164216 (to BWML). None of the funding bodies play any role in the study other than to provide funding.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Sabrina Mahalia Heman-Ackah, University of North Carolina at Chapel Hill, USA; Haiqiang Qin, Beijing Tiantan Hospital, Capital Medical University, China.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Effects of Ginkgo biloba extract EGb761 on neural differentiation of stem cells offer new hope for neurological disease treatment
- Redistribution of nerve strain enables end-to-end repair under tension without inhibiting nerve regeneration
- Transcriptional dysregulation in neurodegenerative diseases: who tipped the balance of Yin Yang 1 in the brain?
- Magnesium: pathophysiological mechanisms and potential therapeutic roles in intracerebral hemorrhage
- Bridging larger gaps in peripheral nerves using neural prosthetics and physical therapeutic agents
- Potential therapeutic molecular targets for bloodbrain barrier disruption after subarachnoid hemorrhage

