GPER agonist G1 suppresses neuronal apoptosis mediated by endoplasmic reticulum stress after cerebral ischemia/reperfusion injury
Zi-Wei Han , Yue-Chen Chang , Ying Zhou Hang Zhang , Long Chen Yang Zhang , Jun-Qiang Si , Li Li
1 Department of Physiology, Medical College of Shihezi University, Shihezi, Xinjiang Uygur Autonomous Region, China
2 Key Laboratory of Xinjiang Endemic and Ethnic Disease, Shihezi University School of Medicine, Shihezi, Xinjiang Uygur Autonomous Region,China
3 Affiliated Teng Zhou Central People's Hospital, Jining Medical University, Jining, Shandong Province, China
4 Department of Physiology, Jiaxing College of Medicine, Jiaxing, Zhejiang Province, China
Abstract Studies have confirmed a strong association between activation of the endoplasmic reticulum stress pathway and cerebral ischemia/reperfusion (I/R) injury. In this study, three key proteins in the endoplasmic reticulum stress pathway (glucose-regulated protein 78, caspase-12, and C/EBP homologous protein) were selected to examine the potential mechanism of endoplasmic reticulum stress in the neuroprotective effect of G protein-coupled estrogen receptor. Female Sprague-Dawley rats received ovariectomy (OVX), and then cerebral I/R rat models (OVX+ I/R) were established by middle cerebral artery occlusion. Immediately after I/R, rat models were injected with 100 μg/kg E2 (OVX + I/R +E2), or 100 μg/kg G protein-coupled estrogen receptor agonist G1 (OVX + I/R + G1) in the lateral ventricle. Longa scoring was used to detect neurobehavioral changes in each group. Infarct volumes were measured by 2,3,5-triphenyltetrazolium chloride staining. Morphological changes in neurons were observed by Nissl staining. Terminal dexynucleotidyl transferase-mediated nick end-labeling staining revealed that compared with the OVX + I/R group, neurological function was remarkably improved, infarct volume was reduced, number of normal Nissl bodies was dramatically increased, and number of apoptotic neurons in the hippocampus was decreased after E2 and G1 intervention. To detect the expression and distribution of endoplasmic reticulum stress-related proteins in the endoplasmic reticulum, caspase-12 distribution and expression were detected by immuno fluorescence, and mRNA and protein levels of glucose-regulated protein 78, caspase-12, and C/EBP homologous protein were determined by polymerase chain reaction and western blot assay. The results showed that compared with the OVX+ I/R group, E2 and G1 treatment obviously decreased mRNA and protein expression levels of glucose-regulated protein 78, C/EBP homologous protein, and caspase-12. However, the G protein-coupled estrogen receptor antagonist G15 (OVX + I/R + E2 + G15) could eliminate the effect of E2 on cerebral I/R injury. These results confirm that E2 and G protein-coupled estrogen receptor can inhibit the expression of endoplasmic reticulum stress-related proteins and neuronal apoptosis in the hippocampus, thereby improving dysfunction caused by cerebral I/R injury. Every experimental protocol was approved by the Institutional Ethics Review Board at the First Affiliated Hospital of Shihezi University School of Medicine, China (approval No. SHZ A2017-171) on February 27, 2017.
Key Words: nerve regeneration; cerebral ischemia/reperfusion injury; estrogen; G protein-coupled estrogen receptor; G1; G15; endoplasmic reticulum stress; glucose-regulated protein 78; caspase-12; C/EBP homologous protein; neuronal apoptosis; neural regeneration
Introduction
Stroke, a cerebrovascular condition, is increasing in prevalence(Shi et al., 2016; Zheng et al., 2018). Given that stroke is a condition in which blood flow to brain areas is disrupted, it is often associated with high rates of severe disabilities and multiple functional impairments. Moreover, reperfusion itself produces reactive oxygen species or overproduction of free radicals that can cause reperfusion injury (Chan, 1996). Despite advancements in the treatment of reperfusion injury in over the past few decades, clinical effects remain unsatisfactory. Notably,women have lower risk for ischemic stroke than men. However, this innate protection diminishes after menopause, which is believed to be associated with a loss of endogenous estrogen.Similarly, female animals demonstrate less tissue damage and improved functional outcome after experimental cerebral ischemia compared with their male or ovariectomized (OVX)female counterparts (Herson et al., 2009).
Apoptosis is a major pathophysiological change after ischemia/reperfusion (I/R) brain injury (Mattson et al., 2001). The endoplasmic reticulum stress pathway is a newly discovered apoptosis pathway that has been shown to induce apoptosis in Parkinson's disease (Silva et al., 2005), atherosclerosis (Scull and Tabas, 2011), Alzheimer's disease (Gwag et al., 2010), and other metabolic diseases (Sano and Reed, 2013).
There are three main types of estrogens in the human body:estradiol (17 beta-estradiol, E2), estriol, and estrone. However,E2 is the most biologically active natural estrogen. Animal experiments have confirmed that an appropriate dose of estrogen can reduce the area of cerebral infarction or hippocampal cell damage resulting from cerebral ischemia (Buttesmith et al., 2009; Lebesgue et al., 2009). However, owing to the special nature of estrogen, it cannot be used in male patients. Thus,clinical application of estrogen has many disadvantages, such as increased risk of cervical and breast cancers in female patients after perimenopause (Imoto et al., 1998).
Estrogen can regulate cell proliferation, apoptosis, and in flammatory response through rapid signal responses mediated by G protein-coupled estrogen receptor (GPER), a recently discovered estrogen-associated receptor (Prossnitz and Barton, 2014). The GPER agonist G1 and antagonist G15 play a significant role in the study of GPER (Morris et al., 1982; Bell et al., 2006). G1 can specifically excite GPER to play a protective role in many diseases and may replace estrogen in the future (Tropea et al., 2015). Several studies have shown that estrogen may protect the brain by inhibiting endoplasmic reticulum stress and apoptosis (Shughrue and Merchenthaler, 2003; Jia et al., 2009). However, the potential relationship between GPER and endoplasmic reticulum stress-induced apoptosis after I/R injury has not been elucidated. Thus, the present study investigated whether GPER protected the brain against I/R injury, and examined the role of the apoptotic mechanism mediated by I/R injury.
Materials and Methods
Animals
A total of 180 female Sprague-Dawley rats aged 8—12 weeks and weighing 230—300 g were purchased from the Experimental Animal Center of Xinjiang Medical University,China [certificate No. SCXK (Xin) 2003-0001]. Two or three rats were housed in each cage and under a reversed 12-hour light/dark cycle. Food and water were provided ad libitum,and rats were weighed twice a week.
Every experimental protocol was approved by the Institutional Ethics Review Board at the First Affiliated Hospital of Shihezi University School of Medicine, China (approval No.SHZ A2017-171) on February 27, 2017.
Establishment of middle cerebral artery occlusion rat models
The middle cerebral artery occlusion model was induced via an intraluminal suture technique under sterile conditions, as previously described (Offner et al., 2006; Hurn et al., 2007).Brie fly, each rat was intraperitoneally anesthetized with 40 mg/kg pentobarbital and allowed to breathe spontaneously.A rectal probe was used to monitor body temperature, and a heat lamp was used to maintain normal body temperature.Surgery was performed on a stereotaxic apparatus. After anesthetization, rats were placed in a supine position, and a midline skin incision was made to expose the right common carotid artery, internal carotid artery, and external carotid artery. A 3-0 mono filament nylon thread with a rounded tip was inserted into the external carotid artery and gently advanced to a distance of 18.5 ± 0.5 mm, which blocked blood flow to the right middle cerebral artery. After 90 minutes,the 3-0 filament was pulled out, resulting in reperfusion. After the animal awoke, reperfusion was considered successful when the rat incurred a neurological behavior score of more than 1. Rats whose nylon filament did not completely block or penetrate blood vessels were excluded. Neurobehavioral scores were evaluated as previously described, with a neurobehavioral score ≥ 1 considered as successful modeling (Liu et al., 2012; Zhang et al., 2018).
Experimental groups and paraffin sectioning
One hundred eighty rats were randomly divided into 6 groups (n = 30). All drug treatment groups were administered once immediately upon reperfusion. (1) OVX group:Female rats with ovaries removed one week before transient focal cerebral ischemia. (2) OVX + I/R group: OVX rats with I/R model established through middle cerebral artery occlusion (Vavers et al., 2016). (3) OVX + I/R + vehicle group:OVX rats with I/R who received a lateral ventricle injection of dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO,USA; high polarity, high boiling point, good thermal stability, miscibility with water, and good solvent for most organic substances). (4) OVX + I/R + E2 group: OVX rats with I/R who received a lateral ventricle injection of E2 (100 μg/kg,Sigma-Aldrich) diluted in DMSO. (5) OVX + I/R + G1 group:OVX rats with I/R who received a lateral ventricle injection of the GPER-specific agonist G1 (100 μg/kg, Sigma-Aldrich).(6) OVX + I/R + E2 + G15 group: OVX rats with I/R who received a lateral ventricle injection of E2 with the GPER-specific inhibitor G15 (100 μg/kg, Sigma-Aldrich).
Six rats were randomly selected from each group for eu-thanization and staining of paraffin-embedded sections for terminal dexynucleotidyl transferase (dUTP)-mediated nick end labeling (TUNEL), Nissl, and immuno fluorescence.
Neurological scoring
Twenty-four hours after I/R, neurological deficits were evaluated blindly through using the Longa scoring system (Longa et al., 1989), which comprises five tests covering spontaneous activity, walking (but failing to fully extend the left forepaw), and mild focal neurological deficits. A score of 0 was assigned to the best test performance, whereas 4 points were assigned to the worst performance. Mean neurological scores were evaluated by two blinded observers for grading.
Brain water content
Six rats were randomly selected from each group at 24 hours after I/R injury. After neurobehavioral scoring, these rats were deeply anesthetized and sacrificed, and their brains were weighed immediately to determine the wet weight.Subsequently, samples were dried at 120°C for 24 hours and weighed again to determine the dry weight. Percentages of brain water content were calculated as [(wet weight - dry weight)/wet weight] × 100%.
2,3,5-Triphenyltetrazolium chloride staining
Twenty-four hours after I/R injury, six rats from each group were administered anesthesia, sacrificed, and their complete brain tissue was quickly removed and frozen in a -50°C refrigerator for 5 minutes. Frozen brain tissues were sliced to 2 mm-thick sections, quickly placed in 2% triphenyltetrazolium chloride (TTC) solution (Sigma-Aldrich), incubated at 37°C for 30 minutes, and fixed in 10% formaldehyde. Slices were taken out, and both sides of each brain slice was photographed with a digital camera. The infarction area of the two sides of each brain section was measured using a CM-2000B medical image analysis system. Red areas indicated normal brain tissue, whereas pale areas indicated the infarct area. Infarct volume percentages were calculated as (infarct volume /total volume) × 100%.
TUNEL staining
TUNEL analysis was performed using an in situ cell death assay kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions in brain tissue at 24 hours after I/R. Stained brain sections were visualized with a confocal microscope (FV-1000; Olympus,Tokyo, Japan) and digital images were captured. For each group, TUNEL-positive cells were manually counted in the CA1 region (50 μm × 50 μm) of three sections (n = 6). Cells were blindly analyzed and TUNEL-positive cells were counted using a 20× objective.
Nissl staining
Twenty-four hours after I/R, randomly selected slices of the hippocampal CA1 region were flushed with 0.01 M phosphate-buffered saline (PBS) and dipped in dimethylbenzene I, II, and III at concentrations of 90%, 80%, and 70%, respectively, for 5 minutes each. Slices were then immersed in 5 g of Nissl solution (Solarbio Science & Technology Co., Ltd.,Beijing, China) at 37°C for 20 minutes. After incubation,slices were washed with distilled water, followed by 95% alcohol for color separation. Microscopic examination showed distinct Nissl bodies. Slices were dehydrated with anhydrous alcohol, rendered transparent with xylene, and mounted with neutral balata.
Immuno fluorescence
Paraffin sections of the hippocampal CA1 region were randomly selected at 24 hours after I/R, washed with PBS,dewaxed separately in xylene and alcohol, then repaired in citrate repair solution for 8 minutes. Sections were then washed three times with PBS for 5 minutes each, and then incubated in 1% bovine serum albumin for 1 hour at room temperature. Each section was soaked in rabbit anti-caspase-12 polyclonal antibody (1:500; Abcam,Cambridge, UK) diluted in PBS (1:125) at 4°C overnight.After washing with PBS, sections were incubated with a FITC-conjugated anti-rabbit secondary antibody (1:100;Santa Cruz Biotechnology, Dallas, TX, USA) for 1 hour at 37°C. After each slice was rinsed three times with PBS, sections were restained with 4′,6-diamidine-2-phenylindole (Sigma-Aldrich). Immuno fluorescence staining of caspase-12 was observed under a confocal laser-scanning microscope (Zeiss,Oberkochen, Germany). Average optical densities were analyzed and calculated by ImageJ software (Media Cybernetics,Rockville, MD, USA). Five visual fields of each sample were randomly selected and analyzed at a high magnification. The fluorescence intensity of each group was analyzed statistically.
Quantitative real-time polymerase chain reaction assay
Twenty-four hours after I/R, the hippocampal CA1 region was taken from six rats of each group for quantitative real-time polymerase chain reaction (qRT-PCR) to determine mRNA expression levels of glucose-regulated protein(GRP78), caspase-12, and C/EBP homologous protein(CHOP). Total RNA was extracted from brain samples using a brain tissue RNA kit (Sangon Biotech, Shanghai, China)according to the manufacturer's instructions. Isolated RNA was used for cDNA synthesis using a reverse transcription system. PCR analysis used the real-time SYBR Green PCR technology and primers shown in Table 1. A thresholding method was performed to analyze relative gene expression.Each sample was measured thrice. The 2-ΔΔCtmethod was used to express relative mRNA levels (Yuan et al., 2018).
Western blot assay
Twenty-four hours after I/R, six rats were euthanized for western blot assay in each group. Homogenates of hippocampal CA1 region were incubated at 4°C for 30 minutes,and then centrifuged at 10,450 × g for 15 minutes after extracting hippocampal proteins. Protein concentration in the supernatant was measured using a protein assay kit.Proteins were transferred to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA) after separationby Tris-glycine denaturing gradient gel electrophoresis. After blocking for 1 hour at 37°C in Tris-buffered saline with Tween (pH 8.0,10 mM Tris-HCl, 150 mM NaCl, and 0.2%Tween 20), membranes were incubated with the following antibodies overnight at 4°C: rabbit monoclonal anti-GRP78(1:500; Abcam), monoclonal anti-mouse CHOP (1:500;Abcam), and rabbit polyclonal anti-caspase-12 (1:1000; Abcam). The blot was washed with Tris-buffered saline with Tween. After incubating with a horseradish peroxidase-conjugated secondary antibody (anti-rabbit or anti-mouse;1:20,000; Santa Cruz Biotechnology) for 2 hours at room temperature, staining was visualized using enhanced chemiluminescence (GE Healthcare, Chicago, IL, USA). Target protein bands were visualized using an enhanced chemiluminescence kit in conjunction with X-ray film. Grayscale values were measured and normalized to the corresponding β-actin in the same sample.

Table 1 Primer sequence
Statistical analysis
Experimental data were processed using SPSS 17.0 statistical software (SPSS, Chicago, IL, USA). Statistical differences between two groups were analyzed by Student's t test. One-way analysis of variance followed by Student-Newman-Keuls post hoc test was used to compare differences between more than two groups. P < 0.05 was considered statistically significant.
Results
Physiological evaluation
Mean arterial pressure (85—125 mmHg), partial pressure of oxygen (PO2, 80—95 mmHg), partial pressure of carbon dioxide (PCO2, 35—45 mmHg), arterial pH (7.35—7.45), and blood glucose levels (95—125 mg/dL) were monitored during the operation. Insignificant differences were observed between groups during the experiment (data not shown).
Neurological scoring
Compared with the OVX group, Longa scores in the OVX+ I/R group were significantly increased (P < 0.05; Figure 1A). However, Longa scores were not significantly different between OVX + I/R + vehicle and OVX + I/R groups. Compared with the OVX + I/R group, scores were significantly increased in the OVX + I/R + E2 group, but significantly reduced in the OVX + I/R + G1 group (P < 0.05; Figure 1A).Compared with the OVX + I/R + E2 group, Longa scores were significantly increased in the OVX + I/R + E2 + G15 group (P < 0.05; Figure 1A).
Effects of E2 and G1 on reduced brain water content after cerebral I/R injury in rats
Approximately 24 hours after I/R, brain water contents were significantly increased in OVX + I/R and OVX + I/R + vehicle groups compared with the OVX group (P < 0.05; Figure 1B). Brain water content was also reduced in OVX + I/R +E2 and OVX + I/R + G1 groups compared with the OVX+ I/R group (P < 0.05; Figure 1B). However, brain water contents were not significantly different between OVX + I/R and OVX + I/R + vehicle groups (P < 0.05; Figure 1B).Compared with the OVX + I/R + E2 group, brain water content was significantly increased in the OVX + I/R + E2 +G15 group (P < 0.01; Figure 1B).
Effects of E2 and G1 on infarct volume after cerebral I/R injury in rats
TTC staining re flected the degree of cerebral ischemia (Figure 2A). Compared with the OVX group, infarct volumes were significantly increased in the OVX + I/R group (P <0.05; Figure 2B). However, infarct volumes were significantly reduced in OVX + I/R + E2 and OVX + I/R + G1 groups compared with the OVX + I/R group (P < 0.01; Figure 2B).Compared with the OVX + I/R + E2 group, infarct volumes were significantly increased in the OVX + I/R + E2 + G15 group (P < 0.01; Figure 2B).
Effects of E2 and G1 on neuronal morphology in the hippocampal CA1 region after cerebral I/R injury in rats
Rats from each group were euthanized 24 hours after cerebral I/R injury. Nissl staining was used to examine morphologic changes in CA1 neurons. In the OVX group, pyramidal neurons in the hippocampal CA1 sub field had clear cell outlines and abundant cytoplasm. Compared with the OVX group, neuronal damage was more severe in the OVX + I/R group, and injury in the hippocampal CA1 subfield was reduced in the OVX + I/R + E2 group. I/R-induced neuronal damage was clearly ameliorated in the OVX + I/R + G1 group compared with the OVX + I/R group. However, when G15 was injected, the neuroprotective effect of E2 was abolished compared with the OVX + I/R + E2 group (Figure 3).
E2 and G1 reduce apoptosis in the hippocampus of rats after cerebral I/R injury
Compared with the OVX group, percentages of apoptotic cells were significantly increased in OVX + I/R and OVX +I/R + vehicle groups (P < 0.05; Figure 4A). However, upon E2 and G1 injection, these groups exhibited significant decreases in numbers of TUNEL-positive cells (P < 0.05; Figure 4A) compared with the OVX+I/R group. A non-signi ficant difference was observed between OVX + I/R and OVX+ I/R + vehicle groups (P < 0.05; Figure 4A). Compared with the OVX + I/R + E2 group, the percentage of apoptotic cells was significantly increased in the OVX + I/R + E2 +G15 group (P < 0.05; Figure 4A).

Figure 1 E2 and G1 reduce neurobehavioral scores and brain water content.

Figure 2 E2 and G1 reduce infarct volume after I/R.

Figure 3 E2 and G1 reduce morphological alterations in the hippocampal CA1 region after I/R, as detected by Nissl staining.

Figure 4 E2 and G1 reduce neuronal apoptosis in the hippocampal CA1 region after I/R.
Effects of E2 and G1 on the endoplasmic reticulum stress pathway after cerebral I/R injury in rats
Low expression levels of GRP78 (Figure 5B), caspase-12(Figure 5C), and CHOP (Figure 5D) were observed in the OVX group. However, mRNA levels were significantly increased in OVX + I/R and OVX + I/R + vehicle groups compared with the OVX group 24 hours after brain I/R injury (P< 0.05; Figure 5). A non-significant difference was observed between OVX + I/R and OVX + I/R + vehicle groups (P >0.05; Figure 5). After E2 or G1 treatment, levels of GRP78,caspase-12, and CHOP were significantly decreased compared with the OVX + I/R group (P < 0.05; Figure 5). Moreover, compared with the OVX + I/R + E2 group, these levels were significantly increased in the OVX + I/R + E2 + G15 group (P < 0.05; Figure 5).
Figure 5E shows the effects of E2 and G1 on mRNA expression of endoplasmic reticulum stress-related factors after cerebral I/R injury in rats. mRNA expression of GRP78,caspase-12, and CHOP increased in OVX + I/R and OVX + I/R + vehicle groups compared with the OVX group after cerebral I/R injury (P < 0.05; Figure 5E). There were no significant differences in mRNA expression of GRP78, caspase-12, or CHOP between OVX + I/R and OVX + I/R + vehicle groups(P > 0.05; Figure 5E). Administration of E2 or G1 alone decreased the expression of GRP78, caspase-12, and CHOP mRNA compared with the OVX + I/R group (P < 0.05; Figure 5E). However, administration of E2 and G15 increased mRNA levels of GRP78, caspase-12, and CHOP compared with the OVX + I/R + E2 group (P < 0.05; Figure 5E).
Detection of the endoplasmic reticulum stress-specific protein caspase-12 by immuno fluorescence revealed signi ficantly increased levels in OVX + I/R and OVX + I/R + vehicle groups compared with the OVX group 24 hours after brain I/R injury (P < 0.05; Figure 6). Injection of E2 and G1 after reperfusion resulted in significantly decreased expression levels of caspase-12 in rat brains compared with the OVX + I/R group (P < 0.05; Figure 6). Notably, caspase-12 expression was lower in the OVX + E2 group than in the OVX + G1 group (P < 0.05; Figure 6), although they were increased compared with OVX + E2 and OVX + G1 groups(P < 0.05; Figure 6). Immuno fluorescence results were consistent with expression trends for these proteins identified by western blot assay (Figure 5A and C).
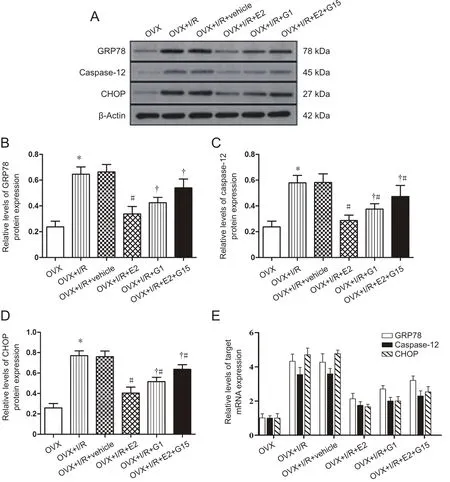
Figure 5 E2 and G1 inhibit expression of endoplasmic reticulum stress-related proteins in the hippocampal CA1 region after I/R.
Discussion
In this study, we examined neuroprotective and anti-apoptotic effects of E2 and GPER activation on I/R injury in an animal model. We demonstrated that activation of GPER,similar to E2, noticeably reduces infarct volume and the percentage of neuronal apoptosis, and improves neurological function. Furthermore, E2 and GPER activation treatments remarkably increased brain water content and infarct volume after cerebral I/R injury in rats. Additionally, apoptosis was obviously attenuated, as expression levels of endoplasmic reticulum stress-related proteins GRP78, CHOP, and caspase-12 were decreased in OVX + I/R + E2 and OVX+ I/R + G1 groups 24 hours after I/R injury. E2 and GPER activation efficiently suppressed apoptosis by inhibiting the endoplasmic reticulum stress-mediated apoptotic pathway.However, the above-mentioned effect of E2 on I/R injury was abolished by the GPER inhibitor G15.
G1 and G15 are highly selective agonists and inhibitors of GPER, respectively, and their discovery promotes the functional study of GPER in animal cells and tissues (Li et al., 2013; Barton and Prossnitz, 2015; Wang et al., 2018a, b).Many studies have shown that GPER activation has the same role as E2 in breast cancer cells, and inhibits the occurrence of breast cancer through the GPER/reactive oxygen species/p38MAPK/p21 signaling pathway; notably, G15 inhibits this effect (Santolla et al., 2015). The neural repair effect of E2 has been observed in ischemic stroke (Simpkins et al., 1997),experimental stroke (Toung et al., 1998), glioma, and neuroblastoma cells in vitro (Bishop and Simpkins, 1994). The results of this study showed that administration of the GPER receptor agonist G1 in the lateral ventricle can remarkably improve neurobehavioral function and reduce brain water content. Other experts have also shown that G1 ameliorates blood-brain barrier permeability after global cerebral ischemia in OVX rats (Lu et al., 2016). According to our results,G1 may also reduce the volume of cerebral infarction after I/R in OVX rats, consistent with the results of Zhang et al.(2010) showing that G1 reduced infarct size after experimental stroke. These results indicate that E2 and GPER activation may have considerable potential for treating neurodegenerative diseases and central nervous system injuries. Therefore,this study explored whether estrogen and activation of GPER have protective functions against I/R injury, and investigated potential mechanisms of these neuroprotective effects.
Since the discovery of GPER in the brain, some scholars have suggested that GPER acts as a classic estrogen receptor that can provide neuroprotection, such as against I/R injury(Owman et al., 1996a; Deschamps and Murphy, 2009; Li et al., 2015). The GPER receptor agonist used here (G1) does not interact with classical nuclear receptors (Bologa et al., 2006b).Thus, it can be used as an alternative drug for postmenopausal women, because it does not elicit adverse effects of hormone replacement therapy (Gillies and McArthur, 2010).Moreover, Nissl staining results showed distinct pyramidal neurons in the hippocampal CA1 subfield and cytoplasmic staining after the addition of G1 treatment. Our findings clearly indicate that G1 treatment can improve neurological deficits. The treatment selectively attenuated infarct damage in the OVX + I/R group, consistent with findings for the E2 treatment. However, these effects were abrogated by the addition of the GPER antagonist G15, thereby confirming that GPER plays an important role for the activity of many estrogen receptors. Further research on this topic is necessary.
To ensure that a high drug concentration was delivered to the damaged brain area, we employed lateral ventricle injection rather than intravenous or intramuscular injection.Given that 100 μg/kg estrogen is the best therapeutic dose for the employed I/R injury model (Yang et al., 2000), we used 100 μg/kg G1 and 100 μg/kg G15 in this preliminary experiment to compare their therapeutic effects with that of E2, considering factors such as the tolerance of rats and best experimental results. The frequency of cortical neuron apoptosis in OVX + I/R + E2 and OVX + I/R + G1 groups was reduced compared with the OVX + I/R group. However, when G15 was added, the therapeutic effect of E2 was noticeably reduced, indicating that GPER plays an important role in the therapeutic benefits elicited by E2.
The present results show that neuronal apoptosis in the hippocampus and expression levels of key apoptotic proteins, such as GRP78, CHOP, and caspase-12, were increased in rat brains after I/R injury. However, the number of apoptotic neurons and level of protein expression decreased after treatment with GPER agonists. Many experimental studies have indicated that estrogen signaling affects stroke-induced injury by modulating apoptotic signaling mechanisms (Dubal et al., 1999; Rau et al.,2003; Liu et al., 2009, 2011). However, this novel study of E2 and GPER indicates that they can protect neurons in the hippocampus through the endoplasmic reticulum stress pathway.GRP78, which is important in cellular responses to stress, is involved in endoplasmic reticulum (ER) glycoprotein trafficking and molecular chaperone activity (Yong et al., 1987; Wooden et al., 1991; Little et al., 1994; Brostrom and Brostrom, 1997). In normal conditions, GRP78 associates with ER-localized transmembrane proteins and maintains an inactive form. However,under pathological conditions, GRP78 dissociates from these receptors, thereby resulting in activation of these proteins.Moreover, when severe endoplasmic reticulum stress disrupts ER function, the ER induces apoptosis by activating downstream signaling molecules, such as CHOP and caspase-12. In the present experiment, E2 and G1 considerably reduced neuronal apoptosis and expression levels of GRP78, CHOP, and caspase-12 in the hippocampus of I/R injury rats. Notably, the therapeutic effect of E2 was slightly stronger than that of G1.However, protective effects were inhibited after the addition of G15. Our results indicate that G1 can reduce apoptosis induced by the endoplasmic reticulum stress pathway.
Rats deficient for the caspase-12 gene were unable to induce apoptosis in endoplasmic reticulum stress. In other words, caspase-12 is essential in the mechanism of endoplasmic reticulum stress-mediated apoptosis and specific to endemic stress in the ER (Nakagawa et al., 2000). Immunofluorescence experiments revealed noticeably increased caspase-12 levels after I/R injury, although G1 injection after reperfusion obviously decreased caspase-12 expression levels in rat brains. This further shows that G1 has a specific effect on endoplasmic reticulum stress.

Figure 6 E2 and G1 reduce expression and distribution of caspase-12 in the hippocampal CA1 region after I/R.
This study has some limitations. The most effective dosages of E2 and G1, and time window of treatment require further investigation. Moreover, potential effects of non-endoplasmic reticulum stress apoptotic proteins or other signaling pathways on this protective mechanism need to be elucidated. Therefore, future studies must focus on the interactions between these pathways.
In summary, GPER activation protects the brain after I/R injury and reduces apoptosis via the endoplasmic reticulum stress-mediated apoptotic pathway. This result provides new insight into treatment of I/R injury. Nevertheless, the results require further validation.
Acknowledgments:We thank the Key Laboratory of Xinjiang Endemic and Ethnic Diseases of Xinjiang Provincial Department of Physiology,School of Medicine, Shihezi University for excellent technical assistance.
Author contributions:Study conception and design: LL and JQS; data analysis and explanation: YZ (Ying Zhou), LC and YZ (Yang Zhang);study implementation and manuscript writing: ZWH, YCC and HZ. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81560175, No. 81260159 (both to LL). The funding sources had no role in study design, conception, analysis or interpretation of data, writing and deciding to submit this paper for publication.
Institutional review board statement:The experimental protocol was approved by the Institutional Ethics Review Board at the First Affiliated Hospital of Shihezi University School of Medicine, China (approval No.SHZ A2017-171) on February 27, 2017. All experimental procedures described here were in accordance with the National Institutes of Health(NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Fabricio Ferreira de Oliveira, Universidade Federal de Sao Paulo, Brazil.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Transcriptional dysregulation in neurodegenerative diseases: who tipped the balance of Yin Yang 1 in the brain?
- Redistribution of nerve strain enables end-to-end repair under tension without inhibiting nerve regeneration
- Choroid plexus tumor necrosis factor receptor 1:a new neuroin flammatory piece of the complex Alzheimer's disease puzzle
- Magnesium: pathophysiological mechanisms and potential therapeutic roles in intracerebral hemorrhage
- Bridging larger gaps in peripheral nerves using neural prosthetics and physical therapeutic agents
- Exogenous neural stem cell transplantation for cerebral ischemia

