Identification of a microRNA switch in spinal commissural axon guidance
The formation of neural circuits is governed by multiple classes of highly conserved extracellular guidance signals such as guidance cues, growth factors, and cell adhesion molecules (Kolodkin and Tessier-Lavigne, 2011; Stoeckli et al., 2018). During embryonic development, vertebrate commissural neurons project axons toward the floor plate and cross the midline of the spinal cord, a process relying upon the coordination of attractive and repulsive guidance cues (Kolodkin and Tessier-Lavigne, 2011; Stoeckli et al., 2018). For instance, floor plate-derived Sonic hedgehog (Shh) and Netrin-1 attract spinal cord commissural axons projecting toward the floor plate and crossing the midline, whereas Slit-mediated repulsion on precrossing commissural axons is silenced. In contrast, postcrossing commissural axons lose responsiveness to Netrin-1/Shh attraction, but acquire responsiveness to repulsion of Slits and Semaphorins (Stoeckli et al., 2018). It is believed that low level of Robo1, one of the Slit receptors, in precrossing commissural axons silence the responsiveness of commissural axons to Slit repulsion before midline crossing. However, Robo1 expression increases in postcrossing commissural axons, triggering Slit repulsion and simultaneously silencing Netrin-1-mediated attraction on commissural axon projection (Long et al., 2004). Although such a differential expression pattern of Robo1 acts as a molecular switch of Slit repulsion to control commissural axon guidance (Long et al., 2004), the molecular mechanisms underlying the fine-tuned regulation of temporal expression of Robo1 in developing commissural axons are still not well understood. MicroRNAs (miRNAs), non-coding small RNA transcripts (~22 nucleotides), bind to the 3′ untranslated region (3′UTR)of target mRNAs and regulate gene expression post-transcriptionally via mRNA decay and/or translational repression (Ambros and Chen,2007). Emerging evidence indicate that miRNAs are involved in axon guidance by regulation of either guidance receptors at transcriptional level or their downstream signaling components at posttranscriptional level (Baudet et al., 2011; Zou et al., 2012; Bellon et al., 2017). However,a key question that remains unanswered is whether miRNAs could directly regulate Robo1 expression in the developing vertebrate spinal cord. Recently, one study from our lab has shown that miR-92, a highly conserved miRNA, may function as a molecular switch to specifically repress Robo1 expression, which further regulates Slit repulsion on precrossing commissural axons and plays an important role in commissural axon guidance in the developing chicken spinal cord (Yang et al., 2018). This finding provides a working model of the regulation of Robo1 expression in Slit-mediated commissural axon guidance in the vertebrate nervous system (Figure 1).
To investigate the potential roles of miRNAs in the post-transcriptional regulation of Robo1 expression in commissural axon guidance, the full length mouse Robo1 3′UTR or chicken Robo1 3′UTR sequence was inserted downstream of the Venus YFP gene of a dual fluorescence reporter, in which two separate CMV promoters drive expression of YFP and RFP (an internal expression control), respectively. We electroporated these dual fluorescence reporters into the developing chicken dorsal spinal cord where commissural neurons reside and monitored expression levels of YFP and RFP in the spinal cord at both precrossing and postcrossing stages. The introduction of either mouse or chicken Robo1 3′UTR dual fluorescence reporters resulted in a dramatic reduction of the YFP/RFP ratio in the spinal cord at the precrossing stages, indicating suppression of Venus YFP-Robo1 3′UTR in precrossing commissural neurons. The expression levels of YFP only in the distal, but not the proximal, region of postcrossing commissural axons were dramatically increased, which is similar to the temporospatial expression pattern of Robo1 protein in the developing spinal cord. These results suggest that endogenous regulators such as miRNAs could regulate temporal and compartmentalized Robo1 expression and/or distribution in commissural axons via targeting the Robo1 3′UTR during midline crossing. Bioinfomatics analysis of the Robo1 3′UTR from multiple species revealed that miR-92, a highly conserved miRNA in the vertebrates, could bind to the Robo1 3′UTR via an evolutionarily conserved miRNA recognition element(MRE) of miR-92. This prompted us to investigate whether miR-92 specifically represses Robo1 expression in the developing spinal cord by targeting the Robo1 3′UTR. Results from in situ hybridization in the developing chicken spinal cord showed that miR-92 was strongly expressed in the dorsal spinal cord at precrossing stages and the expression levels reduced progressively as the development proceeds.At postcrossing stages, miR-92 was barely detected in the dorsolateral spinal cord nor the distal segment of postcrossing commissural axons.Interestingly, miR-92 signals seemed to be restricted in a region along the proximal segment of postcrossing commissural axon trajectories in the ipsilateral side of the spinal cord, which is similar to the repression pattern of the Robo1 3′UTR dual fluorescence reporters in the spinal cord at postcrossing stages. Opposite expression patterns of miR-92 and Robo1 imply that miR-92 may regulate expression of endogenous Robo1 by targeting the Robo1 3′UTR in the developing commissural neurons. To examine the possibility of miR-92-dependent suppression on Robo1 expression, cRobo1 3′UTR dual-luciferase reporters were generated and nucleofected with either control miRNA or miR-92 mimics into HeLa cells. An in vitro luciferase assay showed that expression of miR-92 repressed the luciferase activities of the wild-type Robo1 3′UTR reporter, but not the Robo1 3′UTR reporter bearing a mutated miR-92 MRE. Results from western blot and immuno fluorescence assays confirmed that expression of either miR-92 oligoes or GFP/gga-miR-92 expression constructs reduced endogenous Robo1 protein levels in both dissociated primary neurons and the chicken dorsal spinal cord. To further examine whether endogenous miR-92 is active, a miR-92 Sensor with six repeats of miR-92 MREs downstream of the Venus YFP coding sequence were generated and introduced into the developing chicken spinal cord. Expression of the miR-92 Sensor showed a significant reduction of YFP expression in precrossing commissural neurons compared to the control Sensor(with scramble sequences). Co-expression of the miR-92 Sensor with an anti-miR-92 inhibitor antagonizing the endogenous miR-92 activities in the chicken spinal cord successfully restored the YFP expression. Interestingly, the 3′UTR of chicken Cdc42, a member of the p21 Rho-family of small GTPases involved in Slit/Robo signaling, also has one MRE of miR-92. However, expression of miR-92 failed to repress the luciferase activities of the cCdc42 3′UTR luciferase reporter. Altogether, these results suggested that miR-92 can specifically repress cRobo1 expression in the developing chicken spinal cord.
The generally accepted mechanisms underlying the miRNA-mediated suppression are mRNA degradation and/or translational repression. Previous studies have suggested that the translational repression of target gene expression by miRNAs is a preferred mechanism in developing neurons (Jin and Xiao, 2015). To determine how miR-92 represses cRobo1 expression in the developing spinal cord, we electroporated GFP/gga-miR-92 constructs into the developing chicken neural tube and expression levels of endogenous cRobo1 protein and mRNA in the dorsal spinal cords after electroporation were examined by western blot and quantitative real-time PCR, respectively. As expected, expression of miR-92 reduced endogenous cRobo1 protein levels. However, cRobo1 mRNA levels were not altered in chicken dorsal spinal cord neurons transfected with GFP/gga-miR-92. In addition, in situ hybridization on transverse sections of the chicken spinal cord after electroporation of GFP/gga-miR-92 showed that the Robo1 mRNA levels displayed no significant difference between the electroporated side and unelectroporated side of the spinal cord.These data suggest miR-92 represses cRobo1 expression by translational repression, but not mRNA degradation, confirming a currently favored mechanism of miRNA-mediated suppression of gene expression. Emerging evidence revealed that regulation of local protein synthesis by miRNAs plays an important role in axon guidance (Bellon et al., 2017). Does miR-92 locally regulate Robo1 expression in commissural neurons? The chicken spinal cord electroporated with the cRobo1 3′UTR dual fluorescence reporter showed repression of YFP expression in the proximal, but not the distal, segment of the postcrossing commissural axons nor the dorsal spinal cord where the cell body of commissural neurons locates, suggesting a compartmental regulation of Robo1 expression in commissural axons. Fluorescence in situ hybridization on dissociated precrossing commissural neurons demonstrated expression and/or localization of miR-92 in the axon shaft and the growth cone, further denoting the local activities of miR-92 in precrossing commissual axons. Visualization of de novo cRobo1 local protein synthesis in chicken precrossing commissural axons by expressing a kikGR-based photoconvertible translation reporter carrying the cRobo1 3′UTR demonstrated that miR-92 specifically regulated cRobo1 local protein levels in the axon and/or the growth cone of commissural neurons. Consistent with previous findings, our studies support that the miR-92-dependent regulation of cRobo1 local protein synthesis in the growth cone appears to be a key mechanism in commissural axon guidance.
In the developing nervous system, miRNAs-dependent regulation of guidance signaling molecules plays an important role in controlling axon sensitivities to guidance cues (Baudet et al., 2011; Bellon et al.,2017). To determine whether miR-92 is involved in regulating the responsiveness of commissural axons to Slit repulsion during midline crossing, we performed an open-book turning assay of chicken spinal cord commissural axons after in ovo electroporation. Either inactivation of endogenous miR-92 activities by a miR-92 Sponge or expression of a miR-92-insensitive cRobo1 (a cRobo1 mutant resistant to endogenous miR-92 action) in precrossing commissural neurons resulted in premature responsiveness of precrossing commissural axons to Slit2 repulsion in vitro. Results from a co-culture assay of chicken spinal cord explants with Slit2-secreting HEK293 cell aggregates showed that overexpression of exogenous miR-92 in postcrossing commissural neurons attenuated the Slit2-mediated inhibition on postcrossing commissural axon outgrowth. In addition, inhibition of endogenous miR-92 activities disrupted precrossing commissural axon projection in vivo with less axons reaching the floor plate as well as more misguided axons in the ipsilateral side of the chicken spinal cord. At postcrossing stages, suppression of Robo1 expression by exogenous miR-92 resulted in stalling of commissural axons in the floor plate, which is similar to the phenotype observed in Robo1-/-knockout mice. These results from gain- and loss-function experiments suggest that miR-92 can modulate commissural axon sensitivities to Slit2 repulsion through direct regulation of Robo1 expression to control Slit/Robo1-mediated commissural axon guidance in the developing spinal cord (Figure 1).
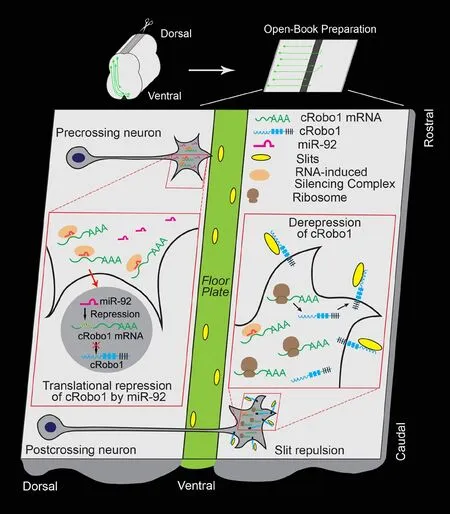
Figure 1 A working model of the regulation of Robo1 expression by miR-92 in spinal commissural axon guidance.
Our study provides a working model of the fine-tuned regulation of Robo1 by miR-92 in developing vertebrate commissural axons to modulate Slit sensitivity during midline crossing: high levels of miR-92 in precrossing commissural neurons repress Robo1 local translation in the growth cone by targeting Robo1 3′UTR, silencing the responsiveness to Slit repulsion and allowing axon projection toward the floor plate, and conversely, loss of miR-92 expression in postcrossing commissural neurons results in upregulation of Robo1 expression, promoting Slit repulsion, triggering commissural axons to exit the floor plate, and preventing them from recrossing the midline(Figure 1). Although our studies suggest that miR-92 could function as a molecular switch to regulate Slit/Robo1-mediated commissural axon guidance, the mechanisms underlying the temporospatial regulation of miR-92 expression in developing commissural neurons remain elusive. Given that the transcription factor c-Myc could induce miR-92 expression in human P493-6 B lymphoma cells (O'Donnell et al., 2005), it is plausible to propose a Myc-dependent transcriptional regulation of miR-92 expression in developing commissural neurons to modulate Slit/Robo1 signaling. Future investigations are required to validate this hypothesis. gga-miR-92 gene is located in the first intron of GPC5 gene encoding Glypican-5, a member of glycosylphosphatidylinositol-anchored heparin sulfate proteoglycans.As heparin sulfate proteoglycans can act as co-receptors for Slits that are required for Slit/Robo signaling (Ypsilanti et al., 2010), miR-92 and Glypican-5 may be co-expressed and regulated by Slits to modulate the Slit/Robo signaling in commissural axon guidance.
Guo-fa Liu*, Tao Yang
Department of Biological Sciences, University of Toledo, Toledo,OH, USA
*Correspondence to:Guo-fa Liu, PhD, MD, Guofa.liu@Utoledo.edu.
orcid:0000-0003-1464-1493 (Guo-fa Liu)
Received:December 5, 2018
Accepted:January 2, 2019
doi:10.4103/1673-5374.251300
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Transcriptional dysregulation in neurodegenerative diseases: who tipped the balance of Yin Yang 1 in the brain?
- Redistribution of nerve strain enables end-to-end repair under tension without inhibiting nerve regeneration
- Choroid plexus tumor necrosis factor receptor 1:a new neuroin flammatory piece of the complex Alzheimer's disease puzzle
- Magnesium: pathophysiological mechanisms and potential therapeutic roles in intracerebral hemorrhage
- Bridging larger gaps in peripheral nerves using neural prosthetics and physical therapeutic agents
- Exogenous neural stem cell transplantation for cerebral ischemia

