Production of liquid bio-fuel from catalytic de-oxygenation:Pyrolysis of beech wood and flax shives
Chetna Mohabeer, Luis Reyes, Lokmane Abdelouahed, Stéphane Marcotte,Jean-Christophe Buvat, Lucette Tidahy, Edmond Abi-Aad, Bechara Taouk,*
(1.Normandie Univ, INSA Rouen Normandie, UNIROUEN, Laboratoire de Sécurité des Procédés Chimiques, LSPC EA-4704, 76000 Rouen, France2.Normandie Univ, INSA Rouen Normandie, UNIROUEN, COBRA UMR-6014, 76000 Rouen, France3.Université du Littoral Cte d’Opale, Unité de Chimie Environnementale et Interactions sur le Vivant, UCEIV EA-4492, 59140 Dunkerque, France)
Abstract: This study presents a detailed analysis of the catalytic de-oxygenation of the liquid and gaseous pyrolytic products of two biomasses (beech wood and flax shives) using different catalysts (commercial HZSM-5 and H-Y, and lab-synthesised Fe-HZSM-5, Fe-H-Y, Pt/Al2O3 and CoMo/Al2O3). The experiments were all conducted in a semi-batch reactor under the same operating conditions for all feed materials. BET specific surface area, BJH pore size distribution and FT-IR technologies have been used to characterise the catalysts, while gas chromatography-mass spectrometry (GC-MS), flame ionisation detection (GC-FID) and thermal conductivity detection (GC-TCD) were used to examine the liquid and gaseous pyrolytic products. It was firstly seen that at higher catalyst-to-biomass ratios of 4∶1, de-oxygenation efficiency did not experience any further significant improvement. Fe-HZSM-5 was deemed to be the most efficient of the catalysts utilised as it helped reach the lowest oxygen contents in the bio-oils samples and the second best was HZSM-5. It was also found that HZSM-5 and H-Y tended to privilege the decarbonylation route (production of CO), whilst their iron-modified counterparts favoured the decarboxylation one (production of CO2) for both biomasses studied. It was then seen that the major bio-oil components (carboxylic acids) underwent almost complete conversion under catalytic treatment to produce mostly unoxygenated aromatic compounds, phenols and gases like CO and CO2. Finally, phenols were seen to be the family most significantly formed from the actions of all catalysts.
Key words: pyrolysis; biomass; catalytic treatment; de-oxygenation; bio-oil upgrading
The transportation industry has long since built a high dependence on fossil sources to provide the elements essential in fuels: aromatic compounds, generally made up of benzene, toluene and xylenes (BTX)[1]. With findings linking fossil use to environmental menaces like global warming, research into finding sustainable, environmentally-friendly sources of aromatics has been boosted. One such alternative is using biomass as feedstock in a process such as catalytic pyrolysis, using catalysts to de-oxygenate the produced oil to produce aromatic compounds. There have been two popular classes of catalysts used throughout literature: zeolite-based and metal-based.
Upgrading bio-oil via the use of zeolites implicates passing the pyrolysis vapours through a microporous structure to convert part of these vapours into desired aromatic compounds and olefins, while the other part is converted into non-condensable gases and coke. The advantage of this process is that high hydrogen pressure is not required[2]. Guda et al[3]worked with pinewood in a packed bed reactor to study effects of acid catalysts on products of pyrolysis; they used an auger reactor at 450 ℃ with nitrogen as carrier gas, testingβ/Al2O3, Si/Al catalyst, H-Y and HZSM-5. Their results affirmed that Si/Al catalyst and beta zeolite contributed to increase of liquid product while HZSM-5 and H-Y led to formation of aromatic hydrocarbons and a higher gas percentage. They also found that the use of HZSM-5 could reduce an oxygen content of 46.4% to 30%, classifying this catalyst as one of the most effective ones for de-oxygenation. Other authors have had the same opinion about the performance of this catalyst[3-6]. Furthermore, literature data prove that using metal-impregnated zeolite materials presents many advantages[6-8].Among the various metals used, iron proves to be active, quite popular as it is highly abundant, low toxicity and cheap[9].
For instance, Mullen et al[10]investigated production of aromatic compounds from catalytic pyrolysis of biomass and some biomass components using Fe-modified HZSM-5 in a pyroprobe reactor. They found that an iron loading of 1.4% produced the largest increase in production of aromatic hydrocarbons from cellulose, hemicellulose and lignin. For the biomass studied, switchgrass, Fe-HZSM-5 loaded with iron at 1.4% Fe produced a similar carbon yield of aromatics as the standard HZSM-5 but higher loadings of Fe decreased the yield. Sun et al[11]compared the catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts in a micro reactor. They found that the Fe/ZSM-5 catalyst demonstrated better activity in conversion of oxygenates and formation of mono-cyclic aromatic hydrocarbons (MAHs) than the ZSM-5 catalyst.
Now, metal-based catalysts are more commonly used in hydro de-sulphurisation (HDS) and hydro de-oxygenation (HDO) with high hydrogen pressure[12], but some authors have made use of these catalysts under other conditions such as a non-sulphated atmosphere, at atmospheric pressure and without hydrogen to study the catalytic de-oxygenation of bio-oil[12-14]. Zhang et al[14]have made use of an acid-base bifunctional catalyst in a fixed bed reactor at either 445 or 500 ℃ to de-oxygenate several bio-oil model components. They have found that a series of de-oxygenation reactions occurred, including decarbonylation, dehydration, and aldol condensation. They also found thatin-situhydrogen was likely formed during the catalysis, which probably participated in the hydro de-oxygenation of small aldehydes/ketones to completely remove oxygen, since olefins were observed in the products.
This study is a direct follow-up of previous research led by our group on the same raw materials[15]. It aims at presenting a detailed analysis of the liquid and gaseous products obtained from the catalytic pyrolysis of flax shives and beech wood using different types of catalysts. To the best of our knowledge, not many research works have tackled such a comparison in one single setup under the same operational conditions. Ultimately, this analysis can help in better understanding the formation of aromatics from catalytic de-oxygenation of biomass and hence, optimise their production.
1 Methods and materials
1.1 Materials used and elemental analyses
Beech wood (BW) used for this study was supplied by ETS Lignex Company and had an average particle size of 400 μm. Flax shives were obtained from “La Coopérative Terre de Lin” and ground and sieved in the laboratory. The particle size was around 1 mm.
Tables 1 and 2 show the ultimate and the proximate analyses of all raw materials used in this study. The total C, H and N were measured on samples using a CHN elemental analyser Flash 2000 (Thermofisher Scientific) while the proximate analysis was conducted by thermogravimetric analysis (TGA) in a STD Q600 equipment, which provided measurement of mass change, according to García et al[16].
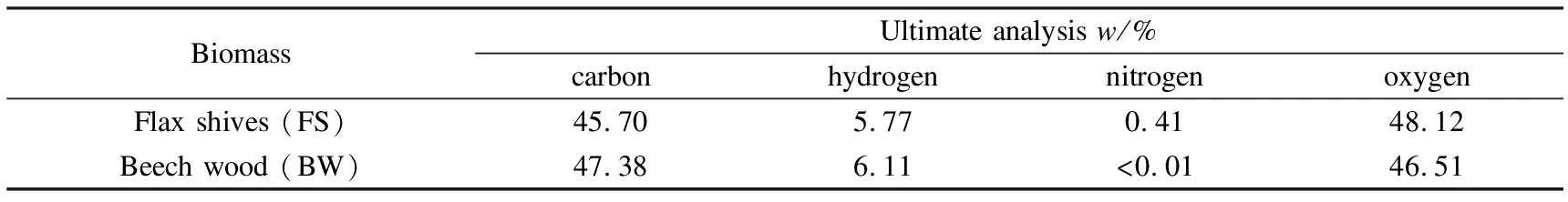
Table 1 Ultimate analysis of the biomasses
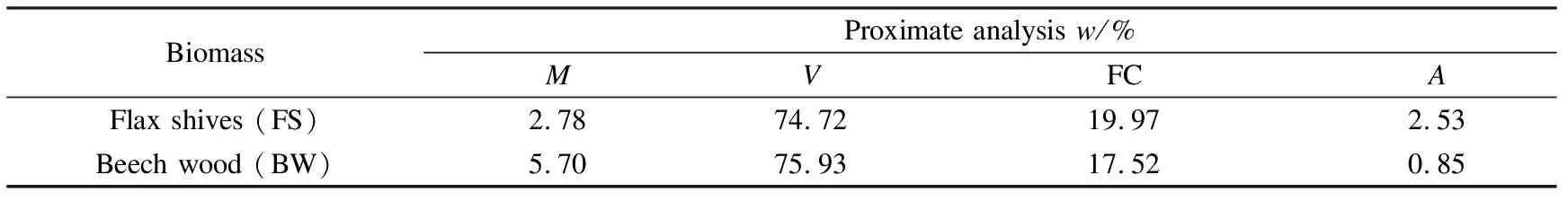
Table 2 Proximate analysis of biomasses based on TGA experiments
1.2 Catalysts preparation and characterisation
1.2.1Preparationofcatalysts
1.2.1.1HZSM-5
The catalyst [hydrophobic HZSM-5 catalyst in its proton form (H+)] was acquired from ACS materials in the form of 3 mm diameter pellets. The SiO2/Al2O3ratio was 38, the specific surface area about 250 m2/g and the pore size about 0.5 nm.The catalyst was triturated and sieved between 1.2 and 1.0 mm. The apparent porosity of this catalyst was 0.61. The catalyst was calcined at 550 ℃ for 4 h , with a heating rate of 2 ℃/min[17]at atmospheric pressure in air, before use.
1.2.1.2H-Y
The H-Y catalyst [4587 Zeolite Y, Hydrogen] was obtained from Alfa Aesar in powder form and had a SiO2/Al2O3ratio of 60 and a specific surface area of 720 m2/g. In order to avoid blockage and any other disadvantage that may be caused by using powders with low particle size, the catalyst was wetted in deionized water and dried overnight in an oven at a temperature of 105 ℃. The dried and compacted catalyst was then triturated and had an apparent porosity of 0.77. Before use, the catalyst was calcined at 550 ℃ using the same procedure as described for HZSM-5.
1.2.1.3Fe-HZSM-5andFe-H-Y
The previously mentioned zeolites, HZSM-5 and H-Y, were used for synthesis of Fe-modified catalysts. An iron nitrate salt, ferric (III) nitrate 9-hydrate (Fe(NO3)39H2O), was purchased from PanReac AppliChem. For each 10 g of catalyst (HZSM-5 or HY), 4.04 g of the iron nitrate salt were diluted in 100 mL of deionized water[17]so as to reach a content of 1.4%[10]. Both catalysts were imbued by the ferric dilution, agitated and heated at 80 ℃ for 10 min. The catalysts were then dried overnight in an oven at 105 ℃. The dried catalysts were afterwards introduced in the tubular furnace to be calcined at 550 ℃, as described above. These catalysts are considered to be bifunctional ones. The apparent porosity was 0.61 and 0.78 for Fe-HZSM-5 and Fe-H-Y, respectively.
1.2.1.4Pt/Al2O3
Tetraamine platinum (II) nitrate [Pt(NH3)4] (NO3)2, containing 1.32% of platinum was acquired from Alfa Aesar in powder form, using gamma-alumina (γ-Al2O3, 99.97%) as support having an apparent porosity of 0.83. For 0.3 g of platinum solution, 10 g of alumina support were required so as to reach a Pt content of 1.32%[13]. The platinum solution and alumina were then added to 20 mL of deionized water and heated at 80 ℃ for 10 min. Following this, the platinum-alumina catalyst was dried and calcined with the same procedure for the afore-mentioned catalysts. In order to reduce the catalyst, it was introduced in the tubular furnace and heated at 5 ℃/min up to 500 ℃ kept for 2.5 h with a gas flow of 500 mL/min of 90% N2and 10% H2.
1.2.1.5CoMo/Al2O3
The bi-metallic catalyst mixture of cobalt and molybdenum was prepared using an atomic relation of Co∶Mo=4∶1; for 1 g of cobalt (II) nitrate hexahydrate 98%, 0.81 g of ammonium molybdate terahydrate 99% and 33.33 g of support were needed[18]. The support used wasγ-Al2O3, 99.97%. The same impregnation, drying, calcination and reduction methods were employed as for the previous catalyst (Pt/Al2O3). The cobalt and molybdenum salts were both purchased from Sigma Aldrich.
1.2.2Catalystcharacterisationmethods
1.2.2.1Specificsurfaceareaandporesize
BET surface area was measured by nitrogen adsorption at -196 ℃ in a Quanta Sorb Junior apparatus (Ankerschmidt). Before analysis, the samples were degassed for 30 min at 200 ℃. Each measurement was repeated three times and an average value was taken.
Nitrogen adsorption-desorption isotherms were obtained on a Micromeritics TRISTAR 3000 analyser at -196 ℃ over a wide relative pressure range from 0.01 to 0.995. The samples were degassed under vacuum for several hours before nitrogen adsorption measurements. The pore diameter and the pore size distribution were determined by the Barret-Joyner-Halenda (BJH) method using the adsorption branch of isotherms.
1.2.2.2Surfaceacidity
The acidity of the catalysts was measured by infrared spectroscopy of adsorbed pyridine. It has been demonstrated in literature that pyridine is the probe molecule of choice to examine the acidity of zeolites as used in biomass pyrolysis applications[19]. To investigate the nature of acid sites and bonding of ions on the surface of catalysts, FT-IR (Spectrum BX, Perkin-Elmer) spectra were obtained in the range of 600-4000 cm-1. The samples for acidity measurement were prepared in powder form, then saturated with small amount of pyridine, and degassed at 100 ℃ for 1 h. The FT-IR spectra in absorbance mode after the pyridine treatment were subtracted with those of the untreated catalysts to obtain the peaks only due to pyridine-acid site interactions[20].
1.3 Pyrolysis experimental and analytical setup
1.3.1Experimentalsetup
The pyrolysis runs were performed in a semi-continuous experimental set up. It comprised of a quartz reactor with a special configuration (Figure 1). The total length of the reactor was 1050 mm. The reactor included two zones: a pyrolysis zone and a catalysis zone, as it has been shown in literature that such a configuration enhances the liquid yield[21]. Both zones were placed horizontally in a tubular furnace. A stainless steel “spoon” could be inserted at one end of the reactor and the other end was connected to a condenser and a flask, placed inside a cold bath to recover the bio-oils formed from condensation of pyrolytic vapours. The non-condensable gases were also recovered for analysis. A flowmeter, placed near the mouth of the reactor, allowed the regulation of the flow of nitrogen, used as carrier gas. The cold bath and the refrigerant were both kept at a constant temperature of -10 ℃.
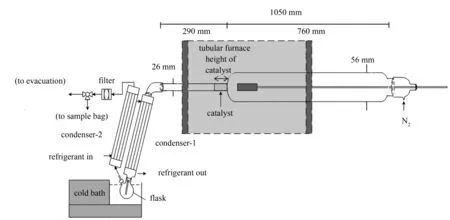
Figure 1 Layout of pyrolysis reactor
The pyrolysis experiments were conducted at 500 ℃ for each biomass used. For one specific test, the reactor was fed with 500 mL/min of nitrogen to create an inert atmosphere. Under these conditions, the residence time is 9.2 min, which means that the pyrolysis regime used was an intermediate one. Indeed, as claimed in literature[22-26], results obtained from fast and intermediate regime are close to each other. About 3 g of biomass were placed onto the spoon, which was kept near the mouth of the resctor. For the experiences comprising the catalysts, a catalyst-to-biomass ration of about 4∶1 was used.After pyrolysis, the oil was recovered by using acetone with 99.98% purity, with a know added amount of nonane,used as internal standard.
1.3.2Bio-oilandnon-condensablegasesanalysis
1.3.2.1GC-MSanalysis
The product analysis methods have previously been reported by Mohabeer et al[15].The analysis of the recovered bio-oil was performed using a gas chromatograph-mass spectrometer instrument GC-MS (Varian 3900-Saturn 2100T). The column was a VF-1701ms (Agilent) (60 m ×0.25 mm × 0.25 μm film thickness). The temperature programme used was the same as Charon et al[27]: the oven was held at 45 ℃ for 4 min then heated to 280 ℃ with a heating rate of 4 ℃/min and held for 20 min. The carrier gas was helium with a constant flow of 1 mL/min. 1 μL of the bio-oil sample diluted in pure acetone was directly injected into the heated split injector (split ratio 30∶1) at 250 ℃. The MS electron ionization energy was 70 eV. The detection was performed in full scan mode. The analysed components were identified with Varian WS (WorskStation) and NIST 2002 software by comparing the mass spectrum obtained to mass spectra from the NIST library. Kovats retention indices of the identified peaks were then calculated and compared to reference values for confirmation of their identity.
1.3.2.2GC-FIDanalysis
Once the peak identity was confirmed, a flame ionisation detector (FID), GC-FID Scion 456-GC Bruker instrument, was used to quantify the components. The column used was the same as the one used for the GC-MS; the temperature programme was also the same except that the final temperature was 240 ℃ instead of 280 ℃. The compounds obtained were then grouped into chemical “families”, each having the same main functional group. A pure reference compound for each family was used for calibration of all peaks of the corresponding family. Standard solutions of each of these reference compounds were prepared by dissolving them in acetone. Five-point straight line calibration curves (withR2>0.99) were established for these pure compounds, using nonane as internal standard.
1.3.2.3GCanalysisofnon-condensablegases
The non-condensable gases were analyzed with a gas chromatograph instrument from Perkin Elmer, Clarus 580, equipped with two detectors, a FID and a thermal conductivity detector (TCD). The instrument also comprised of a Shincarbon St 100 120 column, a methaniser and a hydrogen generator. The oven was regulated from 100-200 ℃. The carrier gas was argon. The TCD was used to determine the components such as H2and N2. The FID detected the carbonated components,except for CO and CO2, which were detected with the help of the methaniser.
1.3.2.4KarlFischer(KF)titrationmethod
The volumetric KF titrations were performed with a Metrohm 870 KF TitrinoPlus apparatus. An aqualine sodium tartrate solution was used as titrating agent, along with Hydranal Composite 5 and Hydranal Methanol Rapid as working media. The sample weight used was in the range of 0.17-0.20 g.
2 Results and discussion
2.1 Defining the pyrolytic temperature
The pyrolytic temperature chosen for this study was 500 ℃. This was based on a series of pyrolytic runs at 450, 500, 550 and 600 ℃ under the same operating conditions. Based on reproducibility of the experiments, a percentage error of 1% was found. Figure 2 demonstrates the different values obtained for the mass balances of the various runs for flax shives (all above 95%), brought to 100%.
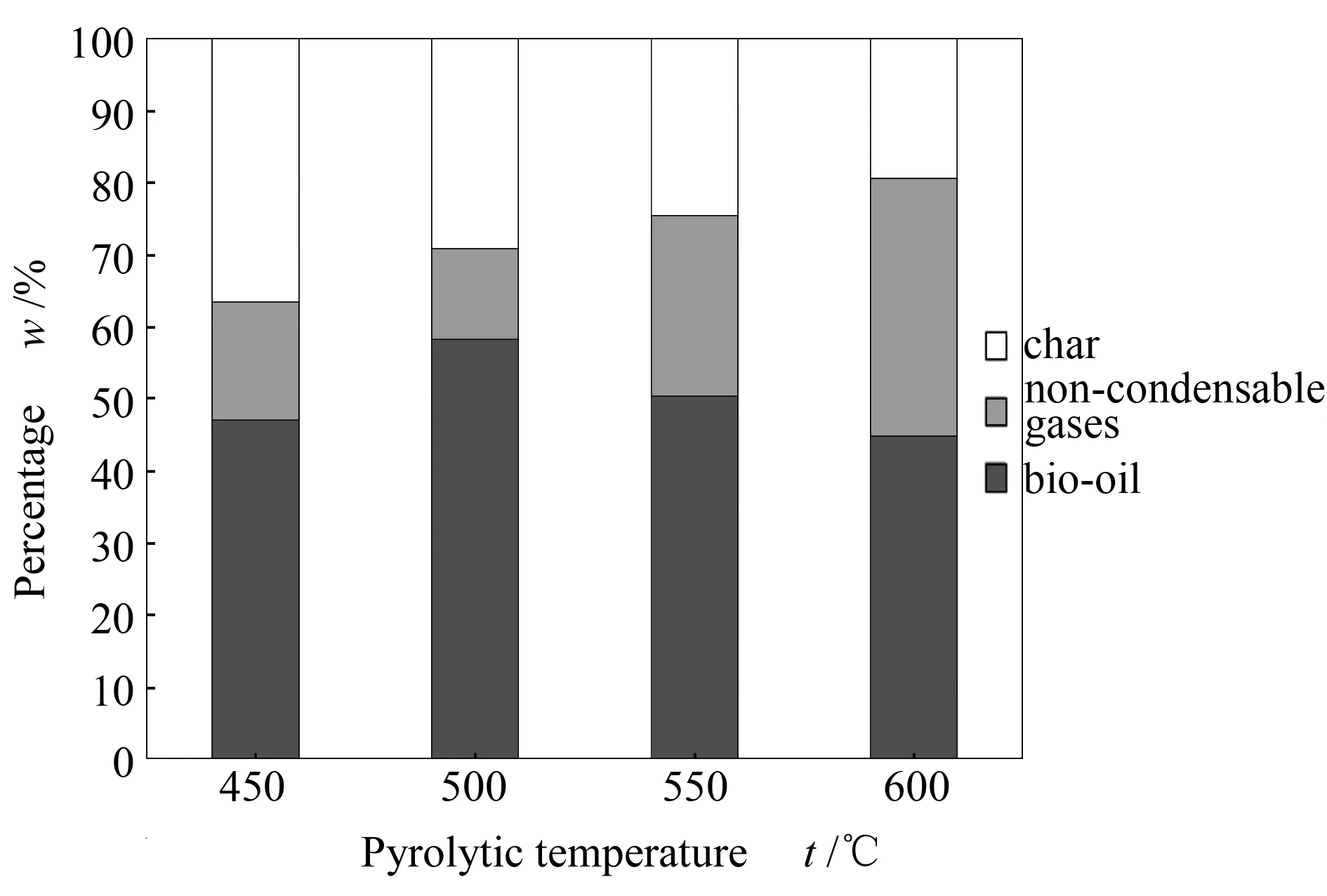
Figure 2 Mass balances for flax shives pyrolysis at different pyrolytic temperatures
Beech wood followed a similar trend, despite the two biomasses having been harvested in a different way and flax shives possessing more mineral content. The maximum yield of bio-oil, 58.18%, wa obtained at 500 ℃ which was hence chosen as the operating temperature for the experimental runs throughout this study.
2.2 Catalyst characterisation results
The values obtained for the specific surface area of the catalysts used are listed in Table 3. The BET surface area decreased after metal modifications. This observation is due to the fact that metal species were deposited on the catalyst surface during impregnation. For the supported catalysts on alumina, the decrease was more pronounced because the surface area ofγ-Al2O3was lower than that of the zeolites and therefore, the dispersion was probably lower. The nitrogen gas adsorption-desorption isotherms for the same representative catalysts samples as mentioned above are illustrated in Figure 3. The specific pore volume for each analysed sample has been included in Table 3. A slight decrease in the specific pore volume of Fe-HZSM-5 as compared to that of HZSM-5 can be observed, corroborating the previous statement claiming a partial coverage of the HZSM-5 pores by Fe. Concerning the isotherms in Figure 3, HZSM-5, Fe-HZSM-5 and H-Y basically show a combination of IUPAC Types I and IV isotherms[28]. Hence, these samples possessed both microporous and mesoporous structures.γ-Al2O3displayed a slightly different isotherm than the others (closer to IUPAC Type III); this may be due to the smaller surface area ofγ-Al2O3relative to the zeolite-based catalysts, which translated to a lower pore volume (0.22 cm3/g).
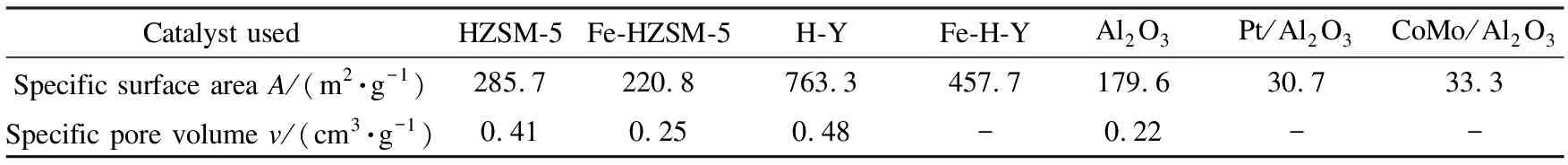
Table 3 Specific surface areas and specific pore volumes of catalysts used
For the acidity characterisation, after pyridine adsorption, the stepwise desorption of the catalysts used was performed at 100, 150, 200, 300 and 400 ℃. Figure 4 represents the spectra obtained for the Fourier transform-infrared (FT-IR) of the catalysts samples at 300 ℃. Catalysts are known to possess two kinds of acid sites. Firstly, for those in the H-form, the hydroxyl groups linking Si and Al atoms have strong Brønsted acid properties. Then, Lewis acid sites are usually associated with extra-framework Al and O species[29]. Concerning the FT-IR spectra obtained for the catalyst supports (HZSM-5, H-Y andγ-Al2O3), the bands at 1436 and 1580 cm-1, characteristic of Brønsted acid sites, as well as bands at 1482 and 1596 cm-1, representing Lewis acid sites, are noted. The principal effect of the metal modification on the supports can be observed as a decrease in the acid bands; however, only those present at 1482 and 1596 cm-1have been completely reduced, providing evidence that the Lewis acid sites were displaced by metal ions. These same observations were previously reported by Lobree et al[30], validating the metal impregnation.
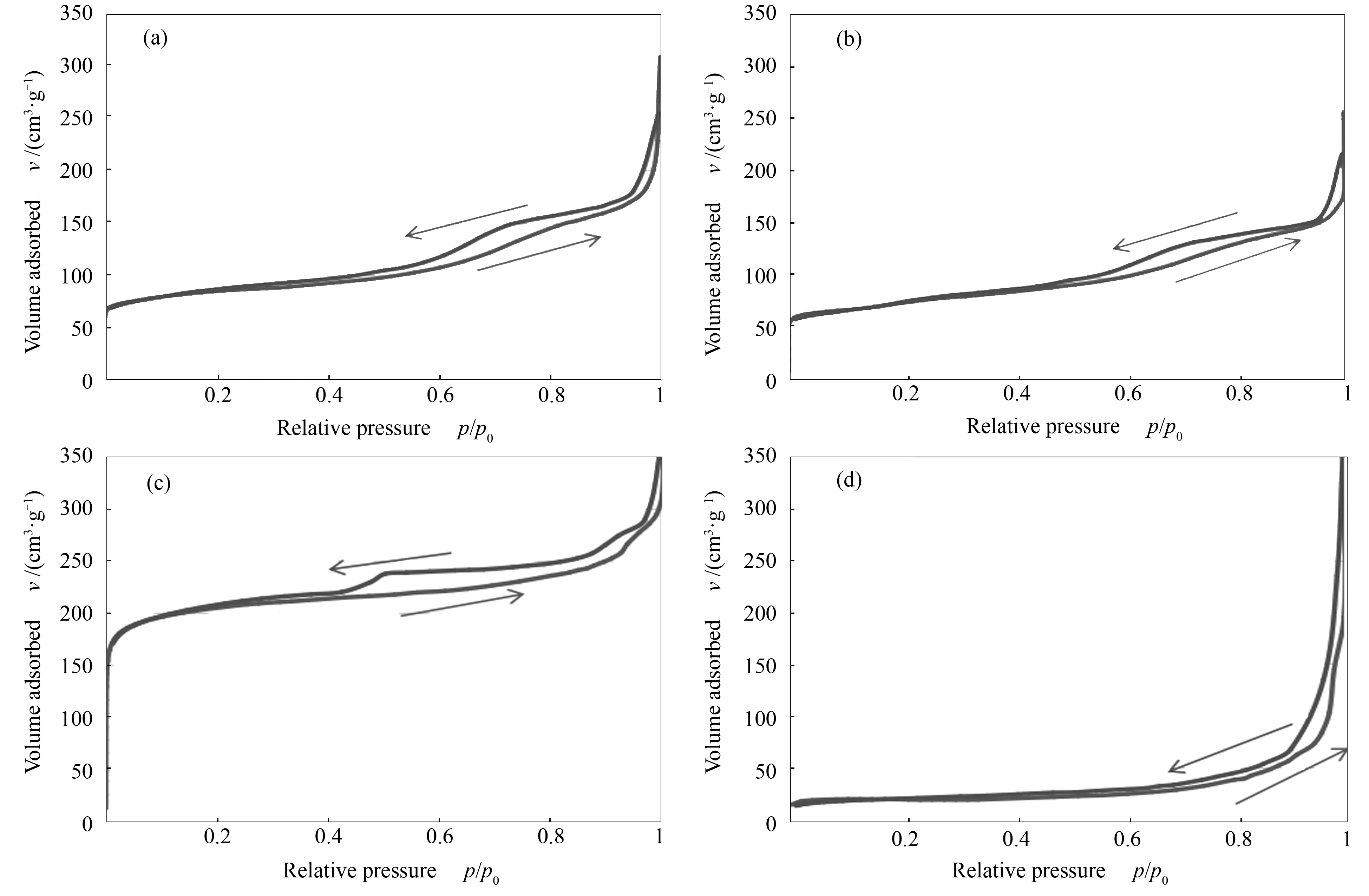
Figure 3 N2 adsorption/desorption isotherms of catalysts and supports
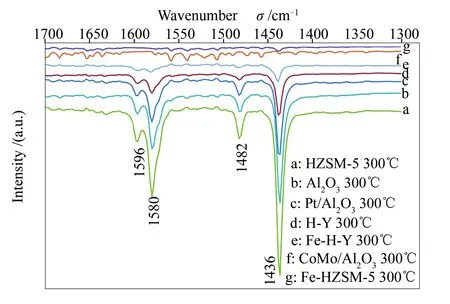
Figure 4 FT-IR spectra of catalysts
2.3 Effect of quantity of catalyst used
Several studies investigating the effect of using different catalyst loadings (catalyst-to-biomass ratios) have been published in literatures[13,27,28]. The reason behind this is that for heterogeneous catalytic systems, the contact time between the catalyst and biomass, or vapour, is an important parameter[31]. Therefore, effect of catalyst loading on the composition of the bio-oil was examined. The Fe-HZSM-5 will be taken as case in point; the parameters are listed in Table 4. It should be noted that the nitrogen flow rate used was 500 mL/min and the apparent porosity of Fe-HZSM-5 was 0.61.

Table 4 Parameters for experimental runs concerning effect of catalyst-to-biomass ratio used
The equation used to calculate the contact time was the following:Firstly, it was observed that by changing the contact time from 1.94 to 3.89 s, the bio-oil was de-oxygenated by a large margin: from 34.33% to 23.84% and 17.31%, respectively (Figure 5). On the other hand, utilising a contact time of 7.77 s did further de-oxygenate the bio-oil produced, but not by a significant amount, causing the oxygen content to drop only to 16.18%.
As for evolution of different chemical families present in the bio-oil samples with different catalyst loadings, the use of higher amounts of catalyst caused the liquid products to decrease and boosted the gaseous products, which is in line with previous studies[32]and acts as a proof that de-oxygenation was indeed taking place. Another major observation was that the phenolic content increased to reach a maximum at the catalyst-to-biomass ratio of 4∶1, while that of other oxygenates decreased (Figure 5). What can be gleaned from this is that using higher catalyst loadings may continually lower the bio-oil oxygen content; however, for catalyst-to-biomass ratios of more than 4∶1, any further improvement remained negligible. More details on the different percentages of the chemical families are given in the Table 5.

Figure 5 Effect of quantity of catalyst used on global oxygen percentage of flax shive bio-oil
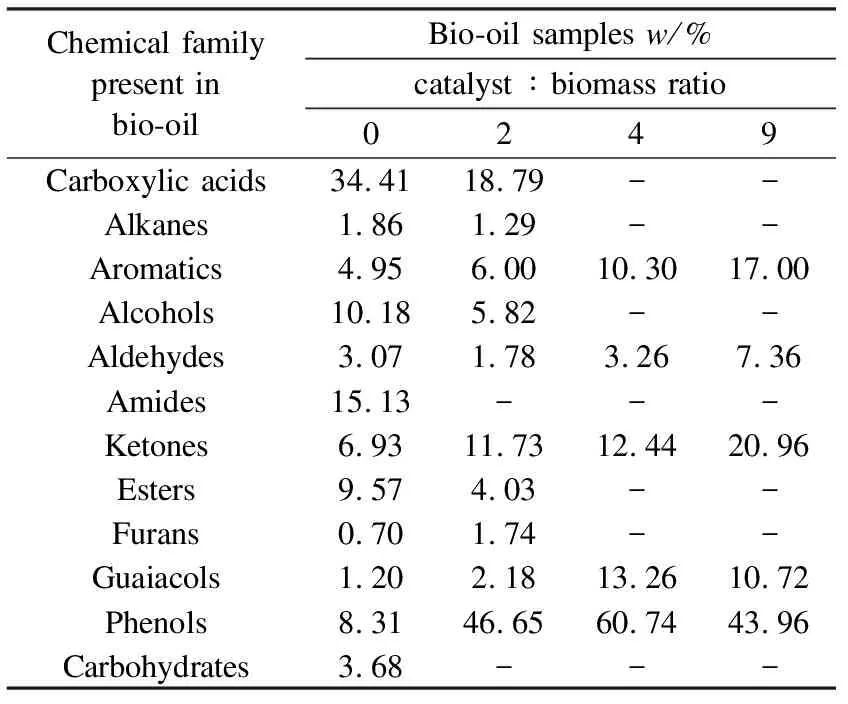
Chemical family present in bio-oilBio-oil samples w/%catalyst ∶ biomass ratio0249Carboxylic acids34.4118.79--Alkanes1.861.29--Aromatics4.956.0010.3017.00Alcohols10.185.82--Aldehydes3.071.783.267.36Amides15.13---Ketones6.9311.7312.4420.96Esters9.574.03--Furans0.701.74--Guaiacols1.202.1813.2610.72Phenols8.3146.6560.7443.96Carbohydrates3.68---
2.4 Performance of catalysts in terms of de-oxygenation activity
It should be noted that the presence of oxygen in biomass-derived oil is the primary reason why the latter is highly functionalised. This elevated functionality diminishes stability of the bio-oils and commonly results in polymerisation[6]. A direct consequence is the increase in viscosity of bio-oils with time,low pH values and low heating values[27,33]. Hence, removal of oxygen (de-oxygenation) of these bio-oils prior to their use is of paramount importance. This is the reason why out of all the catalysts studied during this work, the one which caused the most important drop in oxygen content was considered the most performant one. Figure 6 illustrates the different percentages of oxygen obtained for beech wood and flax shive bio-oils with and without catalytic treatment. It should be noted that the oxygen content denoted here was the one calculated only from the chemical molecules present in the bio-oils.
Figure 6 clearly shows that the most efficient catalyst was Fe-HZSM-5, reducing bio-oil content from 33.82% to 14.50%for beech wood and 34.76% to 13.97% for flax shives. HZSM-5, which was long touted as being the most performant catalyst for de-oxygenation[6], presented the second best efficiency, bringing the oxygen level for beech wood down to 18.40% and to 14.42% for flax shives. The catalysts that followed in terms of de-oxygenation efficiency were Fe-H-Y and H-Y. However, theγ-Al2O3-supported catalysts did not have a very significant impact on the oxygen content of the bio-oils. Despite the fact that some researchers reported successful de-oxygenation through the sole use of these catalysts[34], our results remained nonetheless coherent with what has been presented by the majority of authors[10,12,14,16,31]: these catalysts are better used in hydro de-oxygenation (HDO) processes under high pressure of hydrogen, they are more efficient.
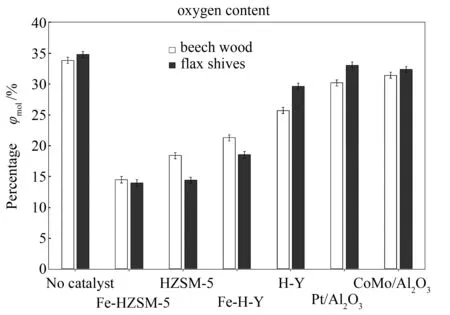
Figure 6 Oxygen content of bio-oil samples obtained with and without catalytic treatment
2.5 Effect of catalytic treatment on pyrolytic products
Judging performance of the catalysts based on oxygen content of the bio-oils gives an idea of their efficiency. However, no knowledge can be garnered concerning the effect that the catalyst utilisation has on the different pyrolytic products. Figure 7 shows the different product (liquid, char, gas) distributions obtained for beech wood and flax shives at 500 ℃ with and without catalyst. The experimental error on the measurement of char mass has been evaluated from the standard deviation and found to be equal to 1.58. Furthermore, the effect of these different catalysts on the bio-oil and non-condensable gas compositions was also investigated. The results gathered concerning the chemical families present in pyrolytic bio-oils and non-condensable gas components from beech wood and flax shives obtained at 500 ℃ with and without catalyst are presented in Tables 6 and 7.
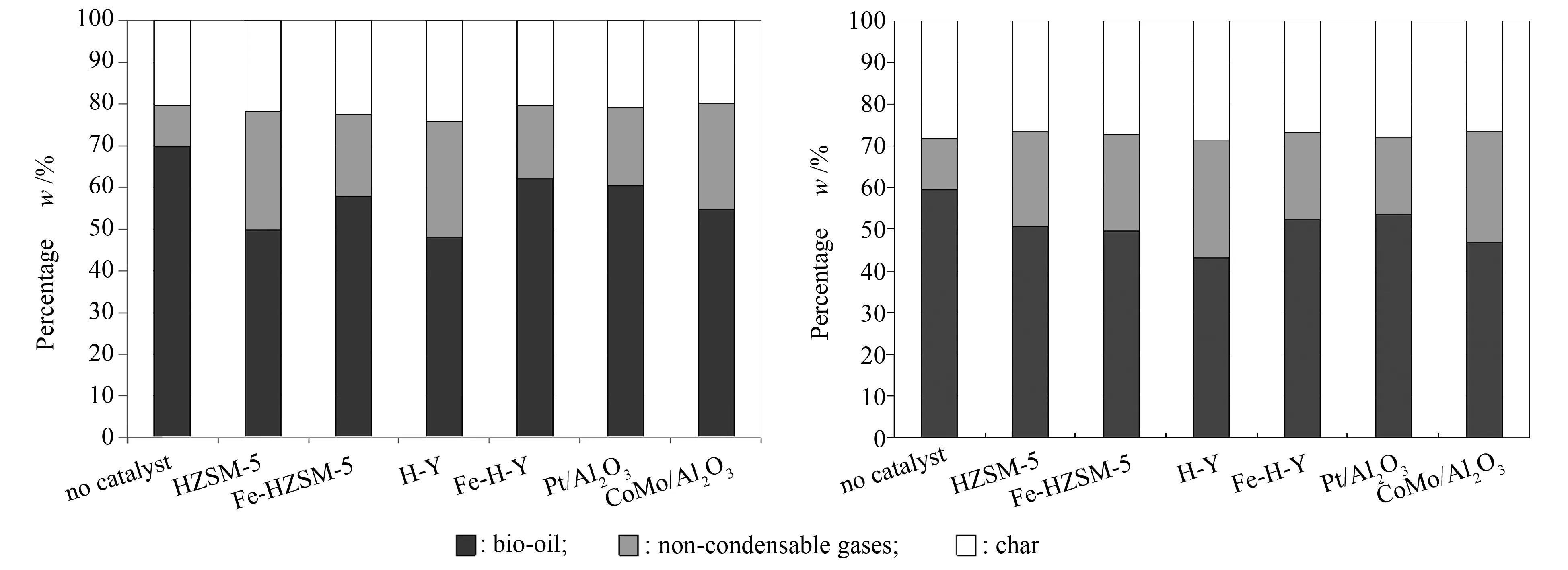
Figure 7 Liquid product distributions for (a) beech wood and (b) flax shives with and without catalytic treatment

Percentage wmol/%beech wood bio-oilsno catalystHZSM-5Fe-HZSM-5H-YFe-H-YPt/Al2O3CoMo/ Al2O3flax shive bio-oilsno catalystHZSM-5Fe-HZSM-5H-YFe-H-YPt/Al2O3CoMo/ Al2O3Carboxylic acids36.367.50-19.8313.9923.5235.3137.26--30.787.8333.6236.25Alkanes0.85----2.912.192.01----2.511.38Aromatics4.8313.288.978.074.897.775.184.5412.1510.517.115.265.194.13Alcohols7.367.475.098.615.8312.288.8811.029.275.799.255.3713.527.29Aldehydes3.621.751.041.871.314.291.813.330.570.821.462.441.840.43Amides3.923.82-5.302.272.361.253.483.221.363.742.242.090.50Ketones10.266.217.305.293.799.6910.378.535.359.043.843.5215.2614.53Esters9.585.42-11.413.5310.835.1710.851.49-11.351.896.553.87Furans2.164.563.993.082.741.032.190.763.045.642.652.541.181.89Guaiacols1.343.062.074.101.440.910.881.301.051.663.211.39-1.06Phenols14.4646.9271.5332.4360.2216.9622.0512.9263.8665.1826.6267.5412.5423.86Carbohydrates5.26----7.454.723.99----5.704.80
In the oils obtained without catalyst, the major chemical family present was carboxylic acids, with 36.36% for beech wood and 37.26% for flax shives. The most significant families following acids were phenols and ketones. It is also worth noting that these were the families contributing most in terms of oxygen content. As for the non-condensable gases, CO2and CO were the major components for beech wood and flax shives, respectively. Also, a table listing the water content of the different bio-oils can be found in Table 8. It was observed that HZSM-5 tended to favour production of water, hence the dehydration reaction, as compared to its iron-modified counterpart. The same observation was made for H-Y as compared to Fe-H-Y. As for the metal-based catalysts, CoMo/Al2O3showed a preference towards the dehydration reaction relative to Pt/Al2O3.

Table 7 Percentages of gaseous components present in non-condensable gas samples with and without catalytic treatment
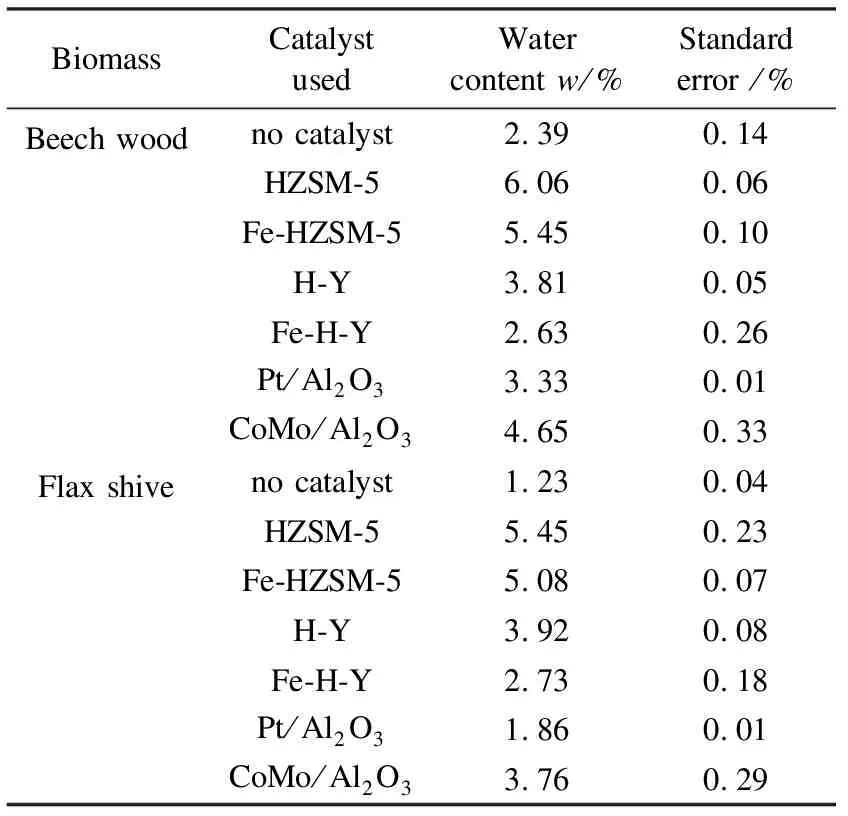
Table 8 Water content of bio-oil samples
The effects of different catalysts with respect to the various chemical groups present in the bio-oil samples and gaseous components in the non-condensable gas samples recovered have been presented in terms of conversion and production percentages in Tables 9 and 10.
The results obtained with both biomasses yielded approximately the same trend, showing that they both reacted in the same manner despite having been collected differently. The zeolites mostly impacted the carboxylic acids, with different degrees of effectiveness; the HZSM-5-based zeolites were the most efficient ones in reducing the acids (-100% for HZSM-5 and Fe-HZSM-5 for the flax shive bio-oils), as compared to the H-Y-based catalysts (-55% and -83% for H-Y and Fe-H-Y, respectively, for the flax shive bio-oils). As for the metal-based catalysts, CoMo/Al2O3was more efficient than Pt/Al2O3in reducing acids (-67% for CoMo/Al2O3vs. -53% for Pt/Al2O3for flax shive bio-oils). However, the degree of effectiveness was very low in contrast to the zeolite-based catalysts. Once more, the reason behind such a dissimilarity is the nature of catalyst itself; metal-based catalysts perform better in the presence of a high pressure of hydrogen in HDO reactions[12].
2.5.1Effectofcatalytictreatmentonliquidproductofbiomasspyrolysis
Now, to better understand the effect of catalytic treatment on the liquid product, the results obtained for each bio-oil has been analysed. Firstly, from Figure 7, the liquid product was the most significant one present. This result is logical given the experimental conditions utilised and is in line with several other researches presented in literature[30,32,34-36]. Similarly, the use of all catalysts impeded the liquid yield and boosted the gas yield. This observation holds true in that the catalysts induced conversion of oxygenated molecules in order to form gas (CO, CO2and so on) and water molecules. This suggests that de-oxygenation was indeed taking place. However, this conversion causes loss of some carbon atoms, albeit along with oxygen ones, to the gaseous components (CO, CO2and so on).
The effect of each catalyst on carboxylic acid, phenol and ketone families was investigated as they were the chemical groups representing the most important proportions in bio-oils (Figure 8). Concerning carboxylic acids, the trend touched upon earlier in Figure 8 (a). As carboxylic acids are the group influencing most of the bio-oil’s characteristics and behaviour[15,36], the efficiency of acid removal is a very important parameter. The efficiency of HZSM-5-based zeolites in reducing carboxylic acid group was very apparent, whileγ-Al2O3-supported catalysts did not have much of an impact on the acid content. The addition of Fe caused the catalysts’ efficiency to reduce acids to increase; the conversion rate increased from -84% for HZSM-5 to -100% for Fe-HZSM-5 and from -69% for H-Y to -84% for its iron-modified version, both in the case of beech wood bio-oil samples. The same trend was observed for the flax shive bio-oils. Thus, the addition of Fe on zeolite supports favoured the occurrence of decarboxylation reaction, and thus the production of CO2.
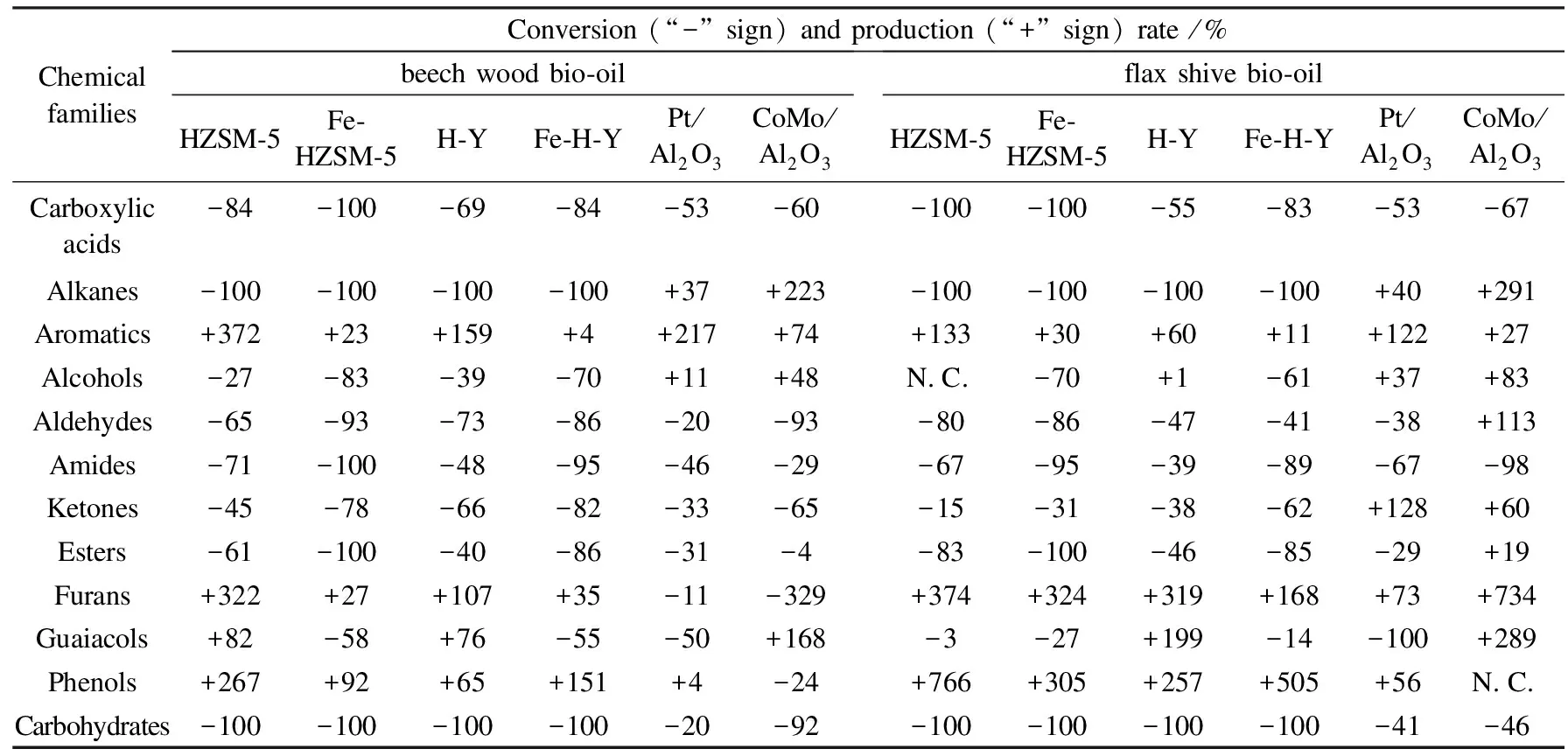
Table 9 Conversion and production rates of chemical families presentin bio-oil samples obtained with and without catalyst use
conversion ratio = (moles obtained without de-oxygenation-moles obtained after de-oxygenation)/moles obtained without
de-oxygenation × 100%
N.C.: no change (same as amount present in non-catalytic sample)

Table 10 Conversion and production rates of non-condensable gas (NCG) componentsobtained with and without catalyst use
conversion ratio = (moles obtained without de-oxygenation-moles obtained after de-oxygenation)/moles obtained without
de-oxygenation × 100%
Prod.: production (produced because of the catalytic treatment, not present in non-catalytic sample);
N.C.: no change (same as amount present in non-catalytic sample)
Moving on to Figure 8 (b), the phenolic contents in the resulting pyrolytic oils after catalytic treatments have increased very importantly. However, from the values from Tables 7 and 9, significant production rates were found for phenols in the case of all catalysts, except CoMo/Al2O3for both biomasses. However, the difference between the biomasses was that flax shives tended to produce more phenols than beech wood. Thus, it can be inferred that flax shives may possess a higher lignin fraction than beech wood, which could result in a higher phenol production. Now, it is important to note that while phenols majorly arise from the lignin fraction, there is also phenol formation coming from the reaction of water and aromatics[37]. Therefore, it may also be stipulated that some of the phenols produced could have arisen from a reaction of a fraction of the water formed by the pyrolysis reaction with aromatics.
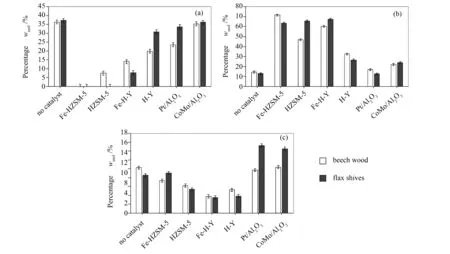
Figure 8 Effect of catalysts on (a) carboxylic acids, (b) phenols and (c) ketones
As for Figure 8 (c), the ketone content was importantly impacted by the catalysts. Gayubo et al[38]have stipulated that acids underwent catalytic de-oxygenation to produce ketones first, which further degraded into alkenes. However, based on Table 7, no production rate was observed for ketones; alkenes (notably, C2H4and C3H6), on their part, experienced mostly production rates. During this study, ketones as intermediates were not observed, contrary to Gayubo et al[38]. Also, another observation is that H-Y-based zeolites seemed to be more efficient in converting ketones. Theγ-Al2O3-based catalysts were the least efficient in converting ketones, and their use even resulted in ketone formation in the case of flax shives.
Figure 9 illustrates evolution of aromatic compound formation from the catalytic de-oxygenation of beech wood and flax shive bio-oils. Formation of aromatics is an important factor to consider when speaking of the upgrading of bio-oils as these are the most interesting bio-oil components. The aromatics percentage in the upgraded bio-oils increased with the use of all catalysts for both biomasses. These findings are in line with previous works presented in literature[4,8,38-40].
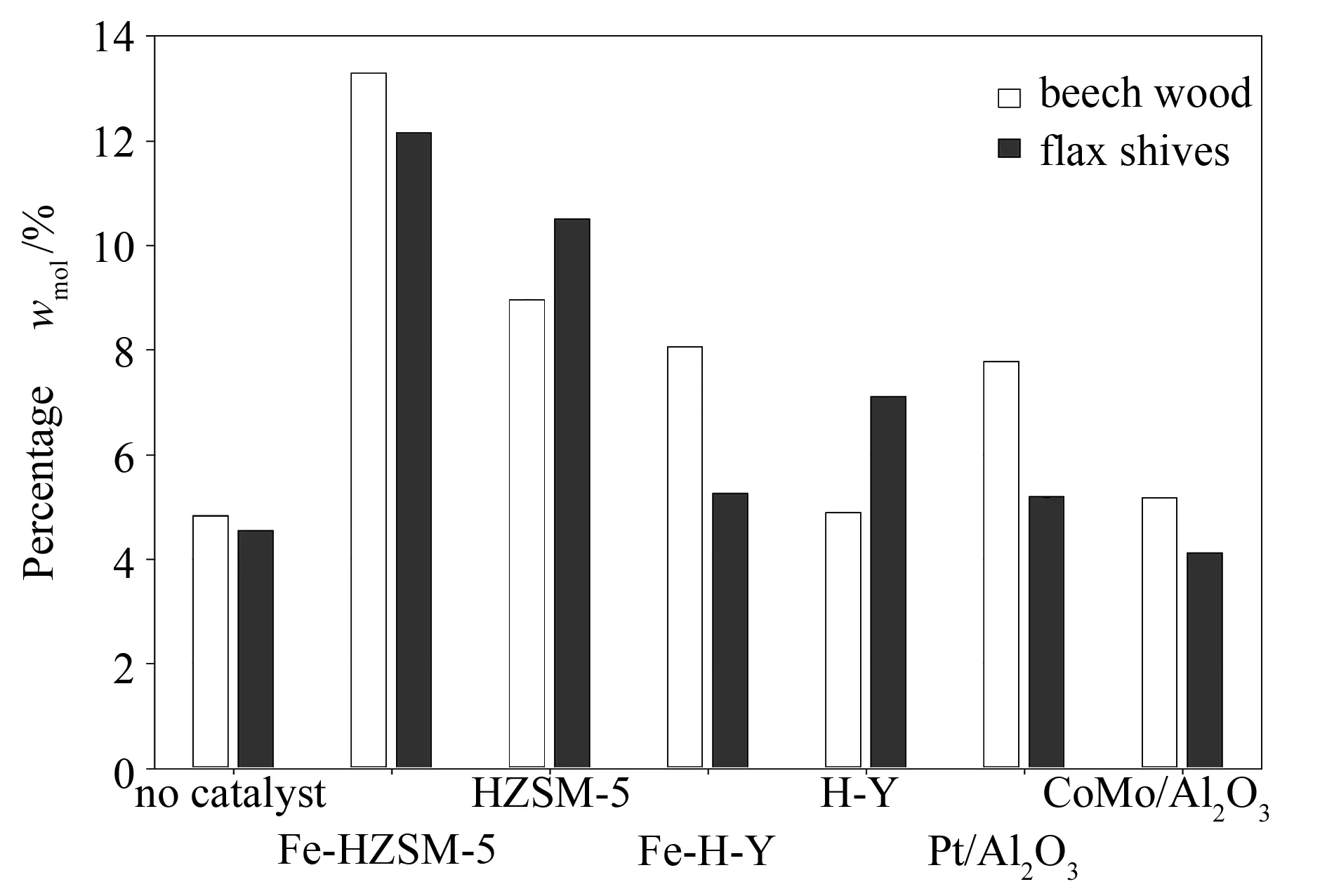
Figure 9 Effect of catalysts on aromatic compounds
However, the interesting point here was that the percentages obtained did not reflect the true happenings of the reaction. Comparing with Table 9, it was observed that HZSM-5 had a higher aromatics production rate than Fe-HZSM-5, as did H-Y relative to its iron-modified counterpart. For the metal-based catalysts, Pt/Al2O3was more efficient than CoMo/Al2O3in producing aromatics. These suggests that addition of a metal on the original catalyst support inhibited formation of aromatic compounds in the enhanced bio-oil.
2.5.2Effectofcatalytictreatmentongaseousproductofbiomasspyrolysis
Almost all the gases analysed demonstrated production rates (Table 10). This is in line with that in literature: the use of catalysts privileged gas formation over liquid production[34]. Figure 10 shows the evolution of CO, CO2, H2and CH4, the major gas components present in the non-condensable gases. CH4demonstrated a rather constant trend for the two biomasses and with the use of all catalysts. Secondly, a notable difference between CO and CO2can be seen; non-condensable gases obtained without any catalytic treatment yielded more CO than CO2for beech wood, while the inverse was detected for flax shives. The emission of these two gases has been associated with hemicellulose and cellulose decomposition[41]; hemicellulose, possessing a higher carboxyl content, emit more CO2, while cellulose produced more CO due to thermal cracking of carbonyl groups. Therefore, based on the previous statement, the major gas contribution was made by the hemicellulosic fraction for flax shives and by cellulose for beech wood. Another observation is that for the same catalyst on the two different biomasses, the same de-oxygenation routes were privileged. For instance, use of HZSM-5 and H-Y privileged decarbonylation route (production of CO): +109% CO vs. +52% CO2for HZSM-5 and +132% CO vs. +42% CO2for H-Y in the case of beech wood. The same trend could be observed for flax shives. Then, addition of Fe to the zeolite catalysts changed de-oxygenation path favoured. Fe-HZSM-5 and Fe-H-Y tended to privilege the decarboxylation pathway (production of CO2): +202% CO2vs. +101% CO for Fe-HZSM-5 and +136% CO2vs. +118% CO for Fe-H-Y, both in the case of beech wood and flax shives.
Concerning H2, an opposite trend to that of CO can be noted. This is interesting as this is related to the reverse water-gas shift reaction. However, it can be stipulated that if it were the latter reaction causing this opposing relationship, the percentage of CO2would have known a similar trend as that of H2, and no specific similarities could be seen here. This can be explained by the fact that it was not only the water-gas shift reaction at play in this case, the reaction also depended on reactivity of the catalyst[31]. Hence, production of CO2from decarboxylation of various chemical families was not constant. Also, the maximum amount of H2gas collected involved the use of Pt/Al2O3, followed by Fe-HZSM-5 and Fe-H-Y. Now, to the extent of our knowledge, not much research has been done on the gases emitted during catalytic de-oxygenation of biomass pyrolytic vapours using Pt/Al2O3, but, it shows that metals supported on catalysts tend to induce hydrogenation/dehydrogenation reactions[42]. Therefore, the presence of Pt and Fe on the catalysts’ surface caused dehydrogenation, emitting more H2gas than the other ones.
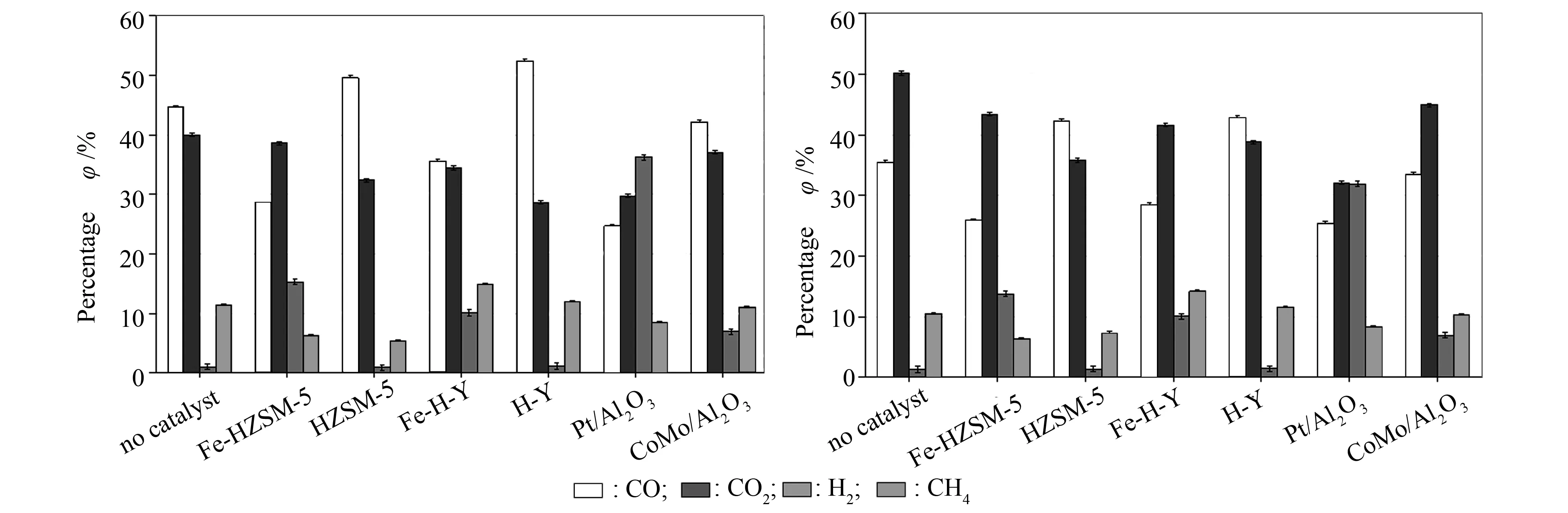
Figure 10 Evolution of CO, CO2, H2 and CH4 with and without catalytic treatment for (a) beech wood and (b) flax shives
3 Conclusions
A detailed analysis of the liquid and gaseous pyrolytic products of catalytic de-oxygenation of two biomasses (beech wood and flax shives) using different catalysts (HZSM-5, Fe-HZSM-5, H-Y, Fe-H-Y, Pt/Al2O3and CoMo/Al2O3) was presented. The different pathways and reactions through which aromatic compounds were produced from catalytic de-oxygenation of flax shives as compared to a conventional lignocellulosic biomass, beech wood, were also examined. Fe-HZSM-5 was deemed to be the most efficient among the catalysts utilised as it helped reach the lowest oxygen contents in both bio-oils. The second best was HZSM-5. Secondly, HZSM-5 and H-Y tended to privilege decarbonylation route (production of CO), whilst their iron-modified counterparts favoured decarboxylation one (production of CO2) for both biomasses. Then, the major bio-oil components (carboxylic acids) underwent almost complete conversion under catalytic treatment to produce mostly unoxygenated aromatic compounds, phenols and gases like CO and CO2. Phenols were seen to be the family most significantly formed from the actions of all catalysts. Finally, at higher catalyst-to-biomass ratios of 4∶1, de-oxygenation efficiency did not experience any further significant improvement.

