家蚕色氨酸羟化酶 (TRH) 基因的克隆及表达特性分析
李田,陈曦,李海银,王计英,孙伟,申琦,鲁成,陈萍
1 西南大学 生物技术学院,重庆 400715
2 西南大学 家蚕基因组国家重点实验室,重庆 400716
3 重庆大学 生命科学学院,重庆 401331
Introduction
The biogenic monoamine 5-hydroxytryptamine(5-HT or serotonin) is an ancient intracellular signaling molecule widely distributed in all animals with nervous systems, and has been implicated in various physiological functions and principal behaviors as a neurotransmitter modulator or a neurohormone.Five-HT was first discovered as a biogenic amine during gastrulation in the developing central nervous system (CNS) of mammals[1-2].It was found to modulate various behaviors such as feeding,sleep, sexual behavior, body temperature, learning and memory[3-5].In insects, 5-HT plays a crucial role in the regulation of salivary gland secretion, heart and oviduct contractions, circadian rhythms and diuresis[6-9].InDrosophila, 5-HT was shown to play a vital role in early embryonic development[10-11], and inManduca sexta, it was found to be indispensable for the development of olfactory glomeruli[12-14].Previous studies inBombyx morihave reported the role of 5-HT in the macroglomerular complex (MGC) and ordinary glomeruli in modulating the response of neuronal populations in the antennal lobe (AL)[15-16].In summary, 5-HT is an essential compound during both behavioral and developmental processes[17].
Five-HT results from a cascade of reactions initiated by tryptophan hydroxylase (abbreviated as TRH in invertebrates and TPH in vertebrates), which is a rate-limiting enzyme that converts L-tryptophan(tryptophan) to 5-hydroxy-L-tryptophan (5-HTP).TRH is a member of the pterin-dependent aromatic L-amino acid hydroxylase family (AAAHs), which includes phenylalanine hydroxylase (PAH, EC 1.14.16.1) and tyrosine hydroxylases (TH, EC 1.14.16.2).Five-HTP is subsequently decarboxylated to generate 5-HT (a final product) by aromatic L-amino acid decarboxylase (DDC, EC.4.1.1.28).TRH, as the rate-limiting enzyme, is known to regulate the concentrations of serotoninin vivoand has been reported to be more stable than 5-HT in insect neurocytes[18].Therefore, TRH represents a specific property of 5-HTergic neurons, and evolutionary analyses have revealed that it is likely to have a broader role in the animal kingdom[19].
In vertebrates, TPH is encoded by two genes, and the two transcripts have tissue-specific patterns;TPH1is expressed in the periphery whileTPH2is expressed in the CNS[20-23].Studies inDrosophila melanogasterhave revealed that the gene products of bothPAHandTRHhave tryptophan hydroxylase activityin vivo, and thatTRHexpressed in the neural tissues has a function similar toTPH2in mice[24-27].PAH, which primarily serves in phenylalanine hydroxylation, is expressed predominantly in the periphery and plays a role similar toTPH1in rats[21].Immunohistochemistry using sheep TPH antibody reveals the wide distribution of TRH protein in the brains of several insect species[25].However, to our knowledge, there is no additional research onTRHin insects, particularly the order Lepidoptera, which includes important pests of agricultural crops.In this study, we cloned and characterizedTRHfrom silkworm (a Lepidoptera model insect) to gain insights into its function in silkworm and other Lepidoptera.
1 Materials and methods
1.1 Silkworm strains
Silkworm DaZao strain was maintained at the Southwest University in China, and fed on mulberry leaves under standard conditions (24–26 °C and 70%–85% RH with a photoperiod of 12:12 LD).
1.2 mRNA isolation and cDNA synthesis
Total RNA was purified using TRIzol reagent(Invitrogen) according to the manufacturer’s instructions.3 μg RNA was reverse transcribed by Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega) to synthesize cDNA.
1.3 cDNA cloning of BmTRH
cDNA from the heads of 5th instar DaZao larvae was used as templates in PCR forBmTRHamplification (the sequence of the primers in Table 1).PCR reaction conditions included 94 °C for 4 min,30 cycles of 94 °C for 40 s, 55.5 °C for 1 min and 72 °C for 1.5 min and a final extension at 72 °C for 10 min.PCR products were purified and cloned into the pMD19-T simple vector (TaKaRa) and sequenced[28].
1.4 Multiple sequence alignment and phylogenetic analysis
Amino acid sequences used for the sequence alignment were identified in the protein database of the NCBI with the amino acid sequence ofBmTRHas template.Multiple sequence alignments of the amino acid sequences were performed with DNAMAN and ClustalX.Transmembrane spanning domains were predicted by TMHMM (genome.cbs.dtu.dk/services/TMMM).Phosphorylation sites and N-glycosylation sites were predicted by NetPhos (www.cbs.dtu.dk/services/NetPhos) and NetNGlyc (www.cbs.dtu.dk/services/NetNGlyc) respectively.Values for identity(ID) and similarity (S) were calculated by BioEdit.We utilized MEGA 6.0 to calculate the genetic distances among different species and to construct neighbor-joining (NJ) trees with 1000-fold bootstrap resampling.
1.5 Semi-quantitative RT-PCR analysis
According to the predicted CDS and EST sequences in the SilkDB, primers for expression analysis were designed forBmPAH, BmTRHandBmDDC(the sequences of the primers in Table 1).Conditions of PCR consisted of 94 °C for 4 min,25 cycles at 94 °C for 40 s, 53 °C for 40 s and 72 °C for 1 min and a final extension at 72 °C for 10 min.Templates for the reaction were cDNA from eleven larval tissues (head, fat body, silk gland, tracheae,central nervous, hemolymph, testis, ovary, integument,malpighian tubule, midgut) from the 3-day-old 5th molting stage.BmActin3was used as an internal control (the sequences of the primers in Table 1).
1.6 Recombinant protein expression and purification
The coding sequence ofBmTRHwas amplified by PCR using head cDNA template (the sequence of the primers in Table 1).After digested withBamHⅠandXhoⅠ(TaKaRa), the PCR product was ligatedinto pET-28a vector.Following with transformation into competentE.coliRosetta (DE3) cells, and induced with 0.6 mmol/L isopropyl-β-D-1-thiogalactopyranoside (IPTG) for 6 h at 37 °C before protein extraction.The recombinant protein containing the 6×His tag was purified by affinity chromatography using a Ni2+column.The purified protein was then quantified using the Bradford method.

表1 本研究所用的引物序列Table 1 The primer sequences used in this study
1.7 Mass chromatographic analysis and antibody preparation
Purified protein was analyzed by MALDI-TOFMS as described previously[29].Data were primarily downloaded from the silkworm genome database(http:silkworm.Swu.edu.cn/silkdb) and data from the NCBI protein database was used as a supplement.Peptide mass fingerprinting was analyzed by GPMAW software.The parameters used were as follows: precision = 0.10%; min.prec = 0.50 Da; min.hits =2; max.overlap = 2.Identification criteria were based on the number and coverage of matched peptides: a minimum of 5 peptides were required to match and the coverage of the matched peptides was about 25%.
A rabbit polyclonal antiserum was prepared against the recombinant BmTRH (Zoonbio, China).Immunizing a healthy male adult rabbits named New Zealand White and the rabbit was immunized every two weeks (three times more).Then collecting blood to gain the antiserum and finally the antibody titer is measured by ELISA (Sigma) and was 1: 6 000.
1.8 Western blotting analysis
The proteins of fat body, silk gland, tracheae,hemolymph, testis, ovary, integument, malpighian tubule and midgut were extracted from 3-day-old 5th Dazao strain.Proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis on 12% gels.After determined with the BCA protein assay, approximately 10 mg total protein was loaded per well.Proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA) with a Mini-ProteinⅡblotting system (Bio-Rad) 200 mA,100 min at 4 °C in a buffer containing 15% methanol.Membranes were blocked with 5% dry milk in Tris-buffered saline containing Tween 20 (TBS-T;10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl,0.01% Tween 20) for 30 min at room temperature.Membranes were probed with affinity-purified anti-BmTRH antibodies (dilution 1: 10 000 in TBS-T)and antigen-preabsorbed affinity-purified antibodies at the same concentration.Membranes were washed with TBS-T, followed by incubation with a secondary antibody conjugated to horseradish peroxidase(1: 2 000; Beyotime, China).Signals were visualized with an enhanced chemiluminescence detection system (ECL; Thermo, USA) and photographed using a Clinx ChemiScope 3400 Mini (China Scientific,China).Experiments were repeated for three times with independently isolated protein samples.
1.9 Immunohistochemistry assay
The testis, ovaries, integument and nervous of 3-day-old 5th instars from the silkworm Dazao strain were moved, and rapidly fixed with 4%paraformaldehyde for 2–4 h at room temperature.Samples were embedded in paraffin following dehydration (a sequential ethanol series: 30%, 50%and 70%, 100%).All the materials were treated vertical embedding.When cooled, samples were continually sectioned with the aid of paraffin section technique and the thickness of slices was all 5 μm.Paraffin sections were deparaffinized in xylene,rehydrated through graded ethanol solutions,quenched by 10 min immersion in 3% hydrogen peroxide, and treated with 0.01 mol/L citrate buffer(pH 6.0) at 92–98 °C for 15 min.Sections were incubated for 1–2 h in 10% goat serum at room temperature, and then incubated with the BmTRH antibody (dilution 1: 10 000) overnight at 4 °C.After rinsing Tween-20-phosphated-buffered saline (TPBS),sections were incubated for 1–2 h with an HRP-labeled goat anti-rabbit antibody (dilution 1: 2 000) at room temperature.The sections were developed using DAB (3,3-diaminobenzidine) and observed under a microscope.Serum of goat injected with PBS (negative serums) served as a negative control.
2 Results
2.1 BmTRH cloning and expression

图1 Bm TRH的克隆和表达鉴定Fig.1 The cloning and expression of BmTRH. (A) The lane of BmTRH is about 1 667 bp for TA cloning.(B) The cloning fragment is inserted into expression plasmid pET-28a.

图2 BmTRH基因结构和Bm TRH蛋白体外表达鉴定。(A) BmTRH基因结构。黑框代表外显子,横线代表内含子。(B)考马斯亮蓝染色12% SDS-PAGE后在约62 kDa大小处出现蛋白条带。(C) BmTRH蛋白质谱分析图。Fig.2 The structure of BmTRH and identification of BmTRH expressed in vitro.(A) The structure of BmTRH.Exons were represented by black boxes and the intron length was represented by lines.(B) 12% SDS-PAGE of proteins stained with Coomassie brilliant blue.The molecular weight of the recombinant protein was about 62 kDa as estimated.(C) Peptide mass fingerprint by MALDI-TOF-MS.
We identified three potential TRH silkworm homologs by performing homology searches of the silkworm database (provide URL) using the nucleotide sequences of tryptophan hydroxylase fromH.sapiensandD.melanogasteras query.Among the three, phenylalanine hydroxylase (BmPAH)[30]and tyrosine hydroxylase (BmTH)[31]have been previously reported.BmTRH, which had the highest homology withDmTRHandTRP, spans 10.31 kb and is located on nscaf1690 in chromosome 1.We cloned the 1 667 bp cDNAs sequence ofBmTRHcontaining the 1 632 bp ORF (KF650639) from the heads of silkworm larvae by using RT-PCR (Fig.1).BmTRHgene contained 10 exons and 9 introns (Fig.2A), and encoded a putative protein containing 543 amino acids(aa) with an expected molecular weight of 62.15 kDa.To investigate ofBmTRHexpression profiles, the complete coding sequence ofBmTRHwas expressed in a prokaryotic expression system.SDS-PAGE analysis showed that the molecular weight of the expressed recombinant protein was about 62 kDa,which was consistent with the predicted weight of BmTRH(Fig.2B, Fig.3).Purity of the protein was≥ 90% and it was identified as BmTRH by peptide mass fingerprinting (Fig.2C).The peptide fingerprint masses were analyzed as GPMAW, which showed 20.1% coverage of matched peptides and 8 matched peptides (average difference = 0.49).These results indicated the successfulin vitroexpression ofBmTRH.
2.2 Sequences analyses
Analysis of theBmTRHnucleotide sequence(http://smart.embl-heidelberg.de/) revealed that the predicted BmTRH protein had all the typical features of the AAAH family: an N-terminal regulatory (ACT)domain (spanning 49–113 aa), a catalytic domain(spanning 147–478 aa) and a C-terminal coiled-coilregion (spanning 465–476 aa) involved in tetramerization (Fig.4).Comparison of the BmTRH amino acid sequence with the known sequences of Homo sapiens TPH (HsTPH) and Drosophila TRH(DmTRH), indicated that the sequences were conserved in the catalytic domain, while the C-terminus containing the regulatory domains diverged among the species (Fig.4).More importantly, all residues that were identified as important for the structural and functional properties of (HsTPH) and DmTRH[27], were also conserved in BmTRH (Fig.4).For example, the phosphorylation sites were located in residues S84, 89 and 300; the characteristic VLMYGS, GYLSP, F281, E313, A349 and Y352 were predicted to bind BH4; the residues H291, 312, 317 and E357 were iron binding sites; the conserved Y275,R297, H312, F353, F358 and S377 were tryptophan interaction sites; and the leucine zipper at the N-termini reported to be involved in protein multimerization (Fig.4).These findings suggested the presence of most typical features of TRH in BmTRH.In addition, the amino acids sequence of BmTRH shared 70% identity with DmTRH, 61% with HsTPH1, 57% with HsTPH2, 52% with BmPAH,DmPAH and HsPAH, 46% with BmTH or HsTH, and 43% with DmTH (Table 2).

图3 BmTRH原核表达及Western blotting检测Fig.3 The prokaryotic expression and Western blotting analysis of BmTRH.(A) The expression of BmTRH in prokaryotic expression system by 0.6 mmol/L isopropylβ-D-1-thiogalactopyranoside on SDS-PAGE gels.Lane 1: marker.Lane 2: precipitate after induction.Lane 3:supernatant after induction.Lane 4: non-induced control.Lane 5: empty vector induction.(B) Specificity of anti-Bm TRH antibody tested on induced proteins.Immunization with anti- BmTRH antibody (1:10 000)used for Western blotting analysis using 3,3′,5,5′-tetramethylbenzidine assay.A single band of ~62 kDa was detected Western blotting of 10 mg induced proteins.
2.3 Phylogenetic analysis
To explore the phylogenetic relationship between members of AAAHs, we constructed a neighbor joining phylogenetic tree with 51 genes from 13 species including 5 vertebrates [Homo sapiens,Musmusculus,Gallus gallus,Anolis carolinensis,Danio rerio]and 8 invertebrates [Tribolium castaneum(Coleoptera),Acyrthosiphon pisum(Hemiptera),D.melanogaster(Diptera),B.mori(Lepidoptera),Apismellifera(Hymenoptera),Lepeophtheirussalmonis(Arthropoda, Crustacea),Helobdella robusta(Annelida, Clitellata) andCaenorhabditis elegans].The AAAH of physcomitrella, a lower plant, was used as an out-group; this protein was reported not only to have PAH function but also TH function and/or TRH function. All AAAHs clustered into three distinctclades, suggesting that the members of AAAH which likely derived from an ancestor differed in their structures and/or function[32].Moreover, the TRH clade was closer to the PAH clade than to the TH clade (Fig.5).The first clade was the TH clade,which included two subclades, the invertebrate TH subclade comprised insect TH as well as other invertebrate TH, which served as the out-group while the vertebrate TH subclade consisted of TH1 and TH2 groups.Second was the PAH clade where vertebrate PAH and insect PAH belonged to different categories, and other invertebrate PAH were the out-group.Third was the TRH clade comprised the invertebrate TRH subclade and vertebrate TPH subclade.The invertebrate TRH subclade contained insect TRH groups where silkworm clustered together withD.melanogasterand other invertebrate TRH.The vertebrate TPH subclade was divided into TPH1 and TPH2 groups, revealing the likely difference in functions between TPH1 and TPH2.Furthermore,TPH2was mainly expressed in the brain stem, whileTPH1was expressed in the gut,pineal gland, spleen, and thymus in human, mouse and rat[20].Interestingly, TPH1 inDaniogenome had two copies corresponding to gene duplication events that occurred specifically in teleost fish[33].
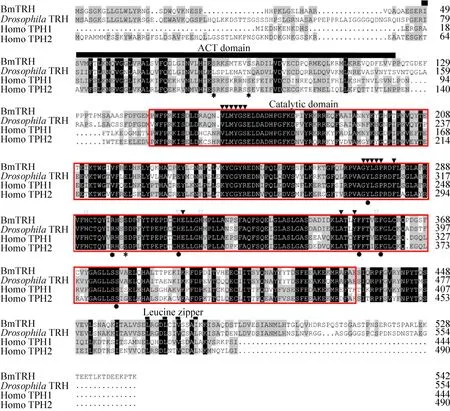
图4 BmTRH与人类TPH1,TPH2和果蝇TRH氨基酸多重序列比对结果Fig.4 Comparison of amino acid sequences alignment of BmTRH with Homo sapiens TPH1, TPH2 and Drosophila TRH.The alignment was generated using ClustalW alignment software.Identical residues were shown as white letters against black,whereas conservatively substituted residues were shaded.Residues implicated in iron binding were marked by a (▽), residues implicated in BH4 binding by a (▼), and residues associated with Leucine zipper by a thinner line.The ACT domains were marked by a bold line, while catalytic domain was marked by a red box.Tryptophan blinding sites were indicated above (●) and phosphorylation sites above (*).The accession numbers of TRH in relevant species in GenBank are listed in Table 3.

表2 家蚕TRH与果蝇 (PAH、TH和TRH)、人类 (PAH、TH和TPH)之间的氨基酸同源比较分析Table 2 Amino acid homology scores among Bombyx TRH with Drosophila (PAH, TH and TRH) and Homo (PAH,TH and TPH)

表3 本研究相关物种的PAH、TH、TRH蛋白GeneBank登录号Table 3 The accession number of PAH, TH, TRH in relevant species in this study
2.4 mRNA expression profile
The TRH activity product, 5-hydroxy-L-tryptophan(5-HTP) is derived from 5-hydroxytryptamine in a reaction that requires aromatic L-amino acid decarboxylase (DDC) that is also involved in the biosynthesis of dopamine[26].We previously reported that the recombinant BmPAH protein had some TRH activity[30], suggesting thatBmDDC,BmPAHandBmTRHcould be related to serotonin synthesis.Thus,the tissue-specific expression patterns ofBmTRH,BmDDCandBmPAHmRNA in eleven larval tissues(including head, fat body, silk glands, tracheae, central nervous, hemolymph, testis, ovary, integument,malpighian tubule and midgut) were investigated by RT-PCR.We found that the 536 bpBmTRHfragment was expressed only in the head and CNS with the PCR product being more intense in CNS than the head.On the other hand, a relatively bright band corresponding to 664 bpBmDDCproduct was detected in the head, fat body, tracheae, CNS, testis,ovary, integument and midgut, while the 567 bp PCRBmPAHfragment was observed in head, fat body,CNS, hemolymph, testis, ovary, integument and midgut.Weak PCR amplification was observed forBmDDCandBmPAHfragments in the hemolymph and malpighian tubule, respectively (Fig.6 and Fig.7).These results showed thatBmTRHmRNA expression was highly restricted in space, and the transcripts of bothBmDDCandBmPAHwere expressed widely in most tissues, indicating their functional importance in various physiological contexts.
2.5 BmTRH protein expression profile
BmTRH was detected in larval tissues using anti-BmTRH antibody.Western blotting showed a specific band of ~62 kDa in the head and ventral chain of larvae, and high expression was observed in the ventral chain than the head.BmTRH was not expressed in fat body, silk glands, tracheae, hemolymph, testis,ovary, integument, malpighian tubule and midgut(Fig.8).These results consistent with the mRNA expression profile ofBmTRH.
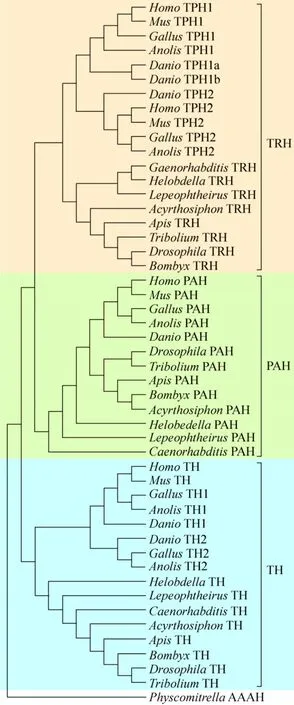
图5 基于氨基酸序列构建的无脊椎动物与脊椎动物TRH、TH和PAH系统进化树Fig.5 Phylogenetic tree of invertebrates and vertebrates TRH (a narrative convenience, is called vertebrate TPH and invertebrate TRH collectivity in here), TH and PAH.There were three distinct clades, TRH clade, TH clade and PAH clade, for all aromatic amino acid hydroxylases in animal.TRH, PAH and TH was indicated in pink, yellow-green and lake blue respectively.The accession numbers of PAH, TH,TRH in relevant species in GenBank are listed in Table 3.

图6 BmTRH、BmPAH和BmDDC基因在家蚕幼虫各组织中的表达模式Fig.6 Expression profiles of BmTRH, BmPAH and BmDDC in larval tissues of silkworm.Lane 1; head; lane 2;central nervous; lane 3; integument; lane 4; fat body; lane 5;hemolymph; lane 6; silk glands; lane 7; midgut; lane 8; testis;lane 9; ovary; lane 10; trachea; lane 11; malpighian tubule;and control; actin control.All of the polymerase chain reaction products were approximately 600 bp.
2.6 Localization analysis of BmTRH Immunohistochemistry
To further analyze the expression of BmTRH,immunohistochemistry was performed on larval tissues embedded in paraffin sections.Clear positive signal was detected in CNS (ganglion at any region and several nerve cords containing brain), whereas weak signal was found in the integument, testis and ovary (Fig.9 and Fig.10).These results suggested that BmTRH expression was restricted to larval nervous tissues.
3 Discussion
In this study, we identified only a single copyof theTRHgene in the silkworm genome, which is consistent with reports in other invertebrates.This is the first time of the cloning of its heterologous expression in a Lepidoptera insect.TheBmTRHgene codes a 61.31 kDa protein consisting of 543 amino acids (aa), similar toDmTRH, which encodes a 61 kDa protein with 555 aa.BmTRH also has conserved residues sharing the structural and functional properties of HsTPH or DmTRH.These findings suggest that theBmTRHcloned here is a functionalTRH.

图7 家蚕幼虫各组织中BmTRH,BmPAH and BmDDC基因表达分析 (泳道1:头;泳道2:中枢神经;泳道3:表皮;泳道4:脂肪体;泳道5:血淋巴;泳道6:丝腺;泳道7:中肠;泳道8:精巢;泳道9:卵巢;泳道10:气管;泳道11:马氏管。BmTRH,BmPAH and BmDDC基因PCR扩增产物大小分别是:536 bp,664 bp和567 bp)Fig.7 Expression profiles of BmTRH, BmPAH and BmDDC in larval tissues of silkworm.Lane 1: head; lane 2: central nervous; lane 3: integument; lane 4: fat body; lane 5: hemolymph; lane 6: silk glands; lane 7: midgut; lane 8: testis; lane 9: ovary; lane 10: trachea; lane 11: malpighian tubule.The polymerase chain reaction products of BmTRH, BmPAH and BmDDC were 536 bp, 664 bp and 567 bp, respectively.

图8 Bm TRH在家蚕幼虫各组织的Western blotting检测Fig.8 Western blotting analysis with the anti-BmTRH antibody in larval tissues of silkworm.Lane 1: tracheal;lane 2: ovary; lane 3: testis; lane 4: silk gland; lane 5:hemolymph; lane 6: fat body; lane 7: integument; lane 8:ventral chain; and lane 9: head.The specific lane was showed in lane 8 and 9.

图9 Bm TRH在家蚕幼虫中枢神经,表皮,精巢和卵巢中的免疫组织化学分析Fig.9 Immunohistochemical analysis of BmTRH in central nervous, integument, testis and ovary of larvae.The arrows indicate positive signals in (A) (ventral ganglion)and (B) (brain).No positive signal was found in (C) (testis),(D) (ovary) or (E) (integument).Incubating with PBS displaced primary antibody was as negative controls.
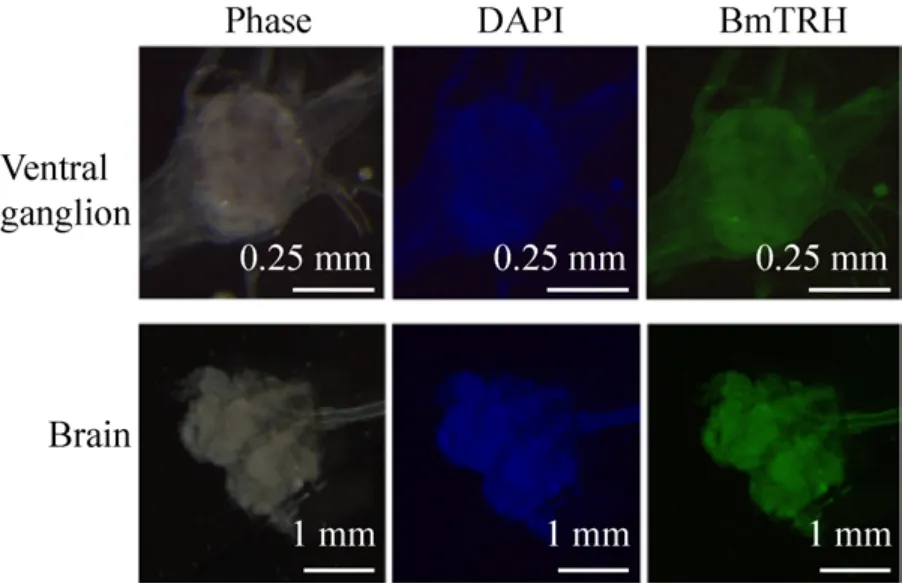
图10 Bm TRH蛋白在家蚕脑和腹部神经节的免疫荧光鉴定 (Bm TRH抗体在脑和腹部神经节中均有表达)Fig.10 Immunofluorescence analysis using BmTRH antibodie in ventral ganglion and brain of B.mori.BmTRH could be detected in ventral ganalion and brain.
InD.melanogaster, DmTRH exhibits high activity with a notable ability to hydroxylate phenylalanine, while DmPAH has strong phenylalanine hydroxylase activity and displays a significant ability to hydroxylate tryptophan.Both DmTRH and DmPAH have been reported, not to have tyrosine hydroxylase activity[24-26].Our phylogenetic analysis indicates that TRH is closer to PAH than TH,and BmTRH together with DmTRH are clustered to a branch with TRH from other insect.Besides, our previous study also showed that BmPAH expressedin vitrowas capable of tryptophan and phenylalanine hydroxylation rather than only tyrosine hydroxylation[30].These results imply that BmTRH,like DmTRH, can hydroxylate both tryptophan and phenylalanine but not tyrosine in silkworm.
TheBmTRHtranscript and BmTRH protein are detected only in the head and CNS with high expression in the CNS. Consistently,immunohistochemistry also shows a strong positive signal only in CNS tissues containing the ventral ganglion and brain.These suggest thatBmTRHcould likely trigger neural activities in silkworm larvae, and is consistent with previous reports that TRH participates in the neural activity in other animals[34-35].
InD.melanogaster, DmPAH and DmTRH are selectively involved in 5-HT synthesis with distinct expression patterns and enzyme activities[36].However, expression analysis in this study shows thatBmTRHtogether withBmPAHandBmDDCcould likely be co-expressed in the head and CNS tissues.These suggest the likelihood of two distinct regulation mechanisms for 5-HT synthesis in CNS, and that BmTRH- and BmPAH-mediated 5-HT biosynthesis pathways may not segregate into neuronal and peripheral tissues in silkworm.A similar phenomenon has been reported inGryllus bimaculatus[37].
4 Conclusion
In this study, we identify and clone a cDNA sequence forTRHgene in silkworm, and analyze the phylogenetic relationships to metazoan as a members of aromatic amino acid hydroxylases (AAAHs).By gene and protein expression analysis, we speculate BmTRH could likely function in the regulation of neural activities.This is the first time that theBmTRHgene is cloned from silkworm and BmTRH protein is expressed and purifiedin vitroin Lepidoptera.
Acknowledgement
Thanks to Feifei Huang, Zhanpeng Dong and Zhouhe Du of Southwest University for providing technical support.

