牛LncRNA-133a对骨骼肌卫星细胞增殖分化的影响
李燕,陈明明,张俊星,张林林,李新,郭宏,丁向彬,刘新峰
牛LncRNA-133a对骨骼肌卫星细胞增殖分化的影响
李燕,陈明明,张俊星,张林林,李新,郭宏,丁向彬,刘新峰
(天津农学院动物科学与动物医学学院,天津 300384)
【目的】探讨长链非编码RNA LncRNA-133a对牛骨骼肌卫星细胞增殖分化过程的影响。【方法】利用测序样品3、6、9月龄胎牛及24月龄成年和牛骨骼肌肌肉组织,qRT-PCR法检测LncRNA-133a的组织时序表达谱。构建牛骨骼肌卫星细胞的体外成肌诱导分化模型,模拟牛骨骼肌的生长发育过程,qRT-PCR法检测LncRNA-133a和肌细胞分化标记因子MyoG、MHC的细胞时序表达谱。利用过表达LncRNA-133载体(pCDNA3.1-EGFP-LncRNA-133a) 或LncRNA-133a抑制物(si-LncRNA 133a) 转染牛骨骼肌卫星细胞,qRT-PCR法检测转染效率以及各转染处理组LncRNA-133a、MyoD、MyoG及MHC基因mRNA的表达水平,Western blotting检测MHC 基因的蛋白表达水平;同时,通过EdU细胞增殖检测、免疫荧光蛋白染色技术检测牛骨骼肌卫星细胞增殖阶段的细胞增殖量和分化阶段的肌管融合程度。【结果】组织表达谱分析发现LncRNA-133a在3月龄胎牛肌肉组织中表达量最高,6月龄胎牛肌肉组织中次之,9月龄胎牛及成年牛肌肉组织中表达量最低,时序表达呈下降趋势;利用成功构建的牛骨骼肌卫星细胞体外诱导分化模型,进行LncRNA-133a、MyoG、MHC的细胞时序表达谱分析,结果发现在牛骨骼肌卫星细胞分化过程中(D0-D3),肌分化标记因子MyoG、MHC的表达水平逐渐升高,LncRNA-133a的表达在分化阶段呈上升趋势,且分化48 h时(D2)表达量最高;成功构建的过表达LncRNA-133a或抑制LncRNA-133a的牛骨骼肌卫星细胞模型,在增殖期(D0):与对照组相比,过表达LncRNA-133a处理组EdU增殖染色检测得到EdU阳性细胞数显著增加(<0.01),而LncRNA-133a抑制处理组EdU阳性细胞数显著减少(<0.01);在分化48 h时(D2):与对照组相比,LncRNA-133a过表达处理组肌细胞分化标记因子MyoD、MyoG及MHC的mRNA表达水平显著升高(<0.05),Western blotting检测MHC蛋白表达量显著增加(<0.01),且MHC蛋白的免疫荧光蛋白染色检测观察到融合肌管的体积占比更大;而LncRNA-133a抑制处理组MyoD、MyoG及MHC的mRNA表达水平均降低,其中MyoG显著降低(<0.05), MHC蛋白表达量显著减少(<0.01),同时MHC蛋白融合肌管的体积占比也降低。【结论】研究证实LncRNA-133a具有促进牛骨骼肌卫星细胞增殖及分化的作用,为进一步挖掘LncRNA-133a调节牛骨骼肌卫星细胞增殖分化调控网络机制奠定了基础。
LncRNA-133a;牛;骨骼肌卫星细胞;增殖;分化
0 引言
【研究意义】骨骼肌作为动物躯体最重要的组织之一,占重比可高达40%[1],因此骨骼肌的发育对经济动物肉牛来说意义重大。肌细胞作为骨骼肌的基本形成单位,它的分化发育直接影响到骨骼肌的发育形成[2]。随着人们对LncRNAs研究的不断深入,研究者已经发现许多LncRNAs可以参与肌细胞的增殖和分化过程[3-5]。【前人研究进展】linc-MD1及lnc-mg作为内源性竞争性RNA(ceRNA)促进肌细胞的分化发育[6-7];lncRNA MAR1发挥其“sponge”吸附作用促进肌分化和再生[8];Linc-YY1则通过与靶基因的互作作用促进肌分化和再生[9];此外,还有Lnc-SEMT,H19等,也都在肌形成过程中发挥其重要的生物学功能[10-12]]。【本研究切入点】有关LncRNAs参与肌肉发育的研究已有很多,但多集中于模式动物小鼠的研究上。近年来,随着生物检测技术及生物信息学分析技术的发展,通过对牛骨骼肌肌肉组织进行高通量测序分析,已经获得了大量与肌肉发育潜在相关的LncRNAs[13-15]。研究者针对这些 LncRNAs开展了一系列的功能研究,并证实了其中一些LncRNA如lncMD[14]、LncRNA-AK143003[15]、LncRNA HZ-5[16]、LncRNA H19[17]等,确实参与调节牛骨骼肌细胞的分化发育。但相较小鼠C2C12成肌细胞生长发育相关LncRNA的报道,参与调节牛骨骼肌细胞生长发育的LncRNAs的相关报道仍然较少,测序获得的与牛肌肉发育相关的海量LncRNAs,还需要研究者不断挖掘和证实它们的功能。【拟解决的关键问题】本研究利用前期在牛肌肉组织中鉴定到的长链非编码RNA LncRNA-133a,通过表达谱分析,并进一步以体外分化牛骨骼肌卫星细胞为模型,通过过表达/抑制LncRNA-133a处理,探究其对牛骨骼肌卫星细胞增殖和分化过程的影响,旨在为牛骨骼肌生长发育相关LncRNAs的功能研究积累更多的有益资料。
1 材料与方法
试验于2017—2018年在天津市农业动物繁育及健康养殖重点实验室完成。
1.1 材料
1.1.1 肌肉组织与细胞来源 肌肉组织为天津市农业动物繁育及健康养殖重点实验室冻存的3、6、9月龄胎牛及24月龄成年和牛骨骼肌肌肉;细胞为该实验室分离冻存的原代牛骨骼肌卫星细胞。
1.1.2 主要仪器与试剂 CO2恒温培养箱(日本三洋);LightCycle 96实时荧光定量PCR仪(Roche);Nano-Drop ND 2000c Spectrophotometer(Thermo- scientific);荧光显微镜(Leica)。
pCDNA3.1-EGFP(武汉淼灵生物科技有限公司);siRNA oligos(上海吉玛基因);Lipofectamine® 3000(Invitrogen);Trizol reagent(Invitrogen);PrimeScript II 1st Strand cDNA Synthesis Kit(Takara);All-in- OneTMqPCR Mix(GeneCopoeia);Hoechst 33342染色液(碧云天);一抗,Anti-Myosin VⅡα antibody ab3481(abcom);二抗,CY3-羊抗兔IgG(BA1032,博士德生物);Cell-Light EdU Apollo 567 In Vitro Imaging Kit(RN:R11053.2,广州市锐博生物科技有限公司);qRT-PCR检测相关引物(苏州金唯智生物科技有限公司)。
1.2 方法
1.2.1 牛肌肉组织中LncRNA-133a时序表达谱鉴定 qRT-PCR检测:3、6、9月龄胎牛及成年牛肌肉组织全基因组检测LncRNA-133a的表达情况,其中3月龄胎牛肌肉组织作为对照。
1.2.2 细胞培养 常规复苏实验室前期冻存的原代牛骨骼肌卫星细胞[18],利用原代牛骨骼肌卫星细胞体外培养模拟牛肌肉的发育进程[19],使用增殖培养基:含体积分数20%的胎牛血清、100 IU·mL-1青霉素和链霉素的DMEM,置于37℃、5%CO2、饱和湿度培养箱中培养。待增殖细胞融合度达80%—90%时将培养基更换为含体积分数2%马血清的DMEM的分化培养基。
1.2.3 牛骨骼肌卫星细胞转染及收集 24孔培养板内增殖期细胞融合度达50%时,按生产商家说明书利用Lipfectamine3000转染已构建的过表达载体pCDNA3.1-EGFP-LncRNA-133a(简写为pcDE-LNC)及抑制物 si-lncRNA133a,以空质粒pCDNA3.1-EGFP(简写为pcDE-NC)及si-NC转染细胞作为对照,转染后24 h时培养基更换为分化培养基。
细胞总RNA及总蛋白收集。增殖期细胞(D0):更换分化培养基前收集;分化期细胞(D1—D3):于更换培养基后24 h(D1)、48 h(D2)、72 h(D3)时收集。按生产商家说明书利用Trizol及蛋白裂解液裂解并提取细胞总RNA及蛋白。
1.2.4 LncRNA-133a、分化标记基因表达水平的检测 qRT-PCR检测:收集24孔板中增殖期(D0)、分化期(D1—D3)未处理细胞、各转染处理细胞以及相应对照组细胞。取4 µg总RNA利用 All-in- OneTM First-Strand cDNA Synthesis Kit 试剂盒反转录为第一链cDNA,再采用qRT-PCR法检测各基因的表达。
qRT-PCR反应体系:10 µmol·L-1上游引物、10 µmol·L-1下游引物、2.0 µL 5x稀释 cDNA、10 µL 2×All-in-OneTMqPCR Mix,Nuclease-free water 将体系补至20 µL。反应条件:95℃ 10 min;95℃ 10 s、60℃ 20 s、72℃ 15 s,重复35个循环。qRT-PCR反应引物信息如表1所示。
1.2.5牛骨骼肌卫星细胞增殖、分化能力的检测 qRT-PCR检测:24孔板中细胞在转染后DM2期时,检测肌细胞分化标记因子MYOD(muscle regulatory factors, MRFs成员)、MYOG(myogenin)及MHC(Myosin heavy chain)的转录组表达情况。
EdU细胞增殖检测:96孔板中细胞在转染后D0期时,按Cell-Light EdU Apollo 567 In Vitro Imaging Kit试剂盒说明书检测各处理细胞的增殖期细胞数。
MHC细胞免疫荧光检测:48孔板中各处理细胞在DM2期时,进行肌卫星细胞分化标记因子MHC的免疫荧光蛋白染色检测。
1.2.6 牛骨骼肌卫星细胞分化标记基因蛋白表达水平的检测 Western blotting检测:6孔板中细胞在DM2期时被蛋白裂解液裂解,收集蛋白,通过Western blotting检测分化标志基因MHC的蛋白表达。

表1 qRT-PCR引物信息
2 结果
2.1 LncRNA-133a的组织表达谱
LncRNA-133a是天津农学院动物分子育种与转基因创新中心实验室前期从3、6、9月龄胎牛肌肉组织的高通量测序数据中筛选鉴定出的组织时序表达呈下降趋势的一条LncRNA。本研究利用qRT-PCR法,验证不同月龄肌肉中LncRNA-133a的时序表达情况。如图1:除在6月龄肋间肌及背腰肌中有相对上调外,LncRNA-133a在3、6、9月龄胎牛及成年牛各肌肉组织中均呈下降的表达趋势。表明测序结果可靠,该LncRNA可进行下一步的功能研究。
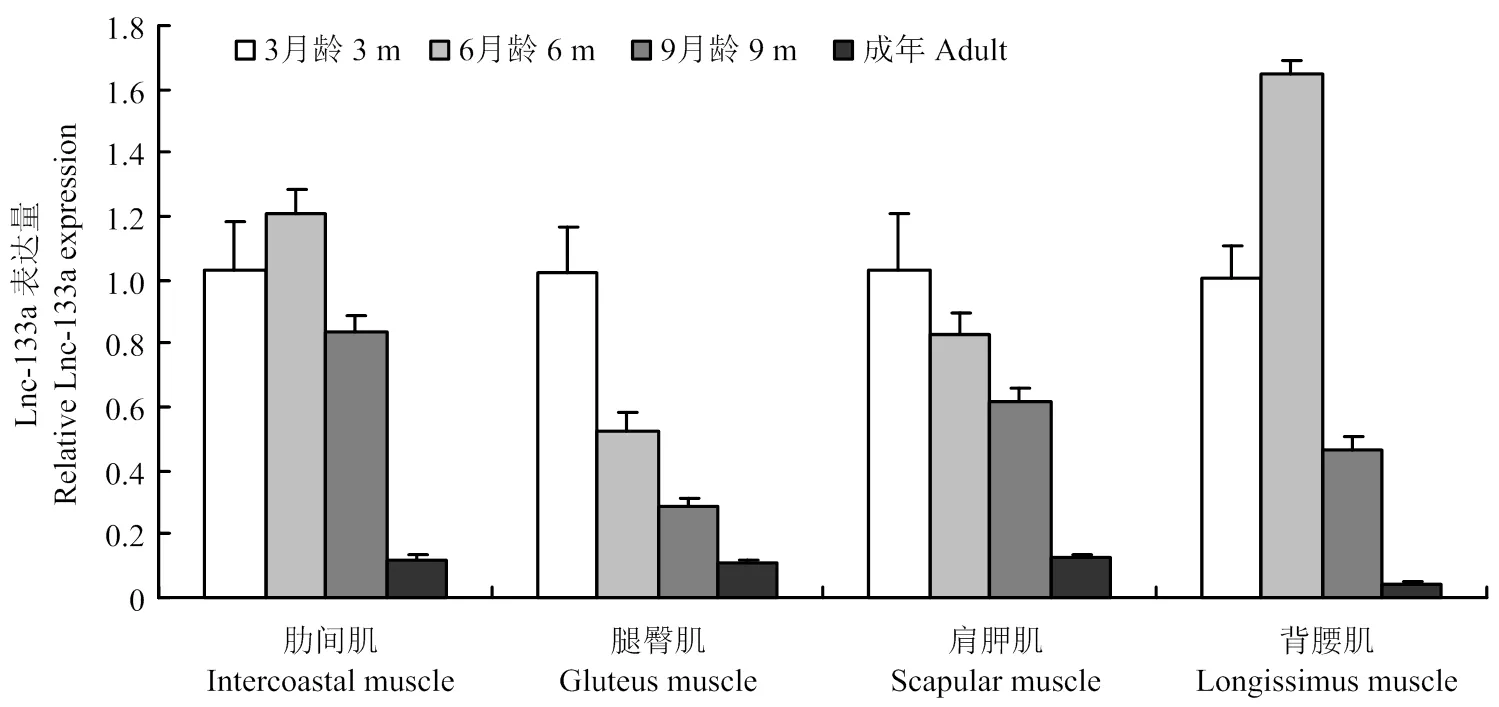
图1 3、6、9月龄胎牛及成年牛不同肌肉组织中LncRNA-133a的表达
2.2 牛骨骼肌卫星细胞LncRNA-133a及肌分化标记因子的表达水平
光镜下观察D0-D3时期牛骨骼肌卫星细胞的分化状态并于对应时期收集细胞总RNA,qRT-PCR法检测LncRNA-133a及肌分化标记因子的正常表达水平。如图2、图3:随着骨骼肌卫星细胞进入诱导分化阶段,牛骨骼肌卫星细胞分化形成的肌管清晰可见(图2),且肌分化标记因子MHC及MyoG均呈上升表达趋势,表明牛骨骼肌卫星细胞的体外诱导分化模型构建成功;LncRNA-133a在牛骨骼肌卫星细胞分化期间高表达,且分化48 h表达量最高,这提示其可能参与牛骨骼肌卫星细胞分化发育的调节过程。
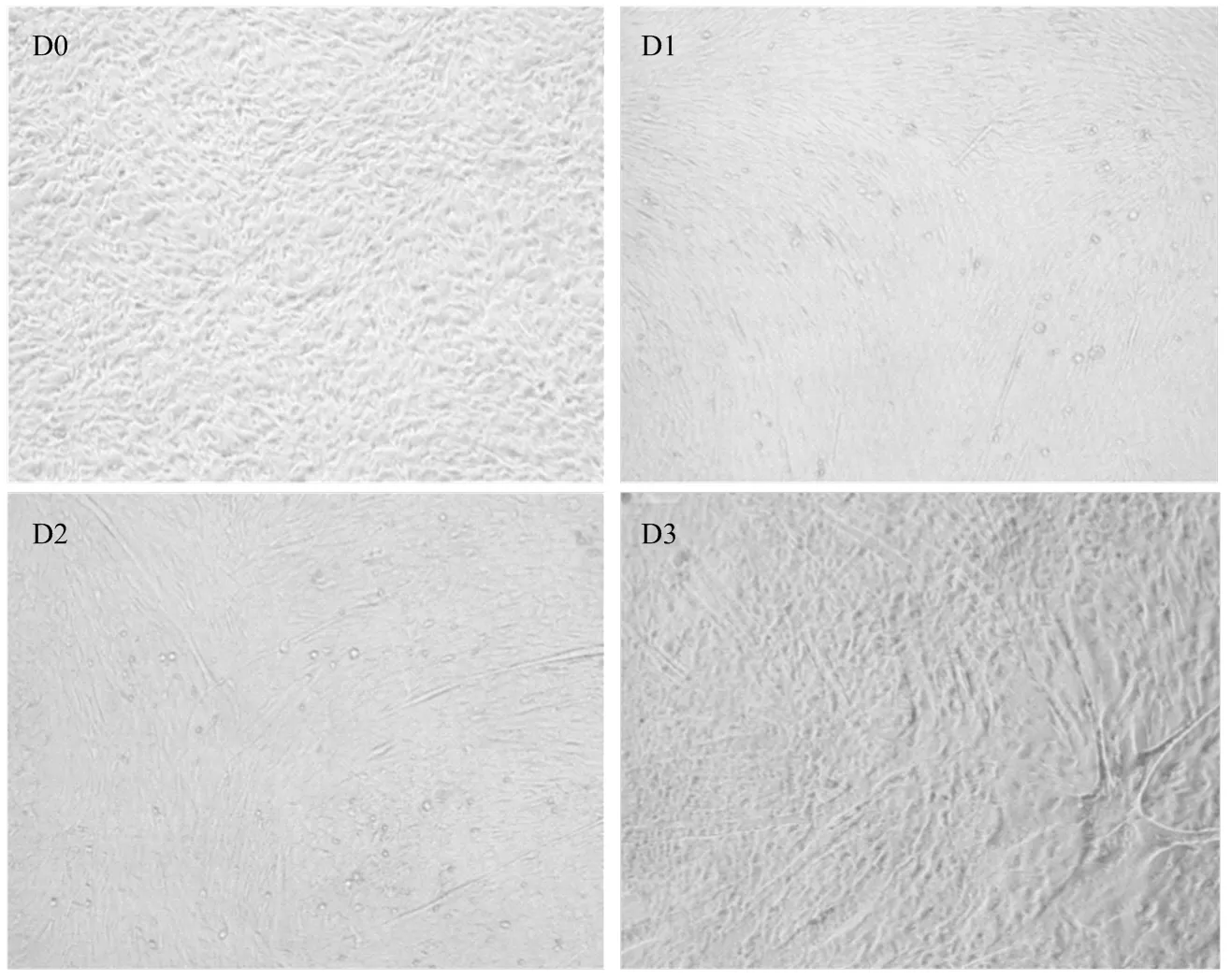
图2 牛骨骼肌卫星细胞分化进程(100×)
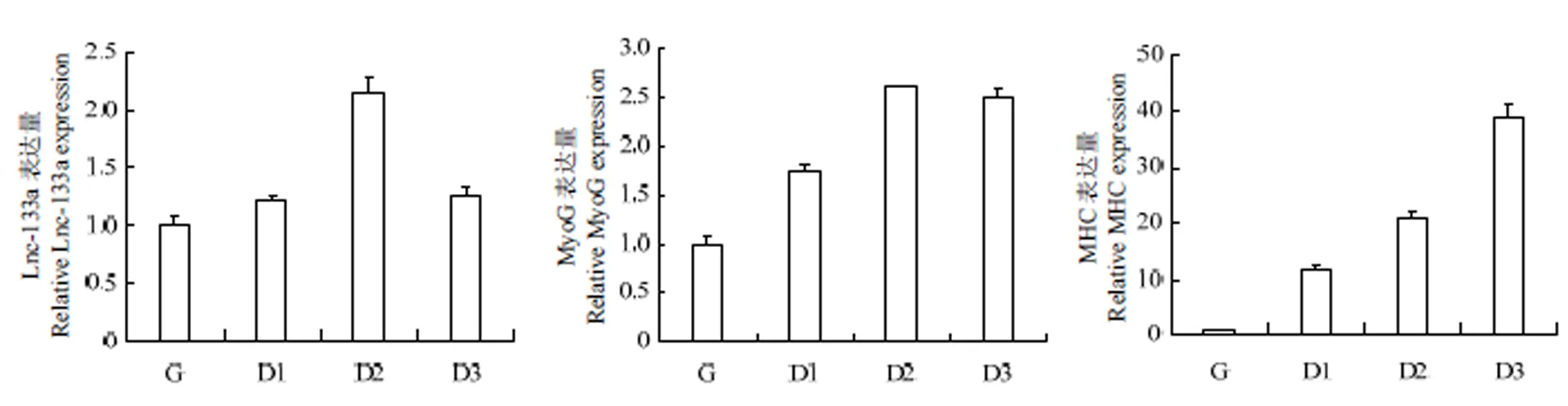
图3 牛骨骼肌卫星细胞分化阶段 LncRNA-133a及分化标记因子的表达
2.3 LncRNA-133a的过表达/抑制效率
牛骨骼肌卫星细胞转染过表达LncRNA-133a载体pcDE-LncRNA-133A或LncRNA-133a的抑制物si-lncRNA133a 及相应对照载体pcDE-NC或对照抑制物si-NC。利用qRT- PCR法检测转染处理后D0及D2期过表达/抑制效率。相比pcDE-NC组,pcDE- LNC组LncRNA-133a在D0和D2期均有显著过表达效果(图4-A);si-lncRNA133a 组相比si-NC组,si-lncRNA133a组LncRNA-133a在D0和D2期均有显著抑制效果(图4-B)。结果表明构建过表达/抑制LncRNA-133a的牛骨骼肌卫星细胞模型成功,可进行下一步试验验证。
2.4 LncRNA-133a对牛骨骼肌卫星细胞增殖能力的影响
过表达/抑制LncRNA-133a 后D0期对牛骨骼肌卫星细胞进行EdU染色检测牛骨骼肌卫星细胞增殖数。结果显示:过表达LncRNA-133a组EdU阳性细胞数显著增多;而抑制LncRNA-133a后,EdU阳性细胞数则显著减少。这表明LncRNA-133a可以促进牛骨骼肌卫星细胞的增殖,对牛骨骼肌卫星细胞的增殖有着正向调控作用(图5)。
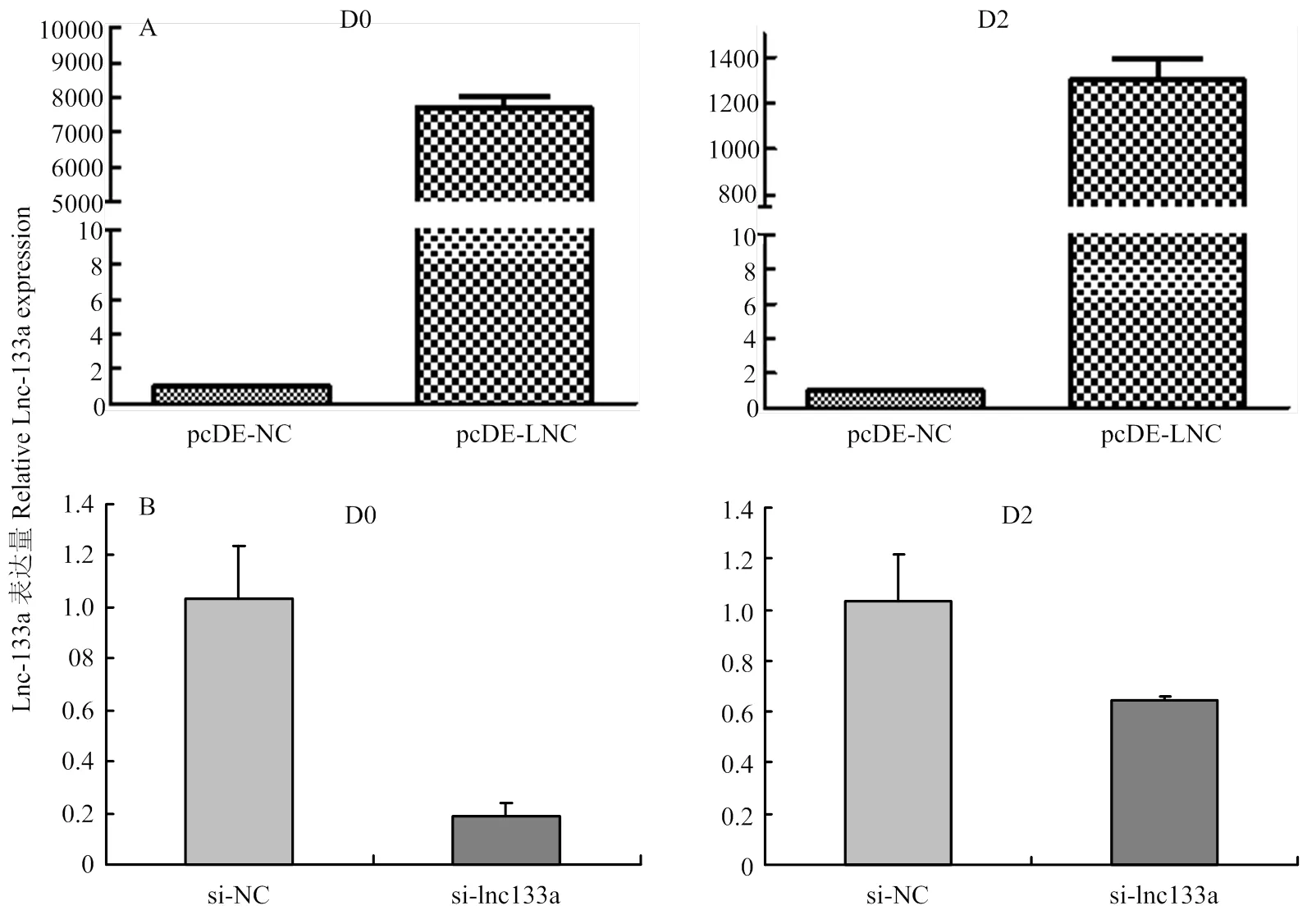
图4 qRT-PCR检测LncRNA-133a的过表达(A)和抑制(B)效率
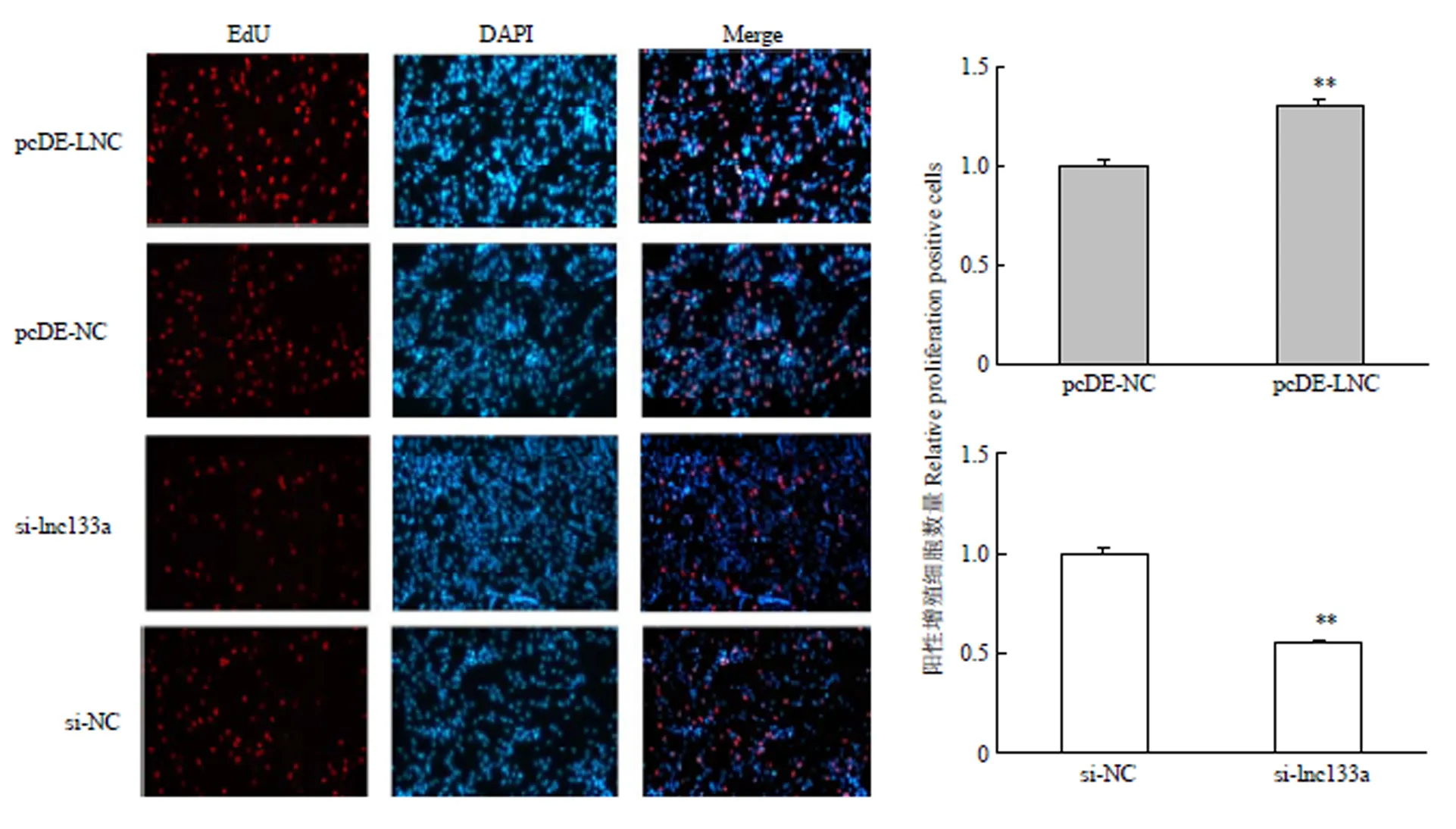
图5 EdU检测过表达/抑制LncRNA-133a后牛骨骼肌卫星细胞的增殖细胞
2.5 LncRNA-133a对牛骨骼肌卫星细胞分化能力的影响
qRT-PCR及Western blotting检测过表达/抑制LncRNA-133a后D2期牛骨骼肌卫星细胞分化标记因子MHC、MyoD及MyoG 的mRNA水平及蛋白水平表达。过表达LncRNA-133a后,MHC、MyoD及MyoG的mRNA水平表达均呈显著上调趋势(图6-A);同时MHC在蛋白水平均也显著上调(图6-B)。而抑制LncRNA-133a后,MHC、MyoD及MyoG的mRNA水平表达均呈下调趋势(图7-A);MHC在mRNA水平虽然下调不显著,但蛋白水平下调显著(图7-B)。此外,通过免疫荧光染色试验检测MHC融合细胞也得到一致结果,如图8:过表达LncRNA-133a后,MHC融合肌管数增多(图8-A);而抑制LncRNA-133a表达后,MHC融合肌管数减少(图8-B)。
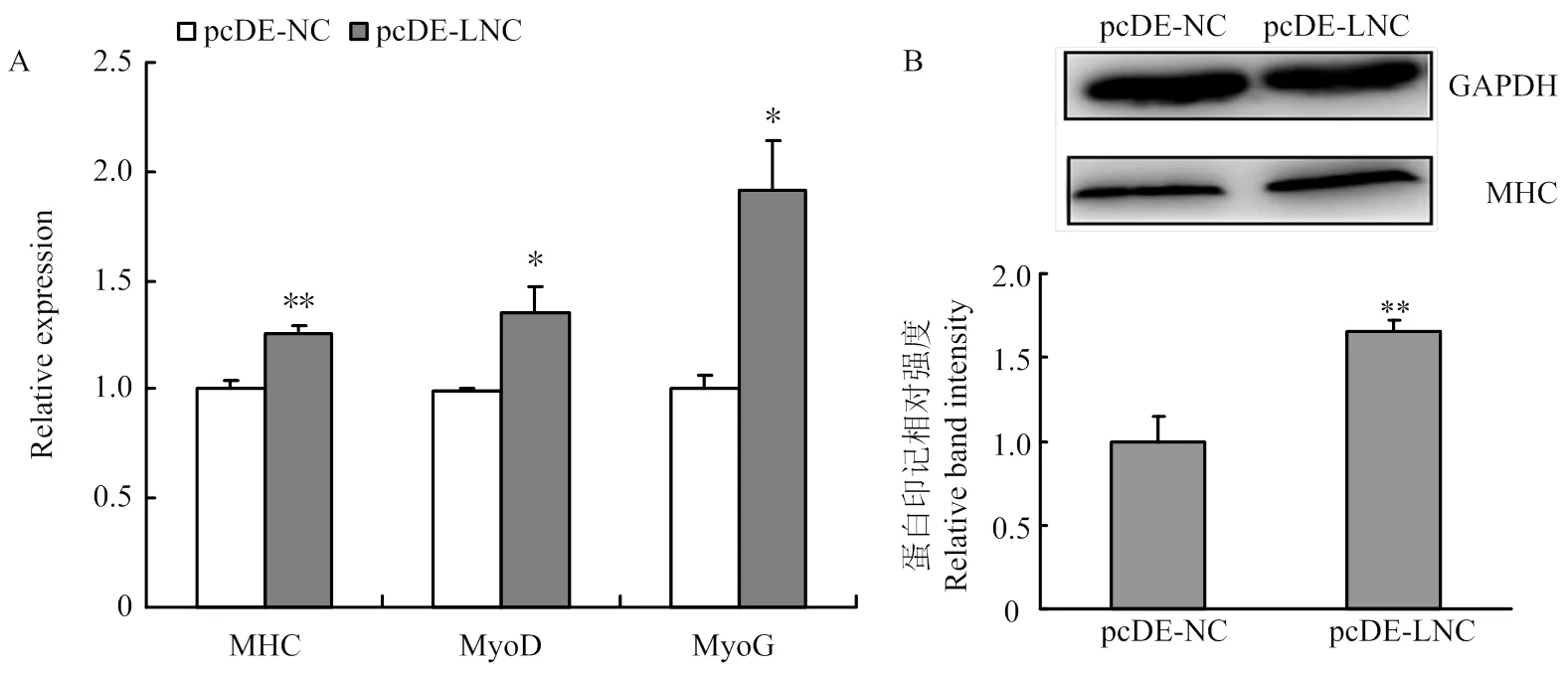
图6 过表达LncRNA-133a促进牛骨骼肌卫星细胞分化
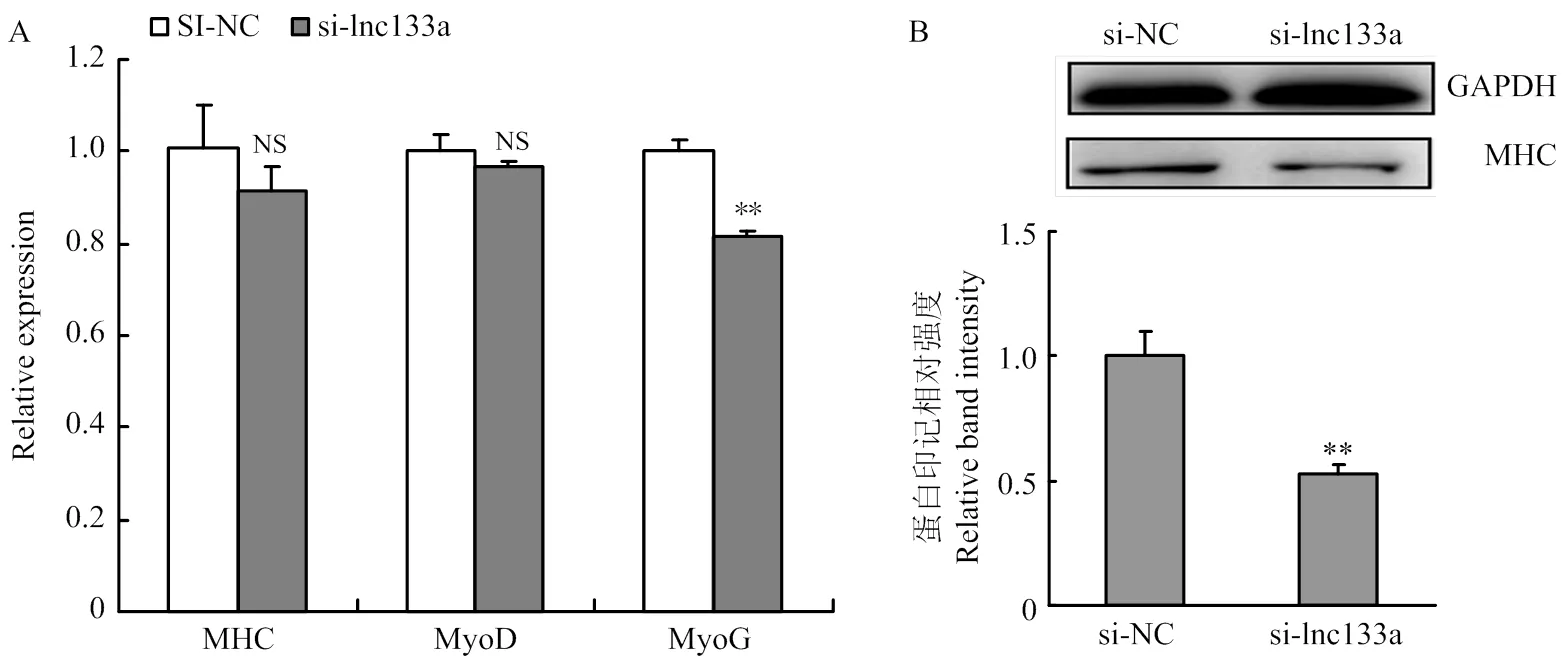
图7 抑制LncRNA-133a表达阻滞牛骨骼肌卫星细胞分化
3 讨论
肌肉发育分化是发育生物学研究的重要主题。肌细胞作为骨骼肌的基本形成单位,它的分化发育直接影响到骨骼肌的发育形成。肌细胞的分化和生长发育过程是内在遗传、表观遗传和外在各种信号互作的结果,受到机体肌肉发育调节因子和调控通路的多层次精细调节。lncRNA作为一类调控因子参与肌肉的发育已是不争的事实,相关报道也不断涌现。
有研究表明通过EdU、CCK8或流式细胞仪检测技术,甚至是细胞划痕试验得到如Sirt1[20]、lncRNA AK017368[21]、长非编码RNA-GTL2[22]等LncRNAs具有促进肌细胞增殖的作用。本研究中,在过表达/抑制LncRNA-133a处理后24h(D0),通过EdU细胞增殖检测初步验证LncRNA-133a具有促进牛骨骼肌卫星细胞增殖的作用。
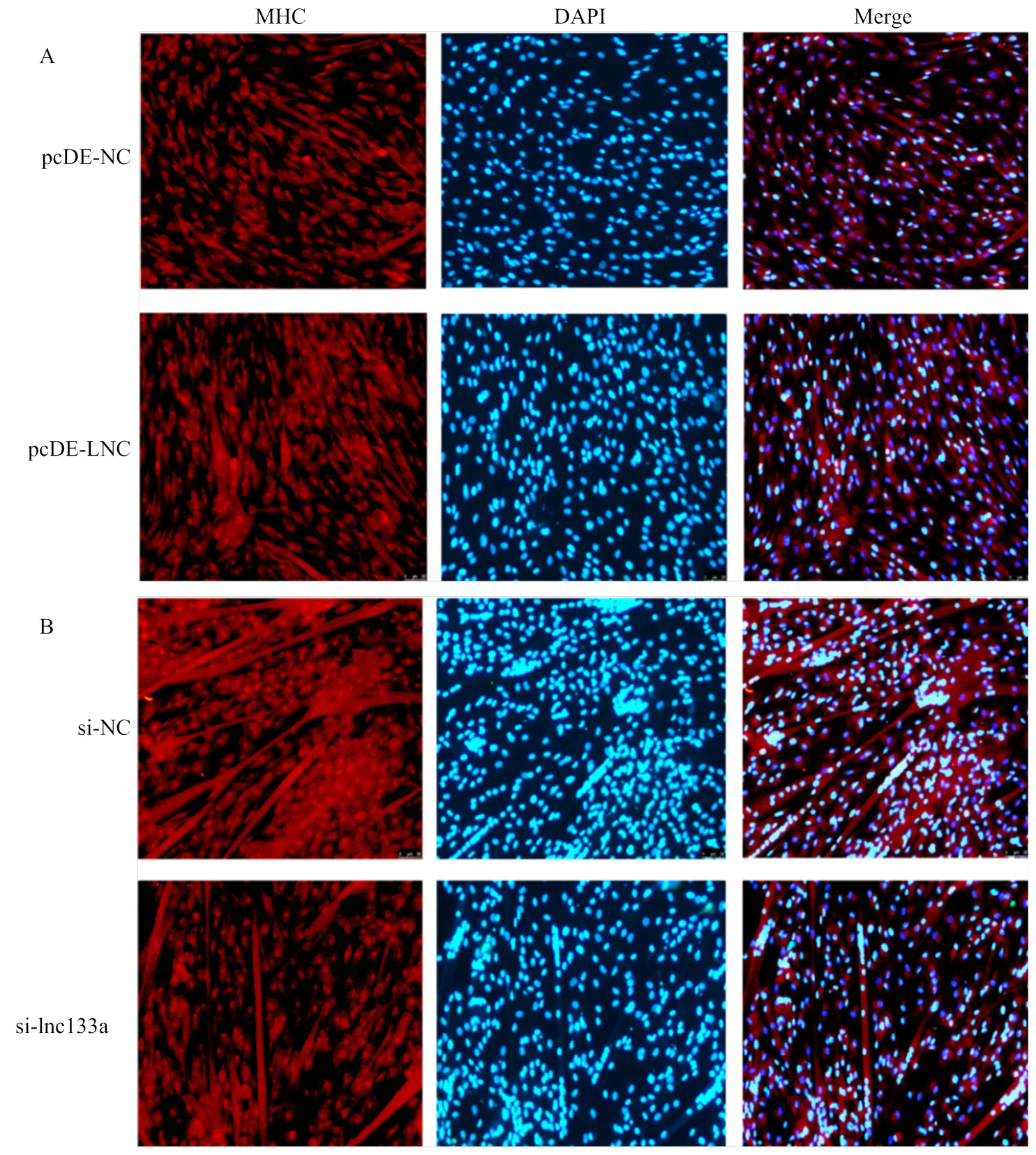
图8 免疫荧光检测过表达(A)/抑制(B)LncRNA-133a牛骨骼肌卫星细胞的分化标记因子MHC
在肌细胞分化过程中,骨骼肌特异性标记基因如MyoD 、MyoG 及MHC 开始表达[23]。其中,MyoD诱导细胞周期的退出同时开启细胞分化[24-25],骨骼肌分化决定因子MyoG受MyoD的启动开始表达,并调控成肌细胞融合和肌纤维形成[26- 27],而作为骨骼肌纤维内粗肌丝主要成分的MHC[28],在肌细胞分化后期表达量逐渐升高。在各肌分化相关的研究中,通常把MyoD、MyoG、MHC作为肌分化标记因子[29-30],利用qRT-PCR、Western blotting及免疫荧光蛋白染色分析等方法,检测它们mRNA水平、蛋白水平及基因蛋白融合表达的变化来验证肌细胞的分化进程。
通过这样的验证手法,现已证实如Linc-YY1、LncRNA Dum、LncMyoD、linc-MD1[9, 31-33]等LncRNAs参与调节肌细胞分化,且这类LncRNAs在成肌细胞分化阶段呈时序性上升表达,所以后续的功能研究基本都集中在这些LncRNAs的高表达时期。此外,Albrecht等[34]研究发现,在3月龄牛胎儿的初级纤维中便可检测到作为肌纤维成熟标记物的肌球蛋白,说明肌纤维的发育主要在妊娠早期。本研究中,通过对LncRNA-133a的时序表达谱分析发现:组织上,LncRNA-133a主要在3月龄胎牛肌肉组织中高表达;细胞上,LncRNA-133a在牛骨骼肌卫星细胞分化早期(D2)表达量最高。据此我们推测LncRNA-133可能参与调节牛骨骼肌卫星细胞的早期分化发育。在牛骨骼肌卫星细胞分化早期(D2)进行LncRNA-133a的功能验证发现,有效过表达LncRNA-133a后,肌分化标记因子如MHC、MyoG、MyoD的mRNA水平均显著上升,同时MHC在蛋白水平也显著上调。同样,在有效抑制LncRNA-133a后,对MyoG mRNA表达水平和MHC蛋白表达水平的下调影响是显著的,对MHC、MyoD mRNA表达水平有不显著的下调影响,这有可能是该检测时期这些分化标记因子自身表达特性的影响。此外,MHC融合细胞的免疫荧光试验分析也得到了一致结果。这些结果均表明LncRNA-133a可以促进牛骨骼肌卫星细胞分化。
现阶段研究对LncRNAs在肌细胞增殖分化过程中扮演的经典调节角色已很明确:在肌细胞的增殖分化过程中,肌生长发育相关LncRNAs对成肌细胞的增殖与分化两个生物学过程的促进或抑制调节作用是相反的,或只参与两者其中一个,但这并不排除LncRNAs 对肌细胞增殖及分化有一致的促进或抑制作用,进而促进或抑制成肌纤维的形成,影响肌肉的生长发育。YUE[35]等研究发现,过表达lncYYW后通过mRNA microarray分析得到,GH1及其下游基因AKT1和PIK3CD上调,上调的GH1激活JAK促进成肌细胞增殖,同时也发现过表达lncYYW促进了成肌细胞的分化。相对于LncRNAs在其他生物学过程中(如肿瘤)调节作用机制的研究,LncRNAs对肌细胞增殖分化的研究仍有短缺,如2017年研究发现与乳腺癌转移相关的LncRNA H19,在肿瘤转移的不同阶段(EMT和MET),通过吸附不同的miRNA均发挥其促肿瘤转移作用[36]。综上,本研究中LncRNA-133a促进肌卫星细胞的增殖分化,可能是LncRNA-133a通过某种互作机制对肌细胞增殖分化相关靶标的调控所致。
4 结论
利用前期鉴定的牛肌肉发育相关的长链非编码RNA LncRNA-133a为研究靶标,经组织表达谱分析发现,LncRNA-133a在3、6、9月龄胎牛及成年牛骨骼肌中时序表达呈下降趋势;进一步利用牛骨骼肌卫星细胞体外分化模型,经过表达和抑制LncRNA-133a后发现,LncRNA-133a对牛骨骼肌卫星细胞的增殖及分化均有促进作用。
[1] 李伯江, 李平华, 吴望军, 李齐发, 黄瑞华, 刘红林. 骨骼肌肌纤维形成机制的研究进展. 中国农业科学, 2014, 47(6): 1200-1207.
LI B J, LI P H, WU W J, LI Q F, HUANG R H, LIU H L. Progresses in Research of the Mechanisms of Skeletal Muscle Fiber Formation.2014, 47(6): 1200-1207. (in Chinese)
[2] Margaret Buckingham, Stéphane D Vincent. Distinct and dynamic myogenic populations in the vertebrate embryo., 2009, 19(5): 444-453.
[3] 魏彩虹, 吴明明, 刘瑞凿, 赵福平, 张莉, 杜立新. 肌肉发育相关LncRNA的研究进展. 中国农业科学, 2014, 47(20): 4078-4085.
WEI C H, WU M M, LIU R Z, ZHAO F P, ZHANG L, DU L X. Research progress in muscular growth and development of long noncoding RNAs., 2014, 47(20): 4078-4085. (in Chinese)
[4] ZHANG H J, YU Y H,JIAKE C. Expression signatures oflncRNAsin skeletalmusclesat the early flow phase revealed by microarray in burned rats., 2016, 22(3): 224-232.
[5] FATICA A, BOZZONI I. Longnon-codingRNAs: new players in cell differentiation and development.2014, 15(1): 7-21.
[6] CESANA M, CACCHIARELLI D, LEGNINI I, SANTINI T, STHANDIER O, CHINAPPI M, TRAMONTANO A, BOZZONI I. A long noncoding rna controls muscle differentiation by functioning as a competing endogenous RNA.2011, 147: 358-369.
[7] ZHU M, LIU J, XIAO J, YANG L, CAI M, SHEN H, CHEN X, MA Y, HU S, WANG Z, HONG A, LI Y, SUN Y, WANG X. Lnc-mg is a long non-coding RNA that promotes myogenesis.2017, 8: 14718.
[8] ZHANG Z K, LI J, GUAN D, LIANG C, ZHUO Z, LIU J, LU A, ZHANG G, ZHANG B T. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration., 2018, https: //doi. org/10. 1002/jcsm. 12281
[9] ZHOU L, SUN K, ZHAO Y, ZHANG S, WANG X, LI Y, LU L, CHEN X, CHEN F, BAO X, ZHU X, WANG L, TANG L Y, ESTEBAN M A, WANG C C, JAUCH R, SUN H, WANG H. Linc-YY1 promotes myogenic differentiation and muscle regeneration through an interaction with the transcription factor YY1., 2015, 6: 10026.
[10] 吴明明. Lnc-SEMT促进绵羊肌肉分化生成的功能研究[D]. 北京:中国农业大学, 2016.
WU M M. Research of Lnc-SEMT function in the process of enhancing sheep muscle differentiation and generation [D]. Beijing: China Agricultural University, 2016. (in Chinese)
[11] KALLEN A N, ZHOU X B, XU J, QIAO C, MA J, YAN L, LU L, LIU C, YI J S, ZHANG H, MIN W, BENNETT A M, GREGORY R I, DING Y, HUANG Y. The Imprinted H19 LncRNA Antagonizes Let-7 MicroRNAs.2013, 52(1): 101-112.
[12] DEY B K, PFEIFER K, DUTTA A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration., 2014, 28(5): 491-501.
[13] LIU X F, DING X B, LI X, JIN C F, YUE Y W, LI G P, GUO H. An atlas and analysis of bovine skeletal muscle long noncoding RNAs., 2017, 48(3): 278-286.
[14] SUN X, LI M, SUN Y, CAI H, LAN X, HUANG Y, BAI Y, QI X, CHEN H. The developmental transcriptome sequencing of bovine skeletal muscle reveals a long noncoding RNA, lncMD, promotes muscle differentiation by sponging miR-125b., 2016, 1863(11): 2835-2845.
[15] GUO Y, WANG J, ZHU M, ZENG R, XU Z, LI G, ZUO B. Identification of myod-responsive transcripts reveals a novel long non-coding rna (lncrna-ak143003) that negatively regulates myoblast differentiation., 2017, 7(1): 2828.
[16] 丁向彬, 张蔚然, 张俊星, 王轶敏, 刘新峰, 郭宏. lncRNA-HZ5对牛骨骼肌卫星细胞成肌分化的调控作用研究. 天津农学院学报, 2017(3): 64-68.
DING X B, ZHANG W R, ZHANG J X, WANG Y M, LIU X F, GUO H. Effects of lnc RNA-HZ5 on myogenic differentiation process of bovine skeletal muscle satellite cells.2017(3): 64-68. (in Chinese)
[17] Xu X, Ji S, Li W, Yi B, Li H, Zhang H, Ma W. LncRNA H19 promotes the differentiation of bovine skeletal muscle satellite cells by suppressing Sirt1/FoxO1., 2017, 22: 10.
[18] 代阳. microRNA-128对牛骨骼肌卫星细胞增殖和成肌分化的调控机制研究[D]. 天津: 天津农学院, 2016.
DAI Y. Study on the regulation mechanism of microRNA-128 in the proliferation and myogenic differentiation process of bovine skeletal muscle satellite cells[D]. Tianjin: Tianjin Agricultural University, 2016. (in Chinese)
[19] PALLAFACCHINA G, FRANOIS S, REGNAULT B, CZARNY B, DIVE V, CUMANO A, MONTARRAS D, BUCKINGHAM M. An adult tissue-specific stem cell in its niche: A gene profiling analysis of in vivo quiescent and activated muscle satellite cells., 2010, 4(2): 77-91.
[20] 王禹. Sirt1 AS IncRNA通过抑制miR-34a的作用促进C2C12细胞增殖[D]. 陕西: 西北农林科技大学, 2015.
WANG Y. Sirt1 AS IncRNA Promotes Proliferation of C2C12 Cells by Inhibiting miR-34a[D]. Shaanxi: Northwest A&F University, 2015. (in Chinese)
[21] LIANG T, ZHOU B, SHI L, WANG H, CHU Q, XU F, LI Y, CHEN R, SHEN C, SCHINCKEL A P. lncRNA AK017368 promotes proliferation and suppresses differentiation of myoblasts in skeletal muscle development by attenuating the function of miR-30c.2017, 32(1): 377-389.
[22] 王子帅. 长非编码RNA-GTL2对C2C12细胞增殖的影响及机制的研究[D]. 北京: 中国农业科学院, 2015.
Wang Z S. Research on impact mechanism long noncoding RNA- GTL2 on proliferation of C2C12 cells[D]. Beijing: Chinese Academy of Agricultural Sciences, 2015. (in Chinese)
[23] 王红娜, 孙洪新, 张英杰, 刘月琴, 谷振慧, 史秀芬. 干扰MSTN对绵羊成肌细胞增殖分化及相关基因表达的影响. 畜牧兽医学报, 2018, 49(01): 46-54.
WANG H N, SUN H X, ZHANG Y J, LIU Y Q, GU ZH, SHI X F. Effects of interfering MSTN on proliferation and differentiation of sheep myoblasts and expression of related genes.2018, 49(01): 46-54. (in Chinese)
[24] DOUCET C, GUTIERREZ G J, LINDON C, LORCA T, LLEDO G, PINSET C, COUX O.Multiple phospho-rylationeventscontrolmitoticdegradation of the muscle transcription factor Myf5., 2005, 6: 27.
[25] TINTIGNAC L A, LEIBOVITCH M P, KITZMANN M, FERNANDEZ A, DUCOMMUN B, MEIJER L, LEIBOVITCH S A. Cyclin E-cdk2 phosphorylation promotes late G1-phase degradation of MyoD in muscle cells., 2000, 259(1): 300-307.
[26] BUCKINGHAM M, RIGBY P W. Gene regulatory networks and transcriptional mechanisms that control myogenesis.2014, 28(3): 225-238.
[27] WIGMORE P M, EVANS D J R. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis.2002, 216 (216) : 175-232.
[28] TAKAGAKI Y, YAMAGISHI H, MATSUOKA R. Factors involved in signal transduction during vertebrate myogenesis.2012, 296: 187-272.
[29] CUSELLA DE AMG, MOLINARI S, LE DONNE A, COLETTA M, VIVARELLI E, BOUCHE M, MOLINARO M, FERRARI S, COSSU G. Differential response of embryonic and fetal myoblasts to TGF beta: a possible regulatory mechanism of skeletal muscle histogenesis.(Cambridge, England), 1994, 120(4): 925-933.
[30] Sabourin L A, Rudnicki M A. The molecular regulation of myogenesis.2000, 5(1): 16-25.
[31] WANG L, ZHAO Y, BAO X, ZHU X, KWOK YK, SUN K, CHEN X, HUANG Y, JAUCH R, ESTEBAN MA, SUN H, WANG H. LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration., 2015, 25(3): 335-350.
[32] GONG C, LI Z, RAMANUJAN K, CLAY I, ZHANG Y, LEMIRE-BRACHAT S, GLASS D J. Long non-coding RNA, LncMyoD, regulates skeletal muscle differentiation by blocking IMP2-mediated mRNA translation., 2015, 34(2): 181-191.
[33] LEGNINI I, MORLANDO M, MANGIAVACCHI A, FATICA A, BOZZONI I. A feedforward regulatory loop between HuR and the long noncoding RNA linc-MD1 controls early phases of myogenesis.2013, 53: 506-514.
[34] ALBRECHT E, LEMBCKE C, WEGNER J, MAAK S. Prenatal muscle fiber development and bundle structure in beef and dairy cattle.2013, 91(8): 3666-3673.
[35] YUE Y W, JIN C F, CHEN M M, ZHANG L L, LIU X F, MA W Z, GUO H. A lncRNA promotes myoblast proliferation by up-regulating GH1.2017, 53(8): 699-705.
[36] ZHOU W, YE X L, XU J, CAO M G, FANG Z Y, LI L Y, GUAN G H, LIU Q, QIAN Y H, XIE D. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR- 200b/c and let-7b.2017, 10(483). DOI: 10. 1126/ scisignal. aak9557
Effects of Bovine LncRNA-133a on the Proliferation and Differentiation of Skeletal Muscle Satellite Cells
LI Yan, CHEN MingMing, ZHANG JunXing, ZHANG LinLin, LI Xin, GUO Hong, DING XiangBin, Liu XinFeng
(College of Animal Science and Veterinary Medicine, Tianjin Agricultural University, Tianjin 300384)
【Objective】The objective of this paper was to investigate the effects of long non-coding RNA LncRNA-133a on the proliferation and differentiation of bovine skeletal muscle satellite cells. 【Method】This study used qRT-PCR to detect the expression level of LncRNA-133a in the skeletal muscle tissues of 3, 6 and 9 months old fetal cattle and 24 months old adult bovine skeletal muscle, and obtained the tissue temporal expression profile of LncRNA-133a. The in vitro induced myoblast differentiation model of bovine skeletal muscle satellite cells was constructed to simulate the growth and development of bovine skeletal muscle. The qRT-PCR was used to detect the cells temporal expression profiles of LncRNA-133a and myocyte differentiation markers MyoG and MHC. The bovine skeletal muscle satellite cells were transfected with LncRNA-133a overexpression vector (pCDNA3.1-EGFP- LncRNA-133a) or LncRNA-133a inhibitor (si-LncRNA 133a), and the transfection efficiency and the mRNA expression levels of LncRNA-133a, MyoD, MyoG and MHC were detected by qRT-PCR in each transfection treatment group, then the protein expression level of MHC gene was detected by western blotting. In addition, the cell proliferation of the bovine skeletal muscle satellite cells and the extent of myotube fusion at the differentiation stage were detected by EdU cell proliferation assay and immunofluorescence protein staining, respectively. 【Result】Tissue expression profiling revealed that LncRNA-133a had the highest expression in the muscle tissue of 3 months old fetal bovine, followed by the 6-month-old fetal bovine muscle tissue, and the lowest expression in the 9-month-old fetus and adult bovine muscle tissue, which demonstrated that the time expression showed a downward trend. Cell-time expression profiles of LncRNA-133a, MyoG, and MHC were analyzed by a successfully constructed bovine skeletal muscle satellite cell differentiation model in vitro, and the results showed that the expression levels of myogenic differentiation markers MyoG and MHC gradually increased during the differentiation of bovine skeletal muscle satellite cells (D0-D3). The expression of LncRNA- 133a increased in the differentiation stage, and the expression level reached the highest at 48 h of differentiation (D2). The bovine skeletal muscle satellite cell model of overexpressing LncRNA-133a or inhibiting LncRNA-133a was constructed successfully, and in the proliferative phase (D0): the number of EdU positive cells in the overexpressed LncRNA-133a-treated group was significantly increased (<0.01), and the number of EdU positive cells in the LncRNA-133a inhibition treatment group was significantly decreased (<0.01), compared with the control group. At 48 h of differentiation (D2): compared with the control group, the results of LncRNA-133a overexpression treatment showed that mRNA expression levels of myocyte differentiation markers MyoD, MyoG and MHC were significantly increased (<0.05). Western blotting showed that the expression of MHC protein was also significantly increased (<0.01), and the immunofluorescence protein staining of MHC protein showed that the volume of fusion myotubes was larger. On the contrary, in the LncRNA-133a inhibition treatment group, the mRNA expression levels of MyoD, MyoG and MHC were decreased, and MyoG was significantly decreased (<0.05). Meanwhile, the expression of MHC protein was significantly decreased (<0.01), and the volume fraction of MHC protein fusion myotubes was also decreased. 【Conclusion】Thus, this study confirmed that LncRNA-133a promoted the proliferation and differentiation of bovine skeletal muscle satellite cells, which laid a foundation for further research on the regulatory network mechanism of LncRNA-133a regulating the proliferation and differentiation of bovine skeletal muscle satellite cells.
LncRNA-133a; bovine; skeletal muscle satellite cells; proliferation; differentiation
10.3864/j.issn.0578-1752.2019.01.013
2018-05-18;
2018-09-28
国家自然科学基金青年项目(31501938)、天津市“131人才工程第二层人选”项目(J01009030725)
李燕,E-mail:lihongjiliyan@163.com。通信作者丁向彬,E-mail:xiangbinding@aliyun.com。通信作者刘新峰,E-mail:lxf20001924036@126.com
(责任编辑 林鉴非)

