基于单-和双-三唑衍生物的三个Cuギ和Cuガ多金属氧簇基配合物的水热合成及光催化性能
刘媛媛 李欣书 张慧敏 丁 斌,2
(1无机-有机杂化功能材料化学教育部重点实验室,天津师范大学化学学院,天津 300387)
(2南京大学配位化学国家重点实验室,南京 210093)
0 Introduction
In recent years,the inorganic-organic coordination polymers have become a unique class of functional materials because of their unique chemical applications and beautiful structural motifs[1].Polyoxometalates(POMs),defined as metal-oxide clusters based on polyanions,have received intensive attention because of their many important applications in electrical conductivity,catalysis,medicine,magnetism,materials science,and biological chemistry[2].As illustrated in the previous literature,some important applications are mainly based on the following reasons:わpolyoxometalates can be employed as electron and proton reservoirs;ぷtheir molecular properties are extremely variable such as shape,size,acidity and charge[3].Recently,many remarkable works about preparing the desired metal-organic frameworks (MOFs)are the utilization of the polyanions coordination ability to bind with versatiletransition-metal organic units,which have been presented in coordination polymers[4-6].This synthetic strategy can bring the merits of each rowmaterial together,such as intriguing structural motifs,good physical and chemical properties[7].
For the rational design and preparation of the POM-based coordination frameworks, judicious selective of organic linkers is quite important.The organic linkers can be divided into two parts:presynthesized and in-situ synthesized linkers[8].The former have been widely utilized in the POM-based reaction systems,including Nitrogen-donor linkers,poly-carboxylate linkers and so on[9]. Among the Nitrogen-containing heterocyclic organic linkers,1,2,4-triazole and its derivatives are intriguing because the linkers combine the coordination geometries of both imidazoles and pyrazoles due to their three heteroatoms arrangement.For instance,the organic linkers have been utilized to construct the open MOFs,which contain unsaturated metal clusters and possess even framework flexibility and high thermal stability[10-11].As depicted in Scheme 1,the multi-dentate mono-and bis-triazole derivatives,namely 4-pyridine-2-1,2,4-triazole (L1),3-(4H-1,2,4-triazol-4-yl)benzoic acid (HL2)and trans-4,4′-azo-1,2,4-triazole (L3),which can afford strong coordination nitrogen donors and carboxylate donors to metal centers.It can be anticipated that L1,HL2and L3can be utilized as multi-dentate coordination donors to coordination metallic centers,which can further construct many novel inorganic-organic POM-based hybrid materials[12].
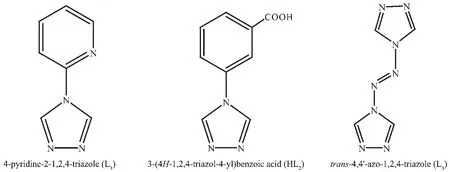
Scheme 1 Three multi-dentate 1,2,4-triazole derivative ligands L1,HL2 and L3

Scheme 2 Hydrothermal assembly of a series of Cuギ and Cuガ hybrid polyoxometalate-based coordination frameworks
Previously we also began to explore the coordination chemistry and functional application of Nitrogencontaining heterocyclic ligands and its derivatives[13-16].In this work under hydrothermal conditions,three novel polyoxometalate (POM)-based Cuギ and Cuガhybrid materials with multi-dentate mono-and bistriazole derivatives,namely{[Cu (L1)2(Mo4O13)]·2H2O}n(1),{[Cu1.5(L2)(HL2)(H2O)(Mo4O13)]·2H2O}n(2),{[Cu2(L3)1.5(Mo4O13)]·H2O}n(3)havebeen designed and synthesized(Scheme 2).Their crystallographic structures have been determined by single crystal X-ray diffraction,FT-IR infrared spectra and PXRD analyses.Photocatalytic activities for decomposition of different organic dyes,namely rhodamine B (RhB),methylene blue (MB)and methyl orange (MO),have been investigated for 1~3.
1 Experimental
1.1 General
Ligands L1(4-pyridine-2-1,2,4-triazole),HL2(3-(4H-1,2,4-triazol-4-yl)benzoic acid)and L3(trans-4,4′-azo-1,2,4-triazole)were prepared according to the literature methods[17]. All the other reagents were commercially available and utilized without further purification.Perkin-Elmer 240 elemental analyzer was ulitilized toperform C,H and Nmicroanalyses.Powder X-ray diffraction patterns were determined on a D/Max-2500 X-ray diffractometer using Cu Kαradiation(λ=0.154 1 nm,U=40 kV,I=40 mA)with a 2θrange of 5°~50°.A Lamdba 900 UV-Vis-NIR spectroscopy was utilized to measure the UV-Vis diffuse reflectance spectra at the ambident temperature.
1.2 Preparation of coordination complexes 1~3
{[Cu(L1)2(Mo4O13)]·2H2O}n(1).CuSO4·5H2O (75.00 mg,0.3 mmol),L1(43.8 mg,0.3 mmol)and MoO3(57.60 mg,0.4 mmol)were added in 10 mL deionized H2O.The mixture were transferred and placed into a Teflon vessel in a steel autoclave,heated at 165℃for 72 h and then cooled to ambient temperature during 24 h.The blue strip-shaped single crystals of 1 were washed by H2O,aether and air-dried.Elemental analysis Calcd.for C14H12CuMo4N8O14.50(%):C,17.31;H,1.25;N,11.53.Found(%):C,17.55;H,1.35;N,11.82.FT-IR (cm-1):3 122 (m),2 615 (w),1 593 (m),1 528(m),1 470(m),1 441(m),1 343 (m),1 253(m),938(w),912(m),813(m),568(m),476(w).
{[Cu1.5(L2)(HL2)(H2O)(Mo4O13)]·2H2O}n(2).CuSO4·5H2O (100.00 mg,0.4 mmol),HL2(94.5 mg,0.5 mmol)and MoO3(14.40 mg,0.1 mmol)were added into 10 mL deionized H2O.Themixtureweretransferred and putted into a Teflon vessel,heated at 165℃for 72 h in a steel autoclave and then cooled to ambient temperature during 24 h.The green strip-shaped single crystals of 2 were washed by H2O,aether and air-dried.Elemental analysis Calcd.for C18H17Cu1.50Mo4N6O19.50(%):C,19.50;H,1.55;N,7.58.Found(%):C,19.73;H,1.63;N,7.73.FT-IR (cm-1):3 180(m),2 629(w),1 704(m),1 548(s),1 459(m),1 401(s),1 332(w),1 270(m),948(w),902(m),820(m),765(m),699(m),552(m),479(w).
{[Cu2(L3)1.5(Mo4O13)]·H2O}n(3).CuSO4·5H2O (125.00 mg,0.5 mmol),L3(49.2 mg,0.3 mmol)and MoO3(28.80 mg,0.2 mmol)were added in 10 mL deionized H2O.The mixture were transferred and placed into a Teflon vessel in a steel autoclave,heated at 165℃for 72 h and then cooled to ambient temperature during 24 h.The red block-shaped single crystals of 3 were washed by H2O,aether and air-dried.Elemental analysis Calcd.for C6H8Cu2Mo4N12O14(%):C,7.33.H,0.82.N,17.10.Found(%):C,7.55;H,0.98;N,17.36.FT-IR (cm-1):3 120(bm),1 513(s),1 296(s),1 176(s),1 100(m),1 048(m),1 000(w),922 (m),804(m),661(m),615(m).
1.3 Photo-catalytic measurementsof coordination complexes 1~3
The photo-catalytic measurements of coordination polymers1~3 were evaluated by the degradation of MB,RhB and MO under irradiation utilizing a 200 W high pressure mercury lamp.The photo-catalytic experiments have been measured in a classical method,5 mg complex 1 or 2 or 3 were suspended in the water solutions containing three organic dyes (10 μmol·L-1,10 mL),respectively.The mixture was stirred in the dark for 30 min for the desorption-adsorption equilibrium,then the mixture was transferred and placed under thelightingof Hglamp (200 W)with continuous stirring.Next,every 20 minutes intervals,aliquots of the mixture samples were extracted and centrifuged.Finally,the sample was analyzed by UV-Vis analytical technique.The photo-catalytic degradation efficiency(D)can be calculated as below:
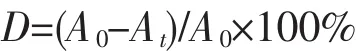
where Atis the absorbance of dye at time t,A0represents the initial absorbance of dyes solution.
1.4 X-ray crystallography
Structure measurements of complexes 1~3 were performed on a computer controlled a Bruker SMART ApexⅡCCD diffractometer equipped with graphitemonochromated Mo Kαradiation with radiation wavelength of 0.071 073 nm by using theω-scan technique.The structures were solved by direct methods and refined with the full-matrix least-squares technique using the SHELXS-97 and SHELXL-97 programs[18-19].Anisotropic thermal parameters were assigned to all non-hydrogen atoms.The organic hydrogen atoms were generated geometrically;the hydrogen atoms of the water molecules were generated geometrically and refined with isotropic temperature factors.Crystal data collection and refinement details for complexes 1~3 are summarized in Table 1.Selected bond lengths and anglesfor complexes1~3arelisted in Table2.Hydrogen bonds analysis was carried out using the PLATON program,all the hydrogen bonds distances and angles are listed in Table 3.
CCDC:1474258,1;1477985,2;1544278,3.

Table 1 Crystal data and structure refinement information for compounds 1~3

a R1=∑||F o|-|F c||/|F o|,wR2=[∑w(F o2-F c2)2/∑w(F o2)2]1/2.

Table 2 Selected bond lengths(nm)and angles(°)for coordination polymers 1~3
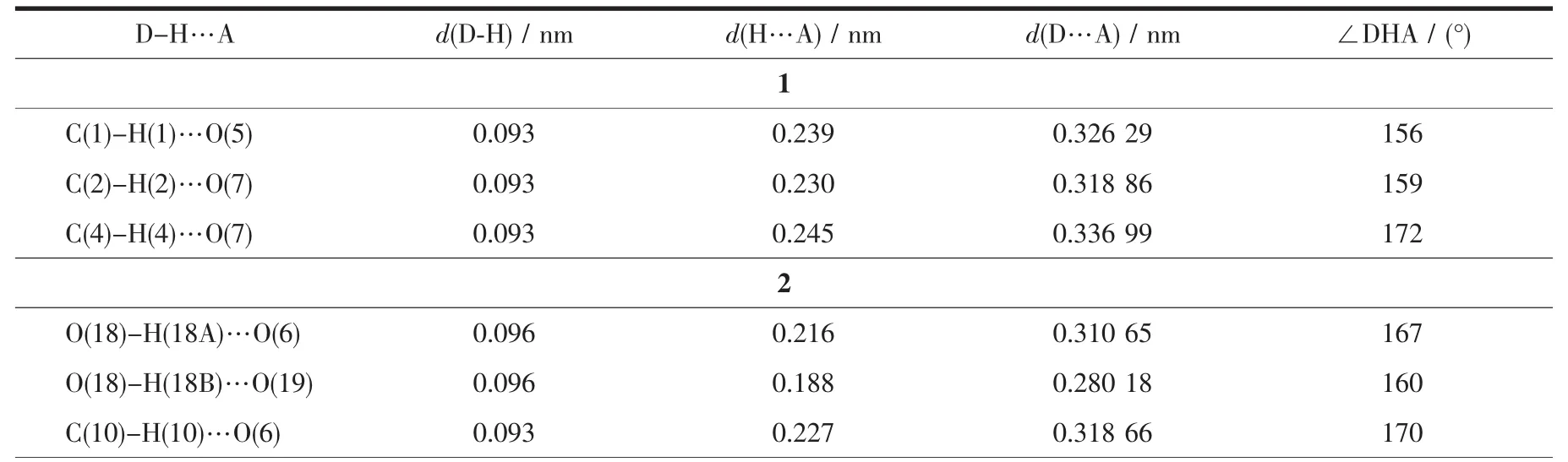
Table 3 Hydrogen bonds for coordination polymers 1~3

Continued Table 3
2 Results and discussion
2.1 Structural description of coordination polymers 1~3
Single-crystal X-ray diffraction analysis demonstrates that coordination compound 1 crystallizes both in Pspace groups and triclinic crystal system.The asymmetric unit of 1 contains one Cuギions,two L1ligands,one Mo4O132-anion and two lattice water molecules.As illustrated in Fig.1,in coordination polymer 1,L1ligands adopt pyrazolate-like[N-N]-bidentate bridging coordination mode.The Cuギcenter (Cu1)is six-coordinated by two nitrogen atoms(N2 and N2i)from triazolyl groups and four oxygen atoms (O1,O1i,O4iand O4ii)from Mo4O132-.The oxygen atom O(1)coming from Mo4O132-anions further links Cu1 atoms.Six-coordinated Cuギ atoms (Cu1)and six-coordinated Mo centers are linked by bidentate L1ligands forming binuclear Cuギ-Mo structural units.As depicted in Fig.2(a),the Mo4O132-anions are inter-linked via vertical oxygen atoms forming 1D chain structures,which are further joined by Cuギcenters and are arranged into the 2D hybrid metal-organic framework along the crystallographic c axis.From side view of the 2D hybrid metal-organic framework (Fig.2(b),it can be found that the pyridine groups of the bidentate L1ligands are not coordinated becauseof steric hindranceeffect,thereforethepyridine groupsof L1aredecorated outsideof the2Dcoordination framework.Numerous non-classical C-H…Ohydrogen bonding interactions (C(1)…O(5)0.326 29 nm,C(2)…O(7)0.318 86 nm,C(4)…O(7)0.336 99 nm)also can be found in the coordination framework of 1,which alsofurther extend 1 intoa3Dsupramolecular network.

Fig.1 Structural unit of 1

Fig.2 (a)Two dimensional layer coordination framework of 1 with Mo4O132-anions linked by Cu(L1)2 structural units;(b)Side view of 2D layer coordination framework of 1
Coordination polymer 2 crystallizes in C2/c space groups and monoclinic crystal system.The asymmetric unit of 2 contains 1.5 Cuギ ions (Cu2 and half of Cu1),one HL2ligand,one deprotonated L2-ligand,one Mo4O132-anion,one bridging H2O molecule (O18)and two lattice H2O molecules (O19 and O20).As depicted in Fig.3,HL2link the neighboring Cu centers(Cu1 and Cu2)through its two nitrogen atoms (N1 and N2)of triazole forming bi-dentate bridging mode.While the deprotonated L2-ligands adopt tridentate bridging coordination mode linking Cu1,Cu2 and Cu1ithrough two triazole nitrogen atoms (N4 and N5)and its one oxygen atom (O15i).The Cu ギ center(Cu1)is six-coordinated by two nitrogen atoms (N1 and N4)from triazolyl groups,three oxygen atoms(O1,O12,O15)from Mo4O132-and one bridging H2O molecule (O18).While the Cuギ center (Cu2)is also six-coordinated by four nitrogen atoms (N2,N2i,N5 and N5i)from four triazolyl groups and two bridging water molecule (O18,O18i).

Fig.3 Structural unit of 2
As depicted in Fig.4,the Mo4O132-anions are inter-linked via the bridging oxygen atoms (O18),bidentate HL2and tri-dentate L2-,which are further joined by Cuギcenters and are arranged into the unique 3D micro-porous hybrid metal-organic framework along the crystallographic c axis.One dimensional microporous channels with 0.479 7(1)nm×0.641 6(1)nm also can be found,in which lattice H2O molecules are located in the 1D channel.Numerous O-H…O and O-H…O non-classical hydrogen-bonding interactions(O(18)…O(6)0.310 65 nm,O(18)…O(19)0.280 18 nm,C(10)…O(6)0.318 66 nm,C(13)…O(4)0.320 71 nm,C(15)…O(2)0.310 97 nm )also can be found in the coordination framework of 2,which also further stabilize the 3D coordination network of 2.
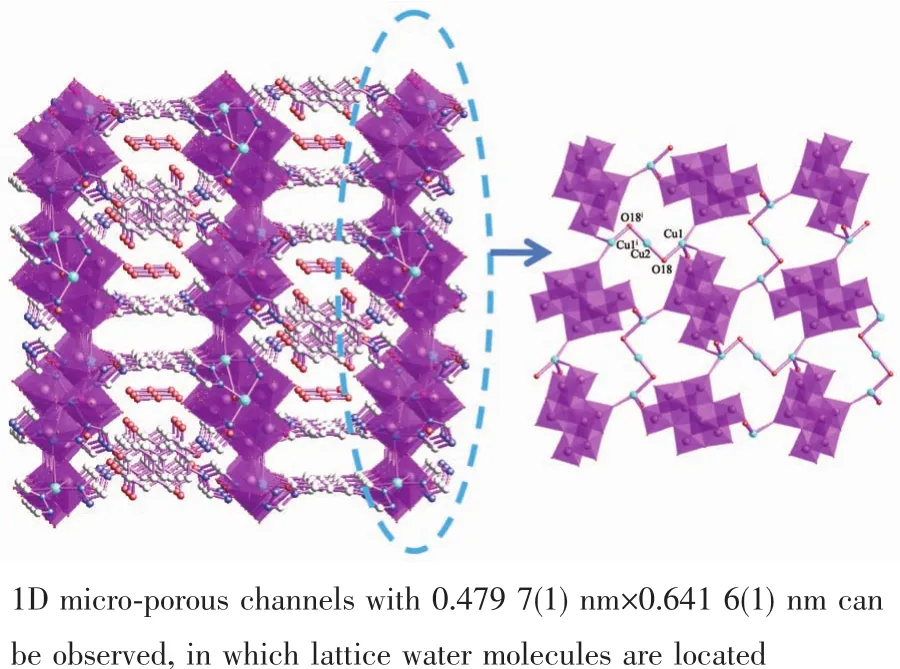
Fig.4 Mo4O132-anions linked by bridging L2-forming 3D micro-porous framework of 2
Coordination polymer 3 crystallizes in P21/c space group and monoclinic crystal system.The asymmetric unit of 3 contains two Cuガ ions (Cu1 and Cu2),1.5 L3ligands,one Mo4O132-and one lattice H2O (O14).As depicted in Fig.5,Cu(1)are four-coordinated by two triazole nitrogen atoms (N2 and N7i)and two oxygen atoms (O3 and O4)forming the tetrahedral coordination mode while Cu2 are four-coordination by four triazole nitrogen atoms (N1,N8i,N9 and N10)forming the tetrahedral coordination mode.The L3ligands are linked via four neighboring Cuギions adopting the tetra-dentate bridging mode.
As depicted in Fig.6(a),the L3ligands bridge neighboring Cuガ centers forming 1Ddouble[Cu2(L3)1.5]chains.Further the Mo4O132-anions are utilized as pillars to link the neighboring 1D double [Cu2(L3)1.5]chains forming the 3D POM-based Cuガ hybrid micro-porous framework.It is noted that,as depicted in Fig.6(b),two POM-based 3D micro-porous frameworks self-interpenetrate with each other,which ultimately form two-fold interpenetrating 3D coordination framework of 3 viewed along the a-axis direction.The C-H…O,N-H…O and O-H…N hydrogen bonding interactions (O(14)…O(2)0.286 23 nm,O(14)…O(4)0.290 84 nm,C(1)…O(6)0.306 73 nm,C(2)…O(9)0.325 61 nm,C(6)…O(14)0.326 94 nm)can be found,which also further stabilize the 3D hybrid framework of 3 (Table 3)[20].

Fig.5 Structural unit of 3
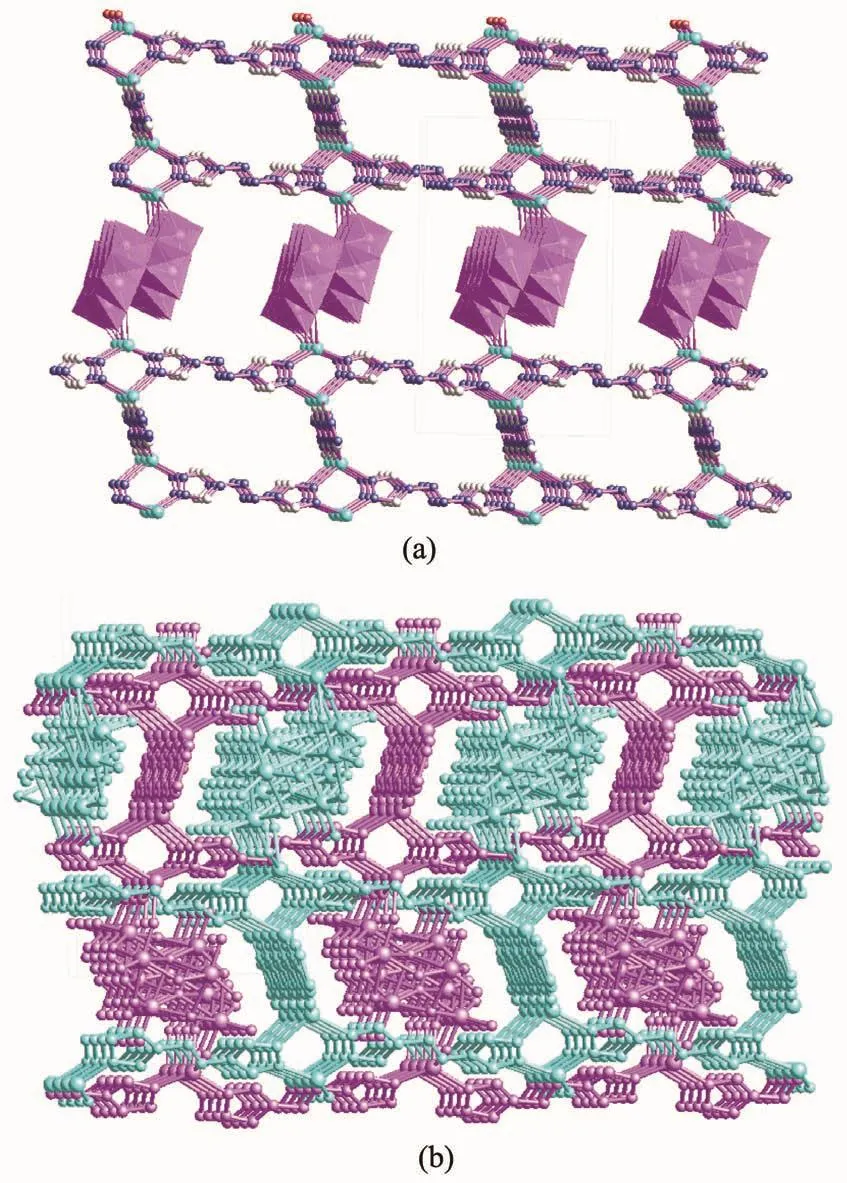
Fig.6 (a)Mo4O132-anions used as pillars to link the neighboring 1D double[Cu2(L3)1.5]chains forming 3D micro-porous framework;(b)Twofold interpenetrating 3D coordination framework of 3 viewed along the a-axis direction
2.2 Powder X-ray diffraction(PXRD)and FT-IR characterizations
PXRD patterns of coordination compounds 1~3 were also determined at ambient temperature to verify the phasepurity.As depicted in Fig.7,the experimental patterns positions were well consistent with those of the theoretical patterns, demonstrating the as-synthesized samples 1~3 are pure phases.The slight differences in reflection intensities between experimental and theoretical PXRD patterns can be ascribed to the crystal orientation variation of the powder samples.

Fig.7 Powder X-ray patterns for coordination framework(a)1,(b)2 and (c)3 confirming purities of the bulky samples
In coordination polymers 1~3,the FT-IR bands at 912,813 cm-1for 1,902,820 cm-1for 2 and 922,804 cm-1for 3 can be assigned to ν(Mo-O)and ν(Mo-O-Μo).FT-IR spectra also demonstrated classical absorption bands for triazole moieties of L.The bands around ca.3 100 cm-1and bands located at 1 100~1 300 cm-1should be correlated with ν(C-H)and ν(CN)orν(N-N)vibrationsof triazole moieties.The triazole out of plane ring absorption is also found at around 600 cm-1(568 cm-1for 1,552 cm-1for 2 and 615 cm-1for 3)[21].
2.2 Photo-catalytic capability for organic dyes by coordination polymers 1~3
As it known to us,large numbers of commercial organic dyes are devoted to our personal life,however,they are also carcinogenic and toxic to us.Therefore how to rational utilize and purify the waste water can be the burning question to solve.Some reported about coordination polymers can show excellent photocatalytic capability in the organic dyes degradation through the UV irradiation method[22].To determine the catalytic capability of coordination polymers 1~3 for dye degradation,three commercial available common organic dyes RhB,MB and MO have been utilized for the photo-catalytic experiments.
The photo-catalytic activities of coordination polymers 1~3 in three different dye solutions are depicted in Fig.8~10,respectively.The variation trend of the concentration ratios of dyes (Ct/C0)against time(min)for coordination polymers 1~3 were plotted,where C0is the initial concentration of dyes after stirred in the dark environment for 20 minutes.As depicted in Fig.8,coordination polymer 1 showed remarkable photo-catalytic capacity for the two organic dyes MO and RhB,and could be almost fully degraded (95.8%for RhB,and 97.5%for MO)in 70 min.As depicted in Fig.9,coordination polymer 2 also showed photo-catalytic capacity for the three organic dyes,and could be almost totally degraded(91.1%for MB,98.6%for RhB and 99.7%for MO)in nearly 70 min.As depicted in Fig.10,coordination polymer 3 showed remarkable photo-catalytic capacity for two organic dyes MOand RhB,and could be nearly degraded completely (97.9%for RhB and 84.1%for MO)in 70 min.The experiment results demonstrate that all the coordination polymers 1~3 also could be excellent candidates in the photo-catalytic degradation of some organic dyes.

Fig.8 Plot of concentration ratios (Ct/C0)against irradiation time of MB,RhB and MO degraded by coordination polymer 1
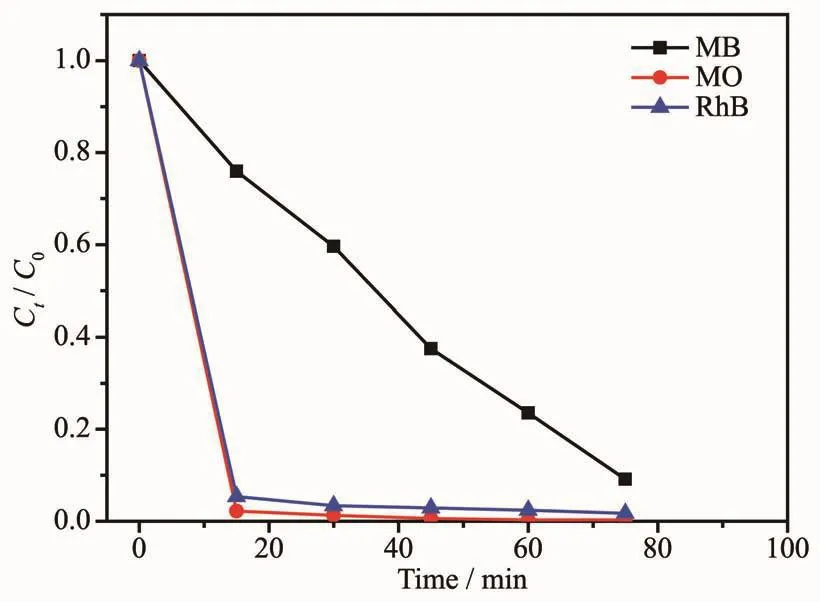
Fig.9 Plot of concentration ratios (Ct/C0)against irradiation time of MB,RhB and MO degraded by coordination polymer 2
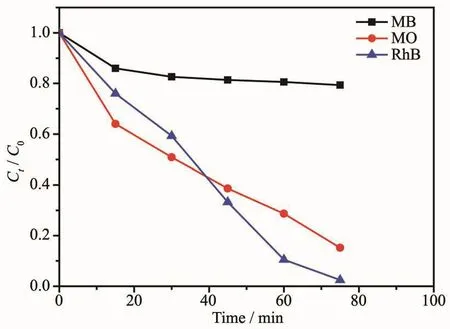
Fig.10 Plot of concentration ratios (Ct/C0)against irradiation time of MB,RhB and MO degraded by coordination polymer 3
With a view to the coordination environment,the types of the central metal ions and the coordinated mode of the ligand have significant influence on the photocatalytic activities.As described in the previous literature[21],during the photocatalytic process of the POM-based hybrid coordination polymers,UV-Vis light can induce POM/organic ligands to produce oxygen and/or nitrogen-metal charge transfer by promoting an electron from the highest occupied molecular orbital (HOMO)to the lowest unoccupied molecular orbital (LUMO).The HOMO strongly demands one electron to return to its stable state.Thus,one electron is captured from H2O molecules,which are oxygenated into the·OH active species,which should further decompose certain organic dyes and effectively complete the photo-catalytic experiment[23-27].
3 Conclusions
In summary,under hydrothermal conditions,three novel polyoxometalate (POM)-based Cuギ and Cuガhybrid materials with multi-dentate mono-and bis-triazole derivatives,namely {[Cu (L1)2(Mo4O13)]·2H2O}n(1),{[Cu1.5(L2)(HL2)(H2O)(Mo4O13)]·2H2O}n(2),{[Cu2(L3)1.5(Mo4O13)]·H2O}n(3)have been designed and synthesized.In 1,the Mo4O132-anions and Cuギcenters are inter-linked by bidentate L1,which are arranged into the 2D hybrid metal-organic framework.In 2,the Mo4O132-anions and Cuギcenters are inter-linked via bridging aqua atoms (O18), bi-dentate HL2and tridentate L2-,which are arranged into the 3D microporous hybrid metal-organic framework.In 3,the L3ligands bridge neighboring Cuガcenters and Mo4O132-anions,which ultimately form the two-fold interpenetrating 3D coordination framework of 3.Photocatalytic activities for decomposition of different organic dyes RhB,MB and MO have been investigated,and the results indicate that 1~3 are good candidates for photocatalytic degradation of the organic dyes.The experiment result also demonstrates that great potential in the construction of the novel POM-based hybrid metal organic frameworks employing different multi-dentate mono-and bis-triazole derivatives and versatile polyoxometalate (POM)-based building blocks.On the basis of this work,further preparation,structural analyses and functional properties investigations of the POM-based hybrid coordination polymers utilizing the versatile building blocks are also under way in our laboratory.

