丙酮/氯离子协同活化过一硫酸盐降解酸性橙
张 珂,许 芬,陈家斌,万 玲,黄天寅
丙酮/氯离子协同活化过一硫酸盐降解酸性橙
张 珂,许 芬,陈家斌*,万 玲,黄天寅
(苏州科技大学环境科学与工程学院,江苏 苏州 215009)
采用丙酮/氯离子(Cl-)协同活化过一硫酸盐(PMS)降解偶氮染料酸性橙7(AO7).研究发现,与Cl-/PMS体系相比,丙酮/Cl-/PMS体系降解AO7的效果显著增强,相应的AO7表观速率常数从0.0767增加到0.1725min-1.考察了丙酮/Cl-/PMS体系降解AO7的主要影响因素(初始pH值、Cl-浓度、PMS浓度、丙酮浓度).结果表明,在碱性环境下,随着Cl-、PMS和丙酮浓度的增加,AO7的降解效果有所增强;研究丙酮/Cl-/PMS体系降解AO7的降解机制.自由基猝灭实验表明,丙酮/Cl-/PMS体系中没有自由基(硫酸根自由基(SO4-•)和羟基自由基(HO•))的产生,而次氯酸根(ClO-)浓度在反应过程中逐渐增加,得出丙酮作为催化剂促进Cl-和PMS产生更多HClO的结论.通过紫外可见光谱分析,表明AO7分子中偶氮键及萘环结构均被破坏.
氯离子;过一硫酸盐;丙酮;酸性橙7;次氯酸
偶氮染料广泛用于现代工业中,如纺织、造纸、制革等行业.由于偶氮染料具有毒性强、含盐量高、致癌、致突变等特点[1-3],若不经处理直接排放会对环境和人类健康造成严重危害.大多数偶氮染料可生化性不高,成分复杂,属于生物难降解,传统的废水处理技术已无法满足染料废水的处理要求[4-6].近年来,基于硫酸根自由基(SO- 4•)的高级氧化技术已得到广泛应用[7-8].硫酸根自由基(SO- 4•)有较高的氧化还原电位(0=2.5~3.1V)[9-11],可以通过加热[12-14]、紫外光照射[15-17]、过渡金属离子[18-21]活化过一硫酸盐(PMS)产生.然而这几种活化方法都具有一定的局限性.加热和紫外光照射均需要提供大量的能量且成本较高.过渡金属离子虽然有易获取、成本低等优点[22-23],但是其较低的回收率和可能造成的二次污染限制了此方法的广泛运用[24].因此,新型过硫酸盐活化方法的研究成为目前过硫酸盐高级氧化技术(AOPs)研究的热点.
氯离子(Cl-)是印染废水中主要的无机成分.据报道,染料废水中含有的无机盐(NaCl)等可高达5~100mg/L[25-26].大量研究表明,氯离子(Cl-)对AOPs有很大影响[27-30].通过研究Cl-对钴/单过硫酸盐体系降解偶氮染料的影响,发现低浓度的Cl-(<5mmol/L)对染料脱色有明显的抑制作用,高浓度Cl-(>50mmol/L)对染料降解的抑制作用会不断减小,甚至会加速染料的脱色[30].
通过研究Cl-浓度对单独PMS体系降解金橙G(OG)的影响,得出Cl-与PMS反应产生具有氧化活性的卤化物HOCl,能够直接降解OG[31].因此,探索PMS活化过程中废水基质(如Cl-)所起到的作用对于该技术用于染料废水处理具有重要意义.
本文在研究新型过硫酸盐活化方法的过程中,发现丙酮/Cl-可以协同活化PMS,加速染料降解.通过改变反应条件,研究了丙酮/Cl-/PMS体系的主要影响因素,并通过自由基猝灭实验及采用荧光猝灭法分析了丙酮/Cl-/PMS体系降解AO7中可能存在的机制.
1 试剂与方法
1.1 实验试剂与设备
过一硫酸盐(HKSO5· 0.5KHSO4· 0.5K2SO4, PMS)、硫酸耐尔蓝A(C40H40N6O6S)、曲拉通X- 100((C2H4O)NC14H22O)购于Sigma-Aldrich; 酸性橙7(AO7)购于国药集团化学试剂有限公司,氯化钠(NaCl) 、丙酮(C3H6O)、亚硝酸钠(NaNO2)、甲醇(CH3OH)、叔丁醇(C4H9OH)、硫酸铵[(NH4)2·SO4]、醋酸(CH3COOH)、磷酸(H3PO4)、硼酸(H3BO3)、硫酸(H2SO4)、氢氧化钠(NaOH)均为分析纯购于国药集团化学试剂有限公司.实验用水为超纯水.实验用搅拌装置购于上海标本模型厂(JB50-D型).
1.2 降解实验
在室温下,将一定量PMS和NaCl注入150mL的锥形瓶中,同时在锥形瓶中加入一定量的超纯水,并用稀H2SO4或NaOH调节pH值,然后迅速加入一定量的丙酮和一定量的AO7溶液,使得总溶液达到100mL,反应体系密封后置于磁力搅拌器混合并启动反应.每隔一段时间取样,迅速加入过量猝灭剂NaNO2终止反应,猝灭后的样品经0.45μm滤膜过滤后,收集滤液24h内测定,每组实验均重复3次以确保实验的重现性,最终结果取三次平均值.
1.3 分析方法
使用Mapada UV-1600(PC)紫外可见分光光度计,于AO7最大吸收波长484nm处测定滤液的吸光度,代入标准曲线求得浓度.次氯酸根浓度采用PerkinElmer LS55荧光分光光度计测定,荧光吸收波长为686nm,激发波长为634nm.应用Excel2016、Origin9.0进行数据分析和作图
2 结果与讨论
2.1 不同体系下AO7的降解效果
图1显示了不同体系下AO7的降解效果.结果表明,PMS单独存在时,AO7在20min内降解了21%,丙酮单独存在时,AO7在20min内几乎不降解.在PMS体系中加入Cl-,AO7在20min内降解了38%;而在Cl-/PMS体系中加入丙酮,AO7在20min内降解了97%.分析原因可能是,Cl-和PMS反应产生HOCl和Cl2,而丙酮催化Cl-/PMS体系产生更多HOCl[见式(1)~(4)][4,32]. HOCl具有强氧化性,可以有效氧化AO7.

图1 不同体系下AO7的降解效果
[AO7]=50μM, [PMS]=1mM, [Cl-]=10mM, [丙酮]=10mM,pH=9.0,= 20℃
HSO
5
-
+ Cl
-
→ SO
4
2
-
+ HOCl (1)
HSO
5
-
+ 2Cl
-
+H
+
→ SO
4
2
-
+ Cl
2
+ H
2
O (2)
HOCl → ClO
-
+ H
+
(3)
HSO
5
-
+ Cl
-

OCl
-
+ H
+
+ SO
4
2
-
(4)
2.2 丙酮/Cl-/PMS体系对AO7的降解机制
为了确定丙酮/Cl-/PMS体系降解AO7的反应机理,向反应体系中加入自由基(SO- 4•、HO•)猝灭剂甲醇(Me)和叔丁醇(TBA)[33]以及HOCl猝灭剂NH4+[34-35],加入Me、TBA浓度均为100mmol/L,NH4+浓度从10增加到100mmol/L.如图2所示,在丙酮/Cl-/PMS体系中加入Me和TBA后,对AO7的降解几乎没有影响,而加入NH4+后,AO7的降解受到明显抑制.当NH4+浓度增加到100mmol/L时, AO7在20min内的降解率从97%下降到19%.说明在丙酮/Cl-/PMS体系中AO7的降解并不是自由基的作用,而是产生的HOCl氧化降解的结果.因此,丙酮对Cl-/PMS体系的促进机理可能是催化作用.
为了进一步证明丙酮对Cl-/PMS体系的催化作用,实验建立了以硫酸耐尔蓝A为探针的荧光光谱法,体系最大激发和最大发射波长分别位于634, 686nm处.实验基于在pH=2.0的缓冲溶液中,ClO-和荧光物质分子硫酸耐尔蓝A借助分子间作用力,在基态时生成不发光的配合物,从而导致荧光强度减弱,ClO-浓度与荧光猝灭程度成正比,通过测量荧光强度的猝灭程度判断ClO-浓度[36-38].实验结果如图3所示,在PMS、Cl-/PMS、丙酮/Cl-/PMS体系中检测到的荧光猝灭强度依次增强,说明与PMS、Cl-/PMS体系相比,丙酮的加入使得反应体系中HOCl的量增加,即丙酮在Cl-/PMS体系中催化Cl-和PMS产生更多HOCl,这与Gallopo等[32]报道的文献结果一致.

图2 叔丁醇、甲醇、NH4+对丙酮/Cl-/PMS体系降解AO7的影响
[AO7]=50μmol/L, [PMS]=1mmol/L, [Cl-]=10mmol/L,[丙酮]=10mmol/L, pH=9.0,= 20℃
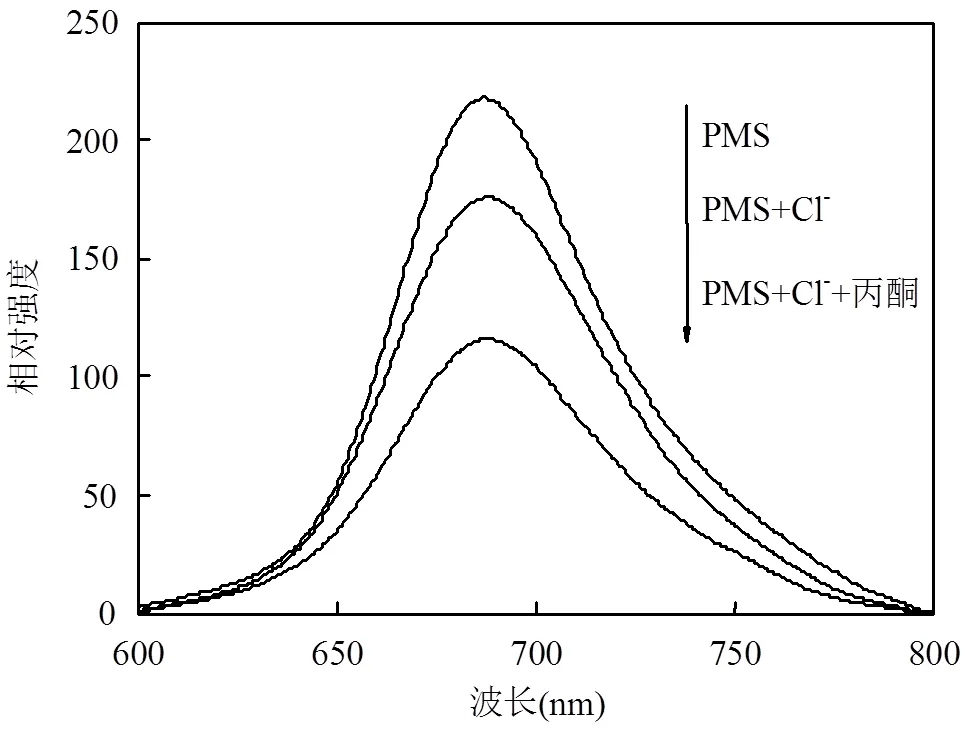
图3 体系的荧光光谱
[AO7]=50μmol/L, [PMS]=1mmol/L, [Cl-]=10mmol/L, [丙酮]=10mmol/L, pH=2.0,= 20℃
2.3 PMS浓度对AO7降解的影响
不同PMS浓度对降解AO7的影响如图4(a)所示,PMS浓度从0增加到0.5mmol/L时,AO7在20min内的降解率增大了88%,当PMS浓度增加到3mmol/L时,AO7的降解率仅10min就达到了97%,如图4(b)所示,通过动力学拟合得出PMS降解AO7符合一级降解动力学,相应的表观速率常数分别为0、0.01304、0.10681、0.22994、0.39969min-1.结果表明,在一定范围内,PMS浓度越高,AO7的降解效率就越快.但当PMS浓度过高时(本实验取PMS浓度为10mmol/L),反应速率没有进一步增大,且与PMS浓度为0.5mmol/L的反应速率相当,相应的AO7的表观速率常数为0.14028min-1.原因可能是在PMS浓度过量的情况下,催化剂和Cl-的含量制约着反应速率,部分PMS不能够被及时活化而发生无效分解.

[AO7]=50μmol/L, [Cl-]=10mmol/L,[丙酮]=10mmol/L,pH=9.0,= 20℃
2.4 丙酮投加量对AO7降解的影响
丙酮投加量对AO7降解效率的影响如图5(a)所示.当投加量分别为0,5,10,20mmol/L时,20min时AO7的降解率分别为41%、73%、96%、100%;继续增大丙酮投加量至100mmol/L,AO7全部脱色仅需要10min.图5(b)显示了,AO7降解的表现速率常数与丙酮投加量的关系.可以看出,随着丙酮投加量的增加,AO7的表现速率常数也在随之增大.这说明丙酮投加量增大,催化Cl-与PMS产生更多的活性氯物质,从而使AO7的降解效率及降解速率均有所升高.

[AO7]=50μmol/L, [PMS]=1mmol/L, [Cl-]=10mmol/L, pH=9.0,= 20℃
2.5 Cl-浓度对AO7降解的影响
保持其他试剂浓度以及反应条件不变,考察不同Cl-浓度对AO7降解效果的影响.如图6(a)所示,从图6可以看出, Cl-浓度对AO7降解效果的趋势与丙酮投加量相似.当Cl-浓度从0增加到100mmol/L时,AO7的降解率从35%增加到100%.图6(b)显示了, Cl-浓度对AO7降解符合一级降解动力学,AO7的表观速率常数分别为0.02095、0.11471、0.18260、0.23335、0.26109、0.75005min-1.由此可知, Cl-浓度越高, Cl-与PMS产生的活性氯物种HOCl越多,丙酮/Cl-/PMS体系氧化降解AO7的速率越快.值得注意的是,当Cl-浓度为0mmol/L时,20min时AO7的降解率为35%,原因可能是丙酮催化PMS分解产生单线态氧分子(1O2)[39-40],对AO7起到一定的氧化降解作用.

[AO7]=50μmol/L, [PMS]=1mmol/L, [丙酮]=10mmol/L, pH=9.0,= 20℃
2.6 初始pH值的影响
pH值是影响PMS活化的重要因素,于是考查了不同初始pH值对丙酮/Cl-/PMS体系氧化降解AO7的影响.通过0.1mol/L的NaOH和稀硫酸调节反应初始pH值,结果如图7所示.从图7可以看出,在初始pH值为5.0、6.0时,AO7几乎不发生降解;当初始pH值为7.0、8.0、9.0时,AO7的降解率分别为42%、46%、100%.由此得出,在pH值为5.0、6.0时的酸性环境下,AO7几乎不脱色,随反应体系初始pH值的升高,染料AO7降解效果逐渐增加. Montgomery[40]曾研究丙酮催化PMS反应的影响,得出在弱碱性环境下,丙酮可以更有效的催化PMS进行一系列的反应.

图7 初始pH值对丙酮/Cl-/PMS体系降解AO7的影响
[AO7]=50μmol/L, [PMS]=1mmol/L, [丙酮]=10mmol/L, pH=9.0,= 20℃
2.7 腐殖酸浓度的影响

图8 HA浓度对丙酮/Cl-/PMS体系降解AO7的影响
[AO7]=50μmol/L, [PMS]=1mmol/L, [丙酮]=10mmol/L, [Cl-]=10mmol/L, pH=9.0,= 20℃
水体中含有大量的有机物,有研究表明自然水体中所含有的溶解性有机物质(DOM)会对水体中污染物的降解有显著影响[41].本实验选取腐殖酸作为研究对象,研究其在丙酮催化Cl-/PMS体系降解AO7中的作用.图8给出了在保持其他试剂浓度及反应条件不变的情况下,不同浓度的腐殖酸(HA)对丙酮/Cl-/PMS体系中AO7降解的影响情况.从图中可以看出,当HA的浓度分别为0,5,10,20mg/L时, 20min时AO7的降解效率分别为100%、96%、95%、94%, AO7并未受到明显的抑制作用,分析原因可能是: (1)AO7与腐殖酸之间作用力很弱,因此不会影响AO7;(2)由于PMS的存在,阻碍了腐殖酸与电子的结合.
2.8 AO7降解过程分析
图9所示为丙酮/Cl-/PMS体系降解AO7过程中紫外可见光谱.从图9可以看出,AO7主要有两处特征吸收峰,分别位于可见光区484nm和紫外光区310nm处,有研究表明[42-44]484nm处对应的是发色基团偶氮键,310nm 处对应的是萘环结构.随着体系反应的进行,AO7在484nm 和310nm处的特征峰强度不断下降,表明AO7的偶氮键和萘环结构不断被氧化断裂,如前文所述,该氧化物质即为HOCl,15min后,偶氮键和萘环的特征峰接近消失.

图9 AO7降解的紫外可见光谱
[AO7]=50μmol/L, [PMS]=1mmol/L, [丙酮]=10mmol/L, [Cl-]=10mmol/L, pH=9.0,= 20℃
3 结论
3.1 在PMS体系中,丙酮或Cl-单独存在,AO7在20min内分别降解了0和38%,而当丙酮和Cl-同时存在,AO7在20min内降解了97%.由此可知,丙酮/Cl-协同活化PMS降解AO7的效果显著,通过荧光光谱法可以证实丙酮催化Cl-/PMS产生更多的HOCl,使AO7脱色.
3.2 体系中的PMS浓度、丙酮浓度、Cl-浓度与AO7的降解率成正相关;初始pH值对AO7的降解有较大的影响,弱碱性如pH=9条件下有利于反应进行;腐殖酸浓度分别为0,5,10,20mg/L时,20min时AO7的降解效率分别为100%、96%、95%、94%.因此,腐殖酸的加入对AO7的降解几乎不产生影响.
3.3 随着体系反应的进行, AO7在484nm和310nm处的特征峰强度不断下降,表明体系中产生的HOCl不断氧化破坏AO7的偶氮键和萘环结构,从而达到降解的目的.
[1] Ji P, Zhang J, Chen F, et al. Study of adsorption and degradation of acid orange 7on the surface of CeO2, under visible light irradiation [J]. Applied Catalysis B Environmental, 2009,85(3):148-154.
[2] Chen K C, Wu J Y, Huang C C, et al. Decolorization of azo dye using PVA-immobilized microorganisms [J]. Journal of Biotechnology, 2003,101(3):241-252.
[3] Xu X R, Li X Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion [J]. Separation & Purification Technology, 2010,72(1):105-111.
[4] Chen J, Zhang L, Huang T, et al. Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: Radical versus non-radical mechanism [J]. Journal of Hazardous Materials, 2016, 320:571-580.
[5] Han D H, Wan J Q, Ma Y W, et al. Enhanced decolorization of Orange G in a Fe(II)-EDDS activated persulfate process by accelerating the regeneration of ferrous iron with hydroxylamine [J]. Chemical Engineering Journal, 2014,256(6):316-323.
[6] Wang M, Liu X, Pan B, et al. Photodegradation of Acid Orange 7in a UV/acetylacetone process [J]. Chemosphere, 2013,93(11):2877-2882.
[7] Qian Y, Zhou X, Zhang Y, et al. Performance of α-methylnaphthalene degradation by dual oxidant of persulfate/calcium peroxide: Implication for ISCO [J]. Chemical Engineering Journal, 2015,279: 538-546.
[8] Anipsitakis G P, Dionysiou D D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt [J]. Environmental Science & Technology, 2003,37(20):4790.
[9] 王 莹,陈家斌,张黎明,等.超声波协同活性碳纤维活化过一硫酸盐降解AO7 [J]. 环境科学学报, 2017,37(4):1404-1412.
[10] Yang S, Yang X, Shao X, et al. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7at ambient temperature [J]. Journal of Hazardous Materials, 2011,186(1):659-666.
[11] Neta P, Huie R E, Ross A B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution [J]. Journal of Physical & Chemical Reference Data, 1988,17(3):1027-1284.
[12] Waldemer R H, Tratnyek P G, And R L J, et al. Oxidation of Chlorinated Ethenes by Heat-Activated Persulfate: Kinetics and Products [J]. Environmental Science & Technology, 2007,41(3): 1010-1015.
[13] Teng Y. Sulfate Radical and its Application in Decontamination Technologies [J]. Critical Reviews in Environmental Science & Technology, 2015,45(16):1756-1800.
[14] Liang C, Su H W. Identification of Sulfate and Hydroxyl Radicals in Thermally Activated Persulfate [J]. Industrial & Engineering Chemistry Research, 2009,48(11):472-475.
[15] Lin Y T, Liang C, Chen J H. Feasibility study of ultraviolet activated persulfate oxidation of phenol [J]. Chemosphere, 2011,82(8):1168- 1172.
[16] Gao Y, Gao N, Deng Y, et al. Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water [J]. Chemical Engineering Journal, 2012,195-196:248-253.
[17] Khataee A R, Pons M N, Zahraa O. Photocatalytic degradation of three azo dyes using immobilized TiO2nanoparticles on glass plates activated by UV light irradiation: influence of dye molecular structure [J]. Journal of Hazardous Materials, 2009,168(1):451-457.
[18] Anipsitakis G P, Dionysiou D D. Radical generation by the interaction of transition metals with common oxidants [J]. Environmental Science & Technology, 2004,38(13):3705-3712.
[19] Liang C, Bruell C J, Marley M C, et al. Persulfate oxidation for in situ remediation of TCE. II. Activated by chelated ferrous ion [J]. Chemosphere, 2004,55(9):1225-1233.
[20] Kusic H, Peternel I, Ukic S, et al. Modeling of iron activated persulfate oxidation treating reactive azo dye in water matrix [J]. Chemical Engineering Journal, 2011,172(1):109-121.
[21] Yang S, Xiao T, Zhang J, et al. Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of Acid Orange 7in aqueous solution [J]. Separation and Purification Technology, 2015,143:19-26.
[22] Wang X, Wang L, Li J, et al. Degradation of Acid Orange 7by persulfate activated with zero valent iron in the presence of ultrasonic irradiation [J]. Separation & Purification Technology, 2014,122(3): 41-46.
[23] Zhao J, Zhang Y, Xie Q, et al. Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zero-valent iron and peroxydisulfate at ambient temperature [J]. Separation & Purification Technology, 2010,71(3):302-307.
[24] Liu C S, Shih K, Sun C X, et al. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. [J]. Science of the Total Environment, 2012,416(507-512):507-512.
[25] Muthukumar M, Selvakumar N. Studies on the effect of inorganic salts on decolouration of acid dye effluents by ozonation [J]. Dyes & Pigments, 2004,62(3):221-228.
[26] Dong Y, Chen J, Li C, et al. Decoloration of three azo dyes in water by photocatalysis of Fe (III)–oxalate complexes/H2O2, in the presence of inorganic salts [J]. Dyes & Pigments, 2007,73(2):261-268.
[27] Wang Z, Feng M, Fang C, et al. Both degradation and AOX accumulation are significantly enhanced in UV/peroxymonosulfate/4- chlorophenol/Cl− system: two sides of the same coin? [J]. Rsc Advances, 2017,7(20):12318-12321.
[28] Yuan R, Ramjaun S N, Wang Z, et al. Effects of chloride ion on degradation of Acid Orange 7by sulfate radical-based advanced oxidation process: implications for formation of chlorinated aromatic compounds [J]. Journal of Hazardous Materials, 2011,196(1):173-179.
[29] 徐 蕾,袁瑞霞,郭耀广,等.氯离子对钴/单过氧硫酸盐体系降解2,4,6-三氯苯酚的影响[J]. 武汉大学学报(理学版), 2013,59(1): 51-56.
[30] Wang Z, Yuan R, Guo Y, et al. Effects of chloride ions on bleaching of azo dyes by Co2+/oxone reagent: kinetic analysis [J]. Journal of Hazardous Materials, 2011,190(1):1083-1087.
[31] 张黎明,陈家斌,房 聪,等.Cl~-对碳纳米管/过一硫酸盐体系降解金橙G的影响[J]. 中国环境科学, 2016,36(12):3591-3600.
[32] Gallopo A R, Edwards J O. Kinetics and mechanism of the oxidation of pyridine by Caro's acid catalyzed by ketones [J]. Chemischer Informationsdienst, 1981,12(37):1684-1688.
[33] Zhou Y, Jiang J, Gao Y, et al. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process [J]. Environmental Science & Technology, 2015,49(21):12941-12950.
[34] Lou X Y, Guo Y G, Xiao D X, et al. Rapid dye degradation with reactive oxidants generated by chloride-induced peroxymonosulfate activation [J]. Environmental Science & Pollution Research, 2013, 20(9):6317-6323.
[35] Deborde M, Von G U. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: a critical review [J]. Water Research, 2008,42(1):13-51.
[36] 段瑞林.荧光光谱法测定次氯酸盐、腺嘌呤和维生素C [D]. 重庆:西南大学, 2016.
[37] Jonnalagadda S B, Parumasur N, Shezi M N. A user-friendly programme 'SIMKINERSQUO; for simulation of kinetics involving complex reaction mechanisms [J]. Computational Biology & Chemistry, 2003,27(2):147-152.
[38] Ensafi A A, Amini M K, Mazloum M. Spectrophotometric Reaction Rate Method for the Determination of Trace Amounts of Vanadium(V) by its Catalytic Effect on the Oxidation of Nile Blue with Bromate [J]. Analytical Letters, 1999,32(9):1927-1937.
[39] Edwards J O, Pater R H, Curclf R, et al. ON The formation and reactivity of dioxirane intermediates in the reaction of peroxoanions with organic substrates [J]. Photochemistry & Photobiology, 2010, 30(1):63-70.
[40] Montgomery R E. Catalysis of peroxymonosulfate reactions by ketones [J]. Journal of the American Chemical Society, 2002,96(25): 7820-7821.
[41] Vione D, Minella M, Maurino V, et al. Indirect photochemistry in sunlit surface waters: photoinduced production of reactive transient species [J]. Chemistry, 2014,20(34):10590.
[42] 王 莹,魏成耀,黄天寅,等.氮掺杂碳纳米管活化过一硫酸盐降解酸性橙AO7 [J]. 中国环境科学, 2017,37(7):2583-2590.
[43] Bauer C, Jacques P, Kalt A. Photooxidation of an azo dye induced by visible light incident on the surface of TiO2[J]. Journal of Photochemistry & Photobiology A Chemistry, 2001,140(1):87-92.
[44] Feng W, Nansheng D, Helin H. Degradation mechanism of azo dye C. I. reactive red 2by iron powder reduction and photooxidation in aqueous solutions [J]. Chemosphere, 2000,41(8):1233-1238.
Acetone and chloride ion Synergistically activate peroxymonosulfate to decolorize acid orange.
ZHANG Ke, XU Fen, CHEN Jia-bin*, WAN Ling, HUANG Tian-yin
(School of Enviromental Science and Engineering, Suzhou University of Science and Technology, Suzhou 215009, China)., 2018,38(11):4159~4165
Acetone and chloride ion (Cl-) were used to synergistically activate peroxymonosulfate (PMS) to decolorize the azo dye, acid orange 7 (AO7). It was found that the first-order rate constant increased from 0.0767 to 0.1725min-1when acetone was added in Cl-/PMS systemIn the acetone/Cl-/PMS system, effect of various factors were explored, including initial pH, Cl-concentration, PMS and acetone dosage. The results indicated that the degradation of AO7 increased with the increase of Cl-、PMS、acetone concentration under alkaline conditions. The degradation mechanism of AO7 in acetone /Cl-/PMS system showed that neither sulfate radical(SO- 4•) nor hydroxyl(HO•) was produced therein. Instead, the HOCl concentration was proved to be increased during the reaction. Hence, acetone could promote the reaction between PMS and Cl-to generate more HOCl. From the analysis of UV-vis spectra, the azo bond and naphthalene ring in AO7 was found being destructed.
chloride ion;peroxymonosulfate;acetone;acid orange 7;hypochlorous acid
X703.1
A
1000-6923(2018)11-4159-07
张 珂(1993-),女,安徽太和人,硕士研究生,主要研究方向为污水处理与回用技术.发表论文1篇.
2018-04-18
国家自然科学基金资助项目(51478283);江苏省研究生实践创新计划项目(SJCX17_0677)
* 责任作者, 讲师, chenjiabincn@163.com

