Screened adamgammadex for reversing rocuronium-induced neuromuscular blockade with greater safety
QI You-mao,QI Ying-bei,YU Bao-chun,LAO Qiao-cong,LI Chun-qi,ZHU Di-feng,JIE Qing
(1.Hangzhou Adamerck Pharmlabs Inc.,Academician Workstation,Hangzhou 310011,China;2.College of Pharmaceutical Sciences,Zhejiang University,Hangzhou 310058,China;3.Hunter Biotechnology Inc.,Hangzhou 310012,China)
Abstract:OBJECTlVE To compare the efficacy and safety of a synthesized candidate adamgam⁃madex with the only marketed selective relaxant binding agent sugammadex.METHODS Forty beagle dogs were divided into 5 groups (8 beagles per group)for efficacy test:normal saline group (normal control),sugammadex 8 mg·kg-1 (positive control)group,and adamgammadex 2,8 and 32 mg·kg-1 groups,respectively.Rocuronium bromide 1.0 g·L-1was given by two separate iv administrations to induce intense neuromuscular blockade (NMB).Beagle dogs received treatment according to groups after the second administration of rocuronium.The time interval from adamgammadex or sugammadex adminis⁃tration to recovery of the fourth twitch to the first twitch of train-of-four(TOF)ratios (T4/T1)to 50%,75%,and 90%was calculated.Zebrafish were divided into 8 groups (30 per group)for sensitizing test by detecting relative levels of tryptase:normal control group (with both zebrafish and substrate),compound 48/80 4 mg·kg-1positive control,sugammadex or adamgammadex 250,500 and 1000 ng per fish groups,respectively.In addition,zebrafish (30 per group)were divided into normal control(treated with ultrapure water),sugammadex or adamgammadex 75,250,or 750 ng per fish groups,respectively.Heart rate (HR)was counted,and a microscope was used to observe bleeding for safety assessment.RESULTS In the beagle dog efficacy test,compared to normal control group,the recovery time of T4/T1 to 50%,75%,and 90%in adamgammadex 2,8 and 32 mg·kg-1groups was decreased (P<0.01),suggesting that adamgammadex had effectively reversed rocuronium-induced NMB.Compared to sugammadex 8 mg·kg-1positive control group,the recovery time of T4/T1to 50%,75%and 90%following adamgammadex 2,8 and 32 mg·kg-1treatment was simillar,suggesting that adamgammadex worked in pretty much the same way as sugammadex in reversing rocuronium-induced NMB.In the zebrafish sensitizing test,compared to sugammadex 250 ng group,the tryptase level in adamgammadex 250 ng group hardly changed,suggesting that there were no significant sensitizing effects.Compared to sugammadex 500 and 1000 ng groups,the tryptase levels in adamgammadex 500 and 1000 ng groups were decreased by 90.6%and 96.4% (P<0.01),indicating significant sensitizing effects and even more serious allergic responses in the higher doses of sugammadex.Zebrafish HR results showed that,compared to normal control group,the HR of sugammadex or adamgammadex 75 ng group was not different or reduced.Compared to normal control,the relative HR of sugammadex 250 ng group decreased by 10% (P<0.01),whereas the HR of adamgammadex 250 ng group stayed stable;the relative HR of sugammadex 750 ng group and adamgammadex decreased by 21%and 11% (P<0.01),respectively.This suggested that adamgammadex had less harmful effect on HR and heart function.Zebrafish bleeding results showed that,compared to normal control,the bleeding events in adamgammadex 75,250 or 750 ng groups were not different,suggesting that the safe level of adamgammadex was same as that of normal control;the bleeding risk of sugammadex 75,250 and 750 ng groups was 0%,0%and 10%,respectively.CONCLUSlON Adamgammadex rapidly and effectively reverses rocuronium-induced NMB.In zebrafish test,adamgammadex shows a similar efficacy to sugammadex but with fewer side effects,suggesting that adamgammadex potentially performs better than sugammadex in safety profile.
Key words:adamgammadex;neuromuscular blockade;sugammadex
Rocuronium bromide (Esmeron®/Zemuron®),a steroidal non-depolarizing neuromuscular blocking agent in modern anesthesia,is widely used to facilitate endotracheal intubation and to provide muscle relaxation during surgery.Cyclodextrinderivative neuromuscular blocking agents (NMBAs)can effectively antagonize the effects induced by muscle relaxants such as rocuronium[1].They encap⁃sulate the relaxants at a ratio of 1∶1 to form tight complexes and block the effects of steroidal muscle relaxants at the neuromuscular junction[1-3].These drugs have demonstrated distinct advantages in preclinical studies by rapidly reversing muscle relax⁃ation and reversing profound muscle relax⁃ation[4-6].The existing cyclodextrin-based selec⁃tive relaxant binding agents (SRBAs)primarily include sugammadex (Bridion®,Merck Sharp&Dohme)and adamgammadex (currently under phaseⅡclinical trial,developed exclusively by Hangzhou Adamerck Pharmlabs Inc.)(Fig.1)[7].
Sugammadex has been marketed for use in more than 80 countries worldwide since its first approval in Europe in 2008[8].It has been recog⁃nized by anesthesiologists proclaiming the drug as a milestone in the field of anesthesiology since its approval in the European market[9].Never⁃theless,sugammadex failed to gain approval from the U.S.FDA in 2008,2013 and 2015,due to major concerns about its possible association with allergic reactions and other side effects[10-11].On Dec.15,2015,FDA finally approved its use in the US,but still warns clinicians of the possibility of a hypersensitivity reaction or anaphylaxis that should be intervened with once it occurs[12].
According to the literature,a drug-induced anaphylactoid reaction is hard to assessin vitroand in conventional animal models such as mice,rats,guinea pigs or beagle dogs[13].Based on the current literature available,the recognized hypersensitivity-related test models for sugam⁃madex by Merck&Co.,Inc.have not successfully shown any hypersensitivity results observed in human beings[14].
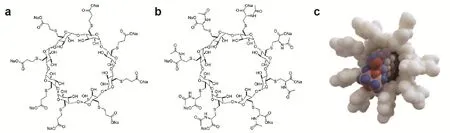
Fig.1 Chemical structures of sugammadex and adamgammadex and chemical encapsulation of rocuronium by adamgammadex.a:chemical structure of sugammadex (C72H104Na8O48S8,CAS No.343306-79-6);b:chemical structure of adam⁃gammadex (C88H128N8Na8O56S8,CAS No.1309580-41-3);c:schematic of an adamgammadex molecule (white)encapsulating a rocuronium molecule (colored).
In the current study,the authors first proposed a new concept that more safety can be achieved by compromising part of potency while maintaining comparable efficacy.With sugammadex as a lead compound,we modified the key structure of its carboxyl groups on their side chains and finally chose a candidate adamgammadex(Aom0498-16 or HS-7)from the numerous resulted compounds.The binding constant of adamgammadex and rocuronium was determined using isothermal titra⁃tion calorimetry,and the results indicate that adamgammadex combines with rocuronium at a 1∶1 molar ratio,suggesting that its mechanism of action is similar to that of sugammadex[15].After confirming the reversalofrocuromiuminduced neuromuscular block by adamgammadex in beagles,the authors first proposed and applied zebrafish (Daniorerio)models to study and evaluate the potential safety of sugammadex and adam⁃gammadex.
1 MATERlALS AND METHODS
1.1 Animal,reagents and equipments
Forty healthy adult beagle dogs of both sexes(Jiaxing Jia′an Laboratory Animal Welfare Co.,Ltd.)were used.Body mass ranged from 9.3 kg to 10.6 kg at the beginning of the test.The beagle dogs were kept under controlled conditions of temperature of 18-26℃,a humidity of 40%-70%,and 12 h light/12 h darkness cycles.Daily temperature difference was no more than 4℃.The cages were ventilated at a constant tempera⁃ture.Animals hadad libitumwater and were fed twice per day.All beagle dogs were sacrificed by overexposure to an anesthetic agent at the end of the test.The test was approved by the Ethics Committee of Laboratory Animal Center,Zhejiang University,China.
Zebrafish embryos were provided by Hunter Biotech,China.Embryos were maintained at 28℃ in fish water (0.2%instant ocean salt in deionized water,pH 6.9-7.2,conductivity 480-510 mS·cm-1and hardness 53.7-71.6 mg·L-1CaCO3).For sensitization and heart rate (HR)change experiments,AB strain zebrafish(a wild type)were used,while the bleeding test involved both AB strain and AL strain zebrafish(albino line whose melanin alleles mutate).All fish in different stages of development were sacrificed by employing overexposure to an anesthetic agent at the end of the test.The test was accred⁃ited by the Association for Assessment and Accredi⁃tation of Laboratory Animal Care International.
All reagents were analytically pure or chemi⁃cally pure.Sugammadex sodium (Bridion®,Merck Sharp&Dohme),adamgammadex sodium (Purity 98%,Hangzhou Adamerck Pharmlabs Inc.),rocuronium injection (N.V.Organon,Oss,Nether⁃lands),0.9%sodium chloride injection (Sichuan Kelun Pharmaceutical CO.,Ltd.),N-benzoyl-dlarginine-4-nitroanilide hydrochloride(BAPNA,911-77-3,Aladdin,as a tryptase-specific substrate),compound 48/80 (C48/80)(120M4040V,Sigma),and ethyl-3-aminobenzoate methanesulfonate(ME SAB,E10521,Sigma).
Equipments included nuclear magnetic reso⁃nance mercury plus-400MHz (Varian,Inc.,USA),Finnigan LCQ-Adavatange mass spectrometer(Thermo Electron,USA),VERTEX 70 FT-IR spectrometer(Bruker,USA),TOF-Watch®SX (N.V.Organon,Netherlands),electronic analytical balance AL104 (Mettler,Switzerland),DWV-ⅡHW constant-temperature animal operating table(Tongchang stainless steel products factory,China),precise micro pump WZS-50F2(Smiths Medical Instrument Co.,Ltd.,China),and animal respirator DW-3000(Huaibei Zhenghua Biological Devices Co.,Ltd.,China),6-and 96-well microplate (Nest Biotech,China),Mithras LB940 multifunction micro⁃plate reader (Berthold Technologies,Germany),SMZ645 stereoscopic microscope (Nikon,Japan),and cell counter(Shenzhen Youte Electronics Co.,Ltd.,China).
1.2 Synthesis of adamgammadex and struc⁃ture confirmation
Adamgammadex was synthesized by using the following procedures[7].Please refer to Fig.2 for the chemical equation for the synthesis.Totally 23.7 g (0.088 mol)N-acetyl-L-cysteine Ⅳ and 160 mL anhydrous DMF was added to a dry flask with three necks and stirred until it was fully dissolved.And 8.81 g sodium hydride (60%)was then slowly added in portions under the protec⁃tion of ammonia when the temperature of the obtained reaction solution in constant tempera⁃ture cold water bath was reduced to approximately-10℃.The mixture was stirred mechanically at<-5℃.The stirring was continued when all of the sodium hydride had been added.When there was no more bubbling,Ⅴ,the sodium salt form ofⅣ,was obtained.With a cold bath controlled at about 5℃,the active intermediate 6-perdeoxy-6-periodo-γ-cyclodextrinⅢ in 8.38 g (3.85 mmol)DMF solution prepared by γ-cyclodextrinⅡwas added into the reaction solutionⅤand mixed for 30 mins under the protection of argon.After that,the reaction solution was first heated gradually to 70℃,then cooled to the room temperature after 12 h,and filtered,where the filter cake was washed twice with DMF and then with acetone to remove triphenylphosphane and triphenylphos⁃phine oxide.After a decompression drying,a crude product was obtained.After dissolution with water,the crude product was refined by a chromatographiccolumn.Then,thefractions were collected,processed by vacuum concentra⁃tion,anddried,adamgammadexwasfinally produced (yield 40%,purity 98% ).For structure confirmation,weperformedelementanalysis(EA)of adamgammadex to determine the composing proportion of C,H,N,Na,O,and S.In electron spray ionization-mass spectrometry (ESI-MS),positive ion source temperature was set to be 250℃with the scanning range from 50 to 3000 amu.In infrared spectroscopy (IR),KBr pellet technique was used with the wave numbers from 4000 cm-1to 400 cm-1.For nuclear magnetic reso⁃nance imaging,1H-NMR,13C-NMR,gCOSY,DEPT135o,gHSQC,and gHMBC were used with D2O as the solvent.
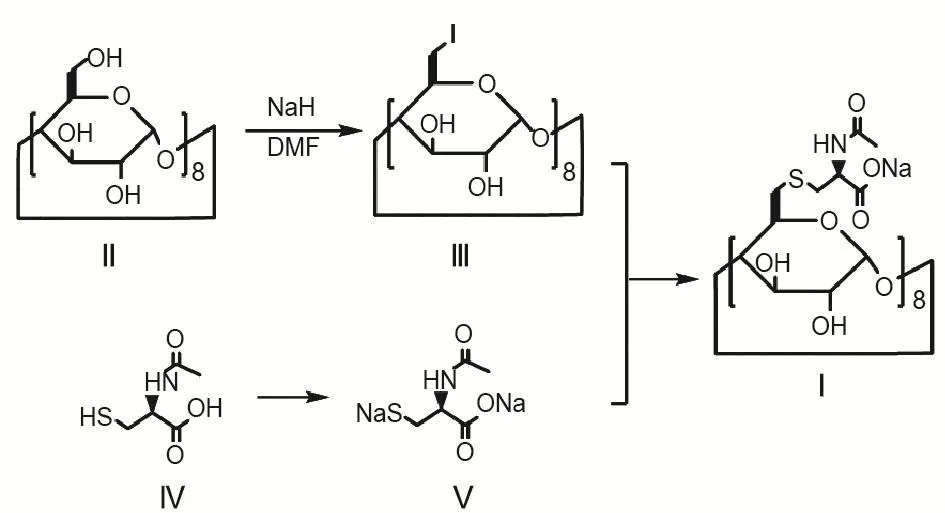
Fig.2 Synthesis of adamgammadex.Ⅲwas obtained by chemical reaction of the primary hydroxyl group of γ-cyclodex⁃trinⅡ.When N-acetyl-L-cysteineⅣ was neutralized with sodium hydrideN-acetyl-L-cysteinedisodium salt,Ⅴwas produced.A crude product was obtained via the direct reaction betweenⅤandⅢ,then washed and refined to obtain the final productⅠ (adamgammadex).
1.3 Efficacy evaluation of adamgammadex by train-of-four(TOF)reading in beagles
TOF-Watch®SX was applied to form 4 consec⁃utive stimuli(one TOF)which evoked twitches at the adductor pollicis muscle.With an increasing degree of NMB induced by NMBAs,all twitches would finally become absent.After the first injection of rocuronium,the first twitch response of TOF(T1)dropped below 20%.The second injection was given and the test drugs were administered immediately,the height of muscle twitch,the T4/T1and the time from administration to T4/T1that reached 0.9 were recorded.The detailed protocol we followed was shown below.
Forty beagle dogs were randomized into five groups (8 beagle dogs per group):normal control group (iv injection normal saline),sugammadex 8 mg·kg-1group,and adamgammadex 2,8 and 32 mg·kg-1groups.After weighing the beagle dogs,anesthesia was induced via intravenously bolus of 3%pentobarbital sodium and a subse⁃quent continuous infusion as needed.The beagle dogs were placed on the constant-temperature(38℃)operational table in a supine position,intu⁃bated endotracheally,and connected to the venti⁃lator(respiratory rate:18 min-1;tidal volume:300 mL).The right jugular vein was separated and intubated for infusion of normal saline (normal control group).It was later used for microinjection of rocuronium when the test started.The left jugular vein was separated for injection of adamgammadex or normal saline.Then,the needle electrodes of TOF-Watch®SX were subcutaneously connected to the two sides of gastrocnemius muscle of the left hind leg.The left hind foot was then placed on the self-controlled air bag table.A skin-tempera⁃ture probe was fixed at the underarm of left foreleg.The temperature under the armpit was kept at no less than 35.5℃.A transducer was fixed on the skin surface of the ankle backend.Continuous stimulation was conducted at 1 Hz.When the twitch height became stable,the continuous stim⁃ulation was maintained for at least 5 min using TOF mode (60 mA,2 Hz,0.2 ms,and 15 s interval).Then,the device was calibrated in the calibration mode and the stimulation was again conducted in a TOF mode.When a stable stimulus response was achieved for at least 5 min,rocuronium solu⁃tion at the concentration of 1.0 g·L-1was iv injected(injection speed:20 mL·h-1)using a micropump and the start time of administration was recorded.When the fourth twitch to the first twitch of train-offour (TOF)ratio (T4/T1)disappeared and T1was reduced below to 20%following the continuous injection of rocuronium,a muscle relaxation model was considered to be successfully established.Rocuronium was then continuously administered 2.5 min later,and the injection was stopped to allow the spontaneous recovery of the neuromuscular function.The stop time of injection was recorded.
Twenty minutes after the full recovery(both T1and T4/T1values were more than 90%),the second iv injection started using the same method as the first injection.Test articles or control solu⁃tions were iv injected according to the grouping at the time the injection stopped.The start and stop time of the second injection of rocuronium and the injection duration of test articles or control solu⁃tions were recorded.With the time of stopping rocuronium as zero,the time taken to reach T4/T150%,75%,and 90%at the first and second injec⁃tions for each animal was obtained.Previous studies found that individuals differ in their sensi⁃tivities to muscle relaxants.As for the recovery time,significant differences exist.Therefore,we decided to evaluate the recovery ratios of the first injection of rocuronium to the second one at 50%,75%,and 90%recoveries,ierepresents the time that T4/T1recovered to 50%after the second injection;tT4/T1-50-1represents the time that T4/T1took to recover to 50%after the first injection).
1.4 Safety evaluation of adamgammadex by tryptase,heart rate and bleeding test in zebrafish
1.4.1 Tryptase measurement
AB strain zebrafish were divided into 8 groups(30 zebrafish per group):adagammadex 250,500 and 1,000 ng per fish,sugammadex 250,500 and 1,000 ng per fish,normal control group(with both zebrafish and substrate),and compound 48/80 positive control (C48/80 4 mg · L-1).After all fish were anesthetized by ME SAB dissolved in water,the corresponding study drugs were microin⁃jected.ME SAB was then washed out.Tryptasespecificsubstrate BAPNA was dissolved into the microplate 1 h after administration.Six zebraf⁃ish were selected randomly from each group to a 96-well microplate.A405nmValues for each group were detected using a multifunctional microplate reader.Increase rate of tryptase (%)=(level of trypt⁃ase in drug treatment group/level of tryptase in normal control group-1)×100%.
1.4.2 Heart rate test
210 zebrafish (30 per group)were adminis⁃treted with sugammadex or adamgammadex in the dose range of 75,250,or 750 ng,respectively.Normal control was treated with ultrapure water.After all fish were anesthetized by ME SAB dissolved in water,the corresponding drugs were microinjected.ME SAB was then washed out using fish water.After 4 h later,10 zebrafish were randomly selected from each group to count heart rate(HR)[16].HRs were measured by a cell counter.
1.4.3 Bleeding test
The grouping and treatments were the same as the HR test.In addition,AL strain zebrafish groups(treated with sugammadex or adamgammadex 750 ng per fish)were set up.After 4 h,Whether there were arterial bleeding and venous congestion occurred,especially heart bleeding and liver conges⁃tion occurred,they were determined by microscop[17].
1.5 Statistical analysis
All statistical analyses were performed using SPSS 16.0 software (SPSS,Chicago USA).For quantitative analysis,all data was presented as.For the beagle dogs test,statistical analysis was performed withStudent′s t-test.For the zebrafish tests,one-way ANOVA followed by theDunnett′stest was used to compare the differ⁃ence between groups.P<0.05 was considered statistically significant.
2 RESULTS
2.1 Spectral data of adamgammadex
The structure of adamgammadex was confirmed by1H-NMR,13C-NMR,ESI-MS,IR,and EA as listed in Tab.1.
2.2 Efficacy of adamgammadex in beagle dogs
As efficacy results showned in Fig.3A,after the first injection of rocuronium,T4/T1decreased sig⁃nificantly.After a period of maintenance when T1 dropped below to 20%,the injection was stopped,T4/T1gradually recovered to the normal range.After the second injection of rocuronium,the test drug was administered immediately.T4/T1rapidly increased to the normal range.The study determined the time taken by T4/T1to recover to 50%,75%,and 90%,and their recovery time ratios of the sec⁃ond injection of rocuronium to the first injectionin a rocuronium-induced NMB beagle dogs model reversed by adamgammadex and sugam⁃madex (Fig.3B).Compared to normal control group,the recovery time of T4/T1to 50%,75%,and 90%of adamgammadex 2,8 and 32 mg·kg-1decreased significantly (P<0.01),suggesting that ad⁃amgammadex effectively reversed rocuronium-in⁃duced NMB.The recovery time of T4/T1to 50%,75%and 90%of adamgammadex 2,8 and 32 mg·kg-1was comparable to that of sugammadex 8 mg·kg-1group (Fig.3C),suggesting adamgammadex performed as well as sugammadex in reversing rocuronium-induced NMB.
As shown in Fig.3B,individuals demonstrate different sensitivities to NMB,making different the recovery time of each animal in the first injection.Because of this,the recovery time ratios of T4/T1between the second injection to the first injection for the same animal(RT4/T1)were further investi⁃gated.Compared to normal control group,the recovery time of RT4/T1to 50%,75%and 90%was significantly decreased in adamgammadex 2,8 and 32 mg·kg-1groups (P<0.01)(Fig.3B).Adam⁃gammadex reached comparable levels in reversing the NMB compared with sugammadex,suggesting adamgammadex successfully reversed rocuroniuminduced NMB.
2.3 Safety of adamgammadex in zebrafish
2.3.1 Sensitizing effect evaluated by tryptase levels
TheA405nmvalue was positively correlated withthe level of tryptase and the value ofA405nmcan be used as a marker for tryptase level.We set up a normal control group which containsed only the zebrafish and the substrate,without the medi⁃cation.The data of other groups were normal⁃ized by that of normal control group.As results shown in Tab.2,after substracting the back⁃ground levelofnormalcontrolgroup,we obtained the following results.The normalized positive control group gave the highest value ofA405nm(P<0.01),suggesting the system was working well.Compared to the sugammadex 250 ng,the tryptase level in adamgammadex 250 ng group was similar,suggesting that there were no signifi⁃cant sensitizing effects in the low dose group.Compared to sugammadex 500 and 1000 ng groups,the tryptase levels in adamgammadex 500 and 1000 ng groups were decreased by 90.6%and 96.4% (P<0.01),suggesting that in the higher dose groups,there were substantial differences in the sensitization side effects,and that adam⁃gammadex groups had significantly lower levels of tryptase.In sum,adamgammadex successfully avoided the sensitization side effects which are commonly seen in sugammadex.
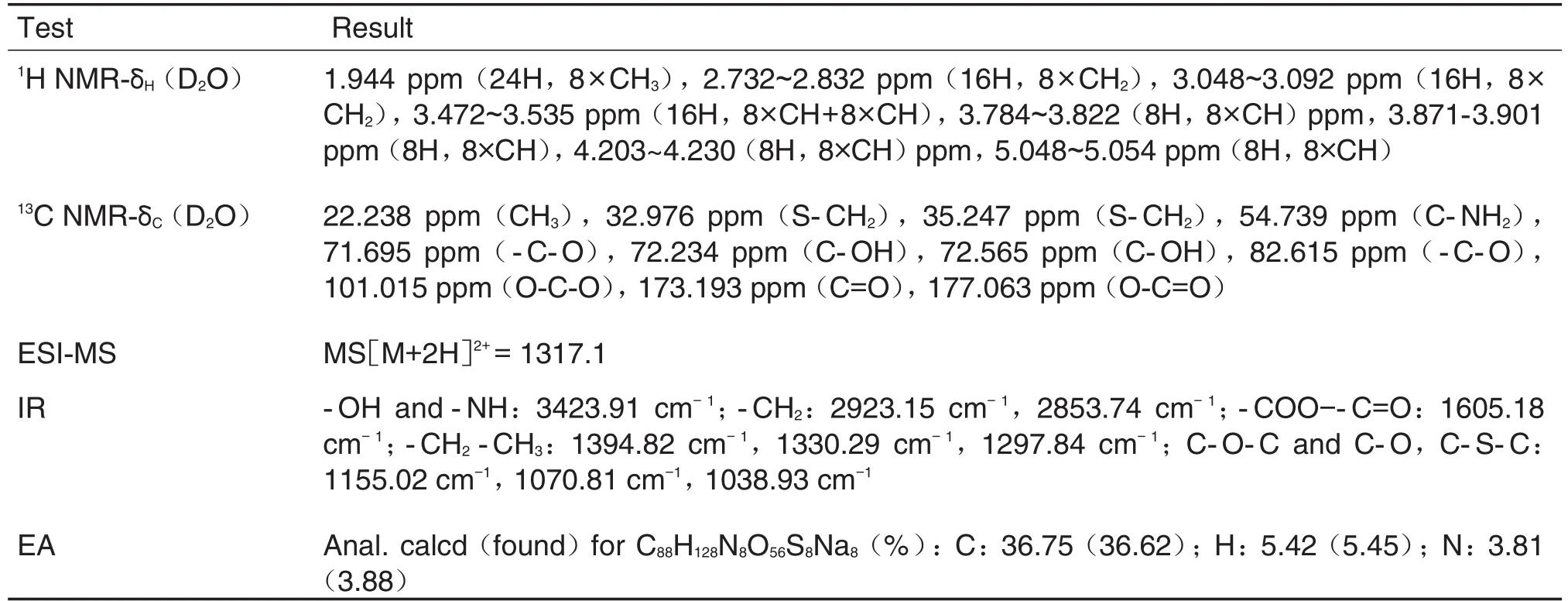
Tab.1 Spectral data of adamgammadex
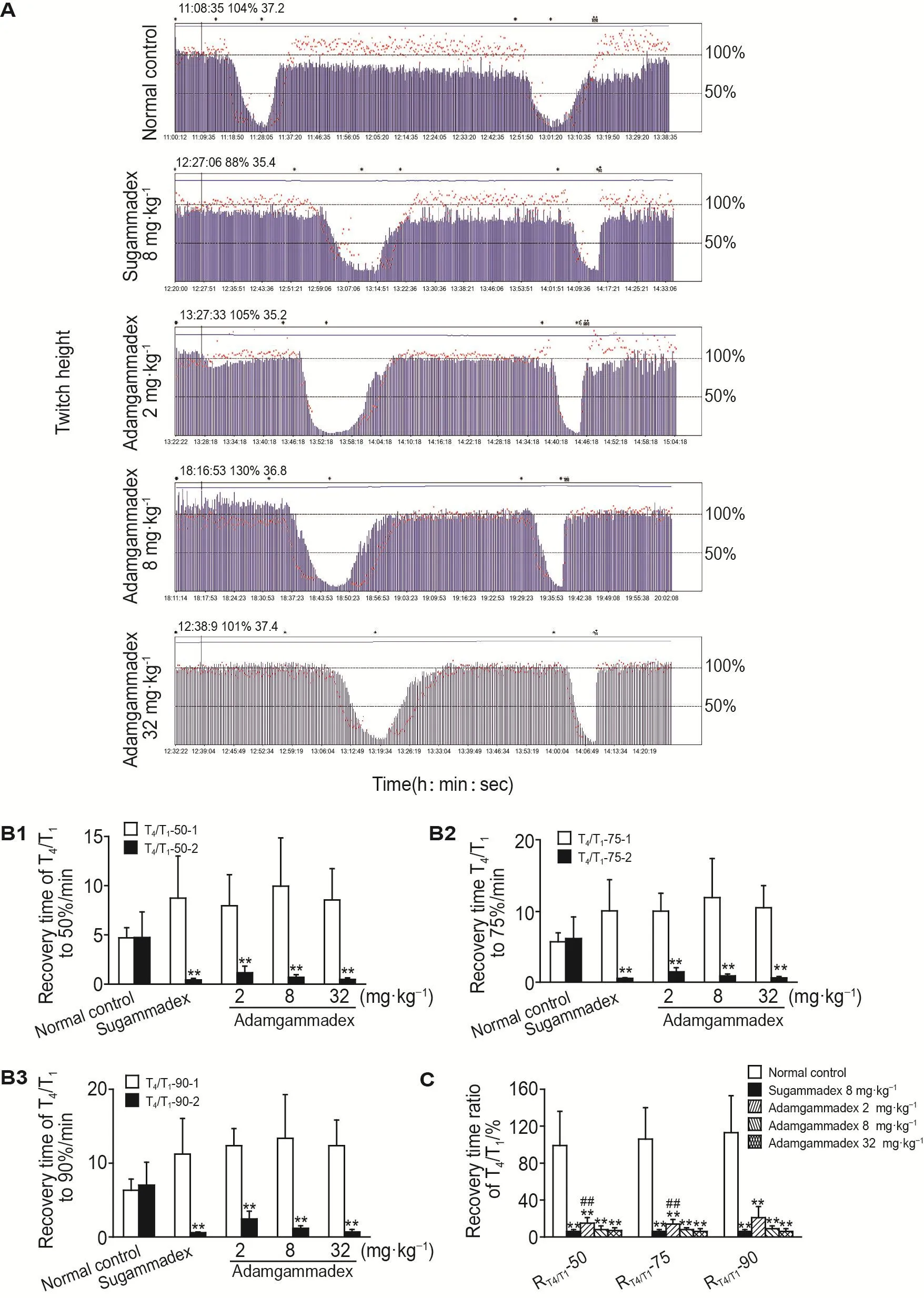
Fig.3 Effect of adamgammadex on reversing rocuronium-induced neuromuscular blockade(NMB)in beagles.Forty beagles were randomly divided into 5 groups(8 beagles per group):normal control(iv injection normal saline),sugammadex 8 mg·kg-1group(positive control),and adamgammadex 2,8 and 32 mg·kg-1groups.Rocuronium 1.0 g·L-1was iv injected(injection speed:20 mL·h-1)using a micropump to establish the muscle relaxation model.After the first injection of rocuronium,the first twitch response of TOF(T1)decreased to below 20%.The second injection was given and the test drugs were administered immediately,the height of muscle twitch,the T4/T1and the time from administration to T4/T1reached 50%,75%,and 90%were recorded.A:typical NMB monitoring results.B1-B3:recovery time of the fourth twitch to the first twitch of train-of-four(T4/T1)reaching to 50%(T4/T1-50-1,T4/T1-50-2),75%(T4/T1-75-1,T4/T1-75-2),and 90%(T4/T1-90-1,T4/T1-90-2).,n=8.**P<0.01,compared with corresponding the first admin⁃istration;C:ratio of T4/T1of the second injection of rocuronium/the first injection of rocuronium(RT4/T1)in two cycles of rocuronium administra⁃tion.,n=8.**P<0.01,compared with normal control group;##P<0.01,compared with sugammadex 8 mg·kg-1group.
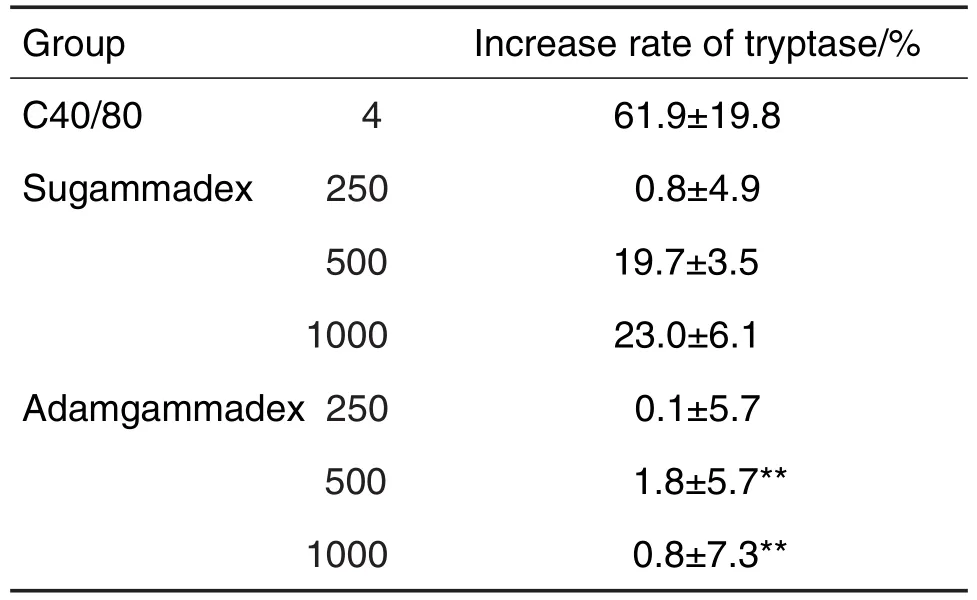
Tab.2 Relative level of tryptase of zebrafish
2.3.2 Heart rate of zebrafish
Compared to normal control group,the HR of both sugammadex 75 ng and adamgammadex 75 ng groups was not different and no reduction occurred in HR.Compared to the control,the rela⁃tive HR of sugammadex 250 ng group decrease by 10% (P<0.01),whereas the HR of adamgam⁃madex 250 ng group stayed stable,compared to the control,the HR of sugammadex 750 ng and adamgammadex 750 ng group decreases by 21%and 11% (P<0.01),respectively (Fig.4),suggesting that adamgammadex had minimal and negligible detrimental effect on HR and heart function.
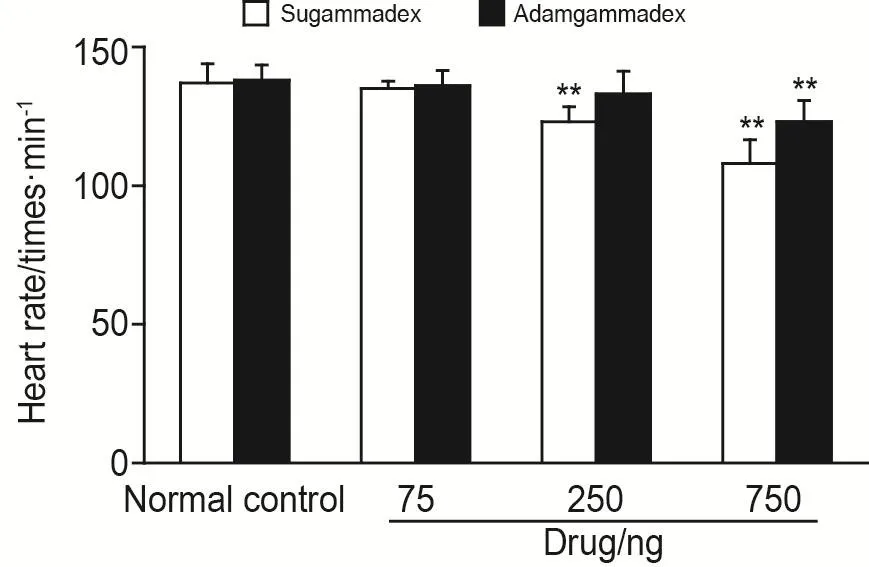
Fig.4 Effects of cyclodextrin-derived selective relaxant binding agents on heart rate (HR)of zebrafish.Thirty zebrafish in each group were microinjected with sugammadex or adamgammadex 75,250 or 750 ng per fish,respectively.Normal control group was microinjected with ultrapure water.4 h later,ten zebrafish were randomly selected from each group to count HR.,n=10.**P<0.01,compared with normal control group.
2.3.3 Bleeding tendency of zebrafish
Compared to the control group,the bleeding events in adamgammadex 75,250 or 750 ng group were not different.The bleeding risk of sugammadex 75,250 or 750 ng group was 0%(0/30),0% (0/30)and 10% (3/30),respectively(Fig.5).Mild venous congestion in sugammadex 250 ng group and apparent pericardial edema and venous congestion in sugammadex 750 ng group were observed.All this suggested a moderate bleeding tendency in sugammadex,but adam⁃gammadex groups had no bleeding risk.Active bleeding was irregular,bright in color,whereas passive venous congestion was vessel-shaped,dark in color.This was due to the different mech⁃anism and different SPO2.
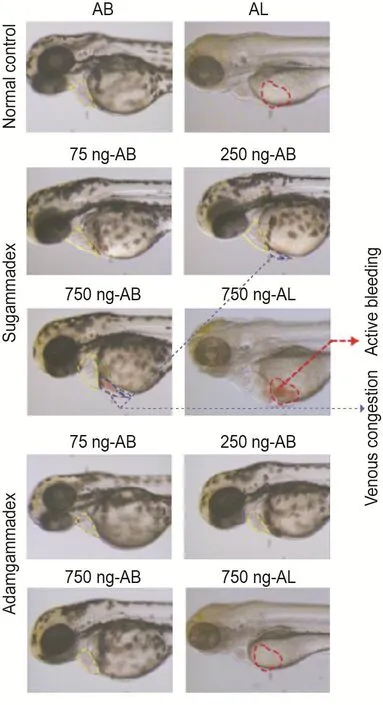
Fig.5 Effect of adamgammadex on venous conges⁃tion and bleeding in zebrafish.See Fig.4 for zebrafish treatment.In addition,AL strain zebrafish groups(normal control,sugammadex or adamgammadex 750 ng per fish)were set up.The areas surrounded by yellow,green,red,and blue dotted line represent the heart,liver,bleeding area and venous con⁃gestion,respectively.
3 DlSCUSSlON
We demonstratedthatadamgammadex effectively reversed rocuronium-induced NMB in a beagle model.The study results show that the NMB induced by rocuronium can be remarkably reversed by adamgammadex.Adamgammadex and sugammadex share the same nucleus in structure with the only difference in their side chains.The introduction of a chiral acetylamino group to the α-carbon next to carboxylic acid of each side chain of adamgammadex may influ⁃ence the effect of adamgammadex to some extent.This explanation is consistent with the reversing effect results that adamgammadex has a lower potency than sugammadex at the dose of 8 mg·kg-1,but it shows the same reversing effect in adam⁃gammadex 32 mg·kg-1as sugammadex 8 mg·kg-1.
At present,the commonly observed side effects of sugammadex in clinical trials and clinical settings include anaphylaxis[18-20],bleeding,arrhythmia,etc,but they were not identified earlier as stated in the introduction part.Therefore,a big chal⁃lenge falling a pharmacologist is that the poten⁃tial allergic responses induced by drugs are not effectively predicted during preclinical studies.It is very important to establish an effective test model in order to avoid the risks a new drug may pose to humans.Zebrafish,a vertebrate model organism with 87%genetic homology and similar early development with humans,are an optimal model organism for use in biomedical studies[31].It has been frequently used to predict the efficacy and safety of drugs.Yanget al[13]successfully found the sensitization of Tween-80 using zebrafish models.Numerousin vivostudies have confirmed substantial similarities in the toxicity profiles exhibited between mammals and zebrafish[21-23].Moreover,a zebrafish larvae model requires a smaller drug dose and can easily be administreed with drugs of interest[13,28].That is the reason why the zebrafish appeared recently as a prophase screening model for the worldwide new drug development.To test the side effects of cyclodextrin-based SRBAs,the authors proposed and applied the zebrafish vertebrate model.
For dosage design,the incidence of allergic reactions among 12 subjects on sugammadex in the high dose groups (8 to 32 mg · kg-1)was 66.7%in total incidence of allergic reactions according to the earlier clinical trials reported in a Japanese review report[14].It is clear that allergic reactions induced by sugammadex are frequent at these dose levels.As we know,the main culprit in allergic reactions is the mast cells (MCs).The level of activation of MCs,is associated with the extent of allergic reactions.Tryptase,the most abundant neutral protease pre-synthesized and stored in MCs,is released by MCs upon degran⁃ulation.This has been established and used as a marker for MCs activation and degranulation due to its localization,storage,and expression in MCs[29-30].Zebrafish MCs have similar structure and functions to mammals,and are involved in the body′s immune response and allergic reaction[16].Therefore,we use tryptase levels in zebrafish as a biomarker of anaphylactoid reactions and dosages of sugammadex or adamgammadex administration to zebrafish groups.This corresponds to the dose range in which a high incidence of allergic reactions induced by sugammadex occurs.The results indicate that the zebrafish model can obvi⁃ously detect the sensitization of sugammadex.
Tab.2 illustrates a high increase in the tryptase level,showing substantial sensitizing effect.This result is consistent with other reports of sugam⁃madex[28-30].More serious allergic reactions in higher doses indicate a dose-dependent manner.This is consistent with the anaphylaxis observed in the clinical trials of sugammadex[28-30].In contrast,the increase in tryptase levels from adamgammadex 250 to 1000 ng has no significant difference with the normal control,indicating no sensitizing effects on zebrafish.From the perspective of chemical structure (Fig.1),we rationalized its mechanisms of anaphylaxis.Relevant studies of penicillin anaphy⁃laxis and cross-reactivity have shown that the betalactam carboxyl group on the side chains of peni⁃cillins is one of the antigenic determinants[31],and side chains that are similar in structure may result in allergic cross-reactions between different peni⁃cillins[31].Sugammadex molecules have a large number of such carboxyl groups which are more likely to react with some tissues or substances such as intrinsic proteins in the body.This may introduce higher risks of allergy to sugammadex and even produce other clinical side effects.However,the chiral acetylamino introduced to the alpha carbon of carboxylic acid of adamgam⁃madex adds 20%of more chiral carbon atoms to the nucleus of γ-cyclodextrin in order to rein⁃force the chiral environment.It also increases the steric hindrance of the carboxyl groups,shielding or restricting part of their effects.Accord⁃ingly,the carboxyl groups have a lower binding rate with the guest molecules and also a lower chance of contacting any body tissues or substances such as proteins,thereby reducing the risk of anaphylaxis induced by adamgammadex.
Moreover,other side effects that may be induced by cyclodextrin-derived SRBAs are on HR(including ventricular fibrillation,ventricular tachycardia,cardiac arrest,and bradycardia)and on the prolongation of activated partial throm⁃boplastin time or prothrombin time[12].These side effects can also be significantly demonstrated in zebrafish.From Fig.4and5,the dose that prompts adamgammadex to induce such side effects is significantly higher than for sugammadex.For instance,the slower HR that was observed in the 250 ng sugammadex group just started to show up in 750 ng adamgammadex group.Venous congestion was found in the doses of 250 ng and 750 ng sugammadex,but none was observed at the same adamgammadex doses.Therefore,a conclusion can be drawn that adamgammadex is significantly superior to sugammadex in safety profiles.
In summary,adamgammadex achieved the same efficacy with sugammadex in reversing rocuronium-induced NMB.The potential side effects such as sensitization,bleeding and HR change of cyclodextrin-derivative SRBAs were quantita⁃tively assessed by means of a zebrafish model in this study.These side effects were all demon⁃strated by sugammadex,whereas adamgammadex,with its efficacy proved in the beagle model and fewer side effects,suggesting that adamgammadex,a newly developed cyclodextrin-derived selective relaxant binding agent,has an optimized pros⁃pect in safety profiles.
Moreover,the results also show that zebrafish tests may be a new way to predict the potential side effects that may occur following administra⁃tion of these drugs.Zebrafish tests are a new and beneficial exploration in drug safety studies,especially for brand-new drugs.
The present study promises an excellent start for our drug development.Up to now,adam⁃gammadex has started clinical trials and the prelimi⁃nary results showed little side effects.Further studies in animal models are required to better characterize the safety profile of the drug,such as more studies of effects on QTc intervals,and further confirmation of bleeding risks in rodents.

