Recent Advances of Intrinsically Conductive Polymers
OUYANG Jianyong
Department of Materials Science and Engineering, National University of Singapore, Singapore 117576.
Abstract: Intrinsically conductive polymers are a class of exciting materials since they combine the advantages of both metals and plastics. But their application is limited due to the issues related to their electronic properties,stability and processibility. For example, although polyacetylene can have electrical conductivity comparable to metals, it degrades fast in air. Most of the conductive polymers in the conductive state, such as polypyrrole and polythiophene, cannot be dispersed in any solvent and cannot be turned to a melt. It is thus difficult to process them into thin films with good quality, while thin films with good quality are important for many applications. In terms of the materials processing, polyaniline (PANi) and poly(3,4-ethylenedioxythiophene)(PEDOT) have gained great attention. PANi doped with some large cations can be dispersed in some toxic organic solvents,and poly(3,4-ethylenedioxythiophene):polystyrenesulfonate (PEDOT:PSS) can be dispersed in water and some polar organic solvents. But the PANi and PEDOT:PSS films prepared from their solutions are usually low. Recently, great progress was made in improving the properties of intrinsically conductive polymers. The conductivity of PEDOT:PSS can be enhanced from 10-1 S·cm-1 to > 4000 S·cm-1 through the so-called “secondary doping”. The high conductivity together with the solution processibility enables the application of conductive polymers in many areas, such as electrodes and thermoelectric conversion. In addition, due to their electrochemical activity, conductive polymers or their composites with inorganic materials can have high capacity of charge storage. Conductive polymers can also be added into the electrodes of batteries, because they can facilitate the charge transport and alleviate the large volume change problem of silicon electrode of batteries. It has been demonstrated that conductive polymers can have important application in many areas,such as transparent electrode, stretchable electrode, neural interfaces, thermoelectric conversion and energy storage system. This article provides a brief review on the enhancement of the electrical conductivity of intrinsically conductive polymers and their application as electrodes and in thermoelectric conversion, supercapacitors and batteries.
Key Words: Conductive polymer; Transparent electrode; Neural interface; Thermoelectric; Energy storage
1 Introduction
Intrinsically conductive polymers have conjugated backbone in oxidized or reduced state. Fig. 1 presents the chemical structures of some conductive polymers. Since the discovery of conductive polyacetylene by doping, great progress has been made in intrinsically conductive polymers. When a conjugated polymer is in neutral state, it has conductivity in the insulator range. The conductivity can increase by even more than 10 orders in magnitude after doping. The doping induces charge carriers in the conjugated polymers. The charge carriers are solitons for polyacetylene, and they are polarons and bipolarons for other conductive polymers. The different charge carriers arise from the symmetry of the conjugated polymer chains. The symmetry of polyacetylene is much higher than other conjugated polymer chains.
In principle, intrinsically conductive polymers can have many unique applications because they combine the merits of both polymers and metals1. They are synthetic polymers made of common elements including C, S, N, O and H. They have low density and high mechanical properties like other plastics, while they are conductive like metals. In addition, the electronic structure and properties of conductive polymers can be tuned by controlling the synthesis conditions, the doping species and doping level and modifying the chemical structure of the conjugated polymer chains2–4. Moreover, they have interesting electrochemical behavior. They can be oxidized or reduced by applying an electrochemical potential5–7. This will not only affect the electrical conductivity but also the optical properties.Therefore, many applications have been reported for intrinsically conductive polymers, such as electrical wires of circuits, electrode of electronic devices, electrostatic coating,electromagnetic shielding, electrode of secondary batteries,electrochromic windows, actuators, sensors, and so on.
However, the practical application of intrinsically conductive polymers is still very limited. The hurdles for the applications include mainly their stability, conductivity and processibility.Polyacetylene can have extremely high conductivity because of its linear chain structure, and its conductivity can be further increased by mechanical stretching8. The conductivity can be up to 105S⋅cm-1, comparable to that of highly conductive metals like Cu and Au. But polyacetylene has a severe problem of poor stability. It degrades rapidly in air as a result of the oxidation by oxygen. On the other hand, conductive polymers with heteroaromatic rings like polypyrrole (PPy), polyaniline (PANi), polythiophene and their derivatives are quite stable in ambient conditions.
Processibality is another big challenge for intrinsically conductive polymers. Because of the rigid conjugated backbone and the strong Coulombic attractions between the polymer cations (or anions) and counter anions (or cations), most of the conductive polymers in conductive state are insoluble and intractable. They degrade at temperature below their melting point. Thus, the conventional processing techniques for plastics,including coating, printing, extrusion and melt spinning, are inapplicable for most of the conductive polymers.
PANi is the first conductive polymer that can be dispersed in a solvent when in conductive state. PANi doped with bulky organic anion like d,l-camphorsulfonic acid (CSA) or dodecyl benzene sulfonic acid (DBSA) can be dispersed in organic solvents like m-cresol and chloroform9,10. The bulky organic anions can effectively lower the Coulombic attraction with the positively charged PANi chains and allow the penetration of solvent molecules into the polymer chains. The interchain interactions among the conjugated polymer chains can be lowered by attaching side chains. When the side chains are anions, the conductive polymers can be doped by the side chain anions11,12. PANi derivatives with self-doping can be dispersed in water or organic solvents as well. But their conductivity is usually much lower than PANi because the side chains inhibit the charge transport across the conjugated polymer chains.

Fig. 1 Chemical structures of some conductive polymers,polyacetylene (PA), polypyrrole (PPy), polyaniline (PANi),polythiophene (PTh) and poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS).
When polymer anions are used as the counter anions of a conductive polymer, the polymer anions can stabilize the conjugated polymer cations in water and polar organic solvent.Such a polymer is a polyelectrolyte. As revealed for polyelectrolytes, the dispersion and stability depends on the ratio of the polymer cations to the polymer anions13,14. Poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) is a polyelectrolyte, and it can be dispersed in water and some polar organic solvents. The dispersion arises from the stabilization of the hydrophobic PEDOT chains by the surfactant PSS anions.This solution processibility together with other merits like high transparency in the visible range and good thermal stability make PEDOT:PSS the most successful conductive polymer today.
The recent research in intrinsically conductive polymers advanced on the improvement of their properties and investigation of their application in various areas, such as optoelectronic devices, neural interfaces, energy conversion and storage devices. These will be reviewed in this article. The author feels sorry to some labs that some of their important achievements may not be included in this article since this is not a comprehensive review article.
2 Conductive polymers with high conductivity
Conductivity is an important parameter for the application of conductive polymers. Two methods were reported to prepare conductive polymer films on substrate. One is to the in-situ polymerization of precursors on substrate. Another is to coat solution of conductive polymer on substrate. The morphology and conductivity of conductive polymer depends on the synthesis conditions. The conductivity of some conductive polymers can be enhanced through a treatment, the so-called“secondary doping”.
Polymer films of PEDOT doped with small counter anions like tosylate (Tso) and trifluoromethanesulfonate (OTf) can be deposited by in-situ polymerization of the monomer, 3,4-ethylenedioxythiophene (EDOT), in solution or vapor phase15–17.The polymerization rate significantly affects the crystallinity and thus the conductivity of the polymer films. Recently, Gueye et al.18prepared PEDOT:OTf films by using iron(III) trifluoromethanesulfonate as the oxidant and a triblock copolymer, PEGPPG-PEG, as the polymerization rate controller in ethanol. N-methyl-2-pyrrolidone (NMP) that was used for the secondary doping of PEDOT:PSS was added into the precursor solution.The addition of NMP can increase the conductivity of PEDOT:OTf. The conductivity is around 1000 S⋅cm-1for the PEDOT:OTf prepared from the solution without NMP, and it increases to 3600 S⋅cm-1when 8% (mass fraction) NMP is added into the precursor solution. A post treatment with sulfuric acid can further enhance the conductivity up to 5400 S⋅cm-1.
The conductivity of some conductive polymers particularly those dispersible in water or organic solvents can be enhanced by “secondary doping”. The first secondary doping was reported on PANi doped with d,l-camphorsulfonic acid (CSA)9. When the PANi:CSA films are cast from its chloroform solution, the conductivity is 10-1S⋅cm-119. The conductivity can be up to 400 S·cm-1when the polymer films are casted from its m-cresol solution. The secondary doping of PANi is attributed to the solvent effect on the conformation of the PANi chains. The polymer chains have a coil conformation in chloroform, while it changes to expanded coil conformation in m-cresol.
Secondary doping methods were reported on PEDOT:PSS as well. Kim et al.20reported the conductivity enhancement of PEDOT:PSS by adding a polar organic solvent into PEDOT:PSS aqueous solution. The conductivity can be enhanced by two orders in magnitude when dimethyl sulfoxide (DMSO) is added,while the conductivity enhancement is only about one order in magnitude when dimethylformamide (DMF) is used.Conductivity enhancement similar to DMSO was found when other polar organic chemicals like ethylene glycol (EG), glycerol and sorbitol were added21-25. Significant conductivity enhancement was also reported when an anionic surfactant or ionic liquid was added into PEDOT:PSS aqueous solution26-28.Apart from the addition of a chemical into PEDOT:PSS aqueous solution, the conductivity can be enhanced by post treatment29-38.In the post treatment, a solvent or solution is dropped onto a PEDOT:PSS film. The conductivity can be significantly enhanced through a post treatment with an organic solvent like DMSO, EG and methanol, cosolvent of water and organic solvent, aqueous or organics solutions of inorganic or organic salts, and acids.
The highest conductivity was observed for PEDOT:PSS through a post treatment with H2SO434. As shown in Fig. 2, the conductivity can be enhanced to higher than 2400 S⋅cm-1when a PEDOT:PSS film is treated with 1.5 mol·L-1H2SO4.Repeating the treatment for 3 times can give rise to a conductivity of ~3100 S⋅cm-1. This conductivity is higher than that of indium tin oxide (ITO) on plastic and comparable to that of ITO on glass. ITO is the most popular material for the transparent electrode of optoelectronic devices. As PEDOT:PSS has high transparency in the visible range, the highly conductive PEDOT:PSS can have important application to replace ITO as the transparent electrode of optoelectronic devices. In addition,the highly conductive PEDOT:PSS exhibits metallic or semimetallic behavior at temperature higher than 250 K. As shown in Fig. 2b, the temperature dependence of the resistance follows the variable-range hopping (VRH) model at temperature below 220 K. The resistance is insensitive to the temperature when the temperature is higher than 250 K. This metallic or semi-metallic behavior indicates the strong interaction among the PEDOT chains. The conductivity can reach ~4400 S⋅cm-1when the PEDOT:PSS films were treated with fumed H2SO438.
Apart from PEDOT:PSS, acid treatment can enhance the conductivity of PEDOT doped with small anions as well17,39.For example, Massonnet et al. found the conductivity of PEDOT:OTf films can be enhanced from ~1200 to ~2270 S⋅cm-1after an acid treatment.

Fig. 2 (a) Conductivities of PEDOT:PSS films after treated with H2SO4 solutions of different concentrations.(b) Analysis of resistance-temperature relationship of a H2SO4-treated PEDOT:PSS film with the VRH model.Reproduced with permission from Ref. 34. Copyright 2012 John Wiley & Sons.
3 Application of conductive polymers as electrode
3.1 Transparent electrode of optoelectronic devices
Intrinsically conductive polymer films can be used as the transparent electrode of optoelectronic devices, such as lightemitting diodes (LEDs), solar cells, and detectors.Optoelectronic devices are important in many areas. They require at least one electrode to be transparent to emit or harvest light. ITO is the conventional material for the transparent electrode. But ITO has problems of scarce indium on earth, high cost and poor mechanical flexibility. The cost of the ITO transparent electrode is much higher than the active materials for many optoelectronic devices. Therefore, novel transparent electrode materials are urgently needed to substitute ITO.Conductive polymers, metal nanowires, graphene and carbon nanotubes have been studied as transparent electrode.
Among the candidates, conductive polymers have the advantage of low cost and simple processing techniques.PEDOT and its derivatives have potential as transparent electrode because of their high transparency in the visible range.PEDOT:PSS have been extensively investigated as the transparent electrode of organic light-emitting diodes (OLEDs)40–43,organic solar cells44–47, perovskite solar cells48–50and electrochromic devices51.
Our lab was the first to report ITO-free perovskite solar cells in 2015. PEDOT:PSS films treated with methanesulfonic acid(MSA) were investigated as the transparent electrode (Fig. 3)48.The devices with the MSA-treated PEDOT:PSS on glass can exhibit a power conversion efficient of 10.6%. When a MSA-treated PEDOT:PSS film on PET was used as the transparent electrode, the perovskite solar cells are mechanically flexible.The flexible perovskite solar cells can exhibit a power conversion efficiency of 8.1%. These values are high in the year of 2015.
3.2 Stretchable electrode

Fig. 3 (a) Photo of a flexible perovskite solar cell with MSA-treated PEDOT:PSS/PET as the transparent electrode.(b) Current density-voltage characteristics of perovskite solar cells with ITO/glass, MSA-treated PEDOT:PSS/glass and MSA-treated PEDOT:PSS/PET as the transparent electrode.Reproduced with permission from Ref. 48. Copyright 2015 American Chemical Society.
Stretchable electrodes are needed in electronic devices and electromechanical systems, such as wearable sensors, wearable circuits and electromechanical soft robotics. However,conductive materials including metals, intrinsically conductive polymers, graphene and carbon nanotubes are not stretchable,while stretchable materials like elastomers are not conductive.Thus, stretchable conductive electrodes reported in literature have serpentine or wavy shape52. Elastomic composites of nanomaterials including graphene, carbon nanotubes, metal particles and metal nanowires can be conductive and stretchable.The concern for these nanocomposites is the toxicity of the nanomaterials.
Conductive polymers have rigid conjugated backbone. Their elasticity is thus less than 5%. Recently, several methods were reported to make stretchable conductive polymers. It was found that PEDOT:PSS is miscible with some soft polymers like waterdispersible polyurethane (WPU)53–55. The PEDOT:PSS/WPU can be elastic and have a strain up to 30%. Their conductivity can be up to 100 S⋅cm-1. WPU is an elastomer, and it is biocompatible. Therefore, PEDOT:PSS/WPU can be stretchable electrode that is particularly important for wearable electronics or bioelectronics systems. Similarly, the stretchability can be increased by attaching soft side chains to the conjugated backbone of conductive polymers56.
An interesting work was recently reported by Prof. Bao’s lab at Stanford University57. Instead of using elastomer, they found that the addition of ionic liquids can dramatically increase the elasticity. The chemical structures of some ionic liquids are shown in Fig. 4. The polymer films can be stretched up to 170%,and the conductivity can be higher than 1000 S⋅cm-1.
3.3 Electronic textiles
Wearable electronic devices can be used for convenient and long-term health and body movement monitoring. It is convenient to use textile as the electrical wires for the electronic devices. There are mainly two major techniques to coat conductive polymers onto textile yarns, solution coating and chemical solution/vapor polymerization.
The solution coating techniques include screen printing, dip coating, ink jet printing, and so on. Sinha et al.58prepared electronic textiles by screen-printing PEDOT:PSS on commercially finished textiles. They used it for electrocardiography (ECG) as PEDOT:PSS is both ionically and electronically conductive. Ding et al.59prepared polyurethane (PU)fibers via electrospinning and then converted them to be conductive by dip coating PEDOT:PSS (Fig. 5). The conductive fabric can have a sheet resistance of 35–240 Ω·□-1. Ryan et al.60prepared conductive yarns by dyeing silk with PEDOT:PSS. The yarns have a Young’s modulus of ~2 GPa and an electrical conductivity of ~14 S⋅cm-1. They have good wearing and washing stability, and the conductivity is not affected by bending or mechanical wear. The yarns can withstand both machine washing and dry cleaning. Guo et al.61fabricated textiles by inkjet printing PEDOT:PSS onto nonwoven polyethylene terephthalate (PET) fabric. A post treatment with ethylene glycol(EG) can enhance the conductivity of PEDOT:PSS fibres62.Abbasi et al.63coated PPy on E-glass by vapor polymerization of pyrrole with FeCl3as the oxidizing agent.
3.4 Neural interface
Bioelectronic devices, such as cochlear implants, retinal prostheses, pacemakers and deep brain stimulators can detect electrical signals like voltage, current or impedance from biological systems or apply electrical stimulus to biological systems. They can be used to cure diseases like Parkinson’s disease, depression, deaf and blind. An electrode of the bioelectronic devices is put in contact with a nerve. The neural interface has significant effect on the performance of the bioelectronic devices. The electronic devices and wires are made of hard materials like metals and inorganic semiconductors,while biological tissues like neural tissue and muscle tissue are soft. In addition, metals and inorganic semiconductors are conductive due to the electron transport, while the charge conduction of biological tissue arises from the ionic transport.Conductive polymers can be perfect materials for the neural interface because they are soft and both electronically and ionically conductive. As shown in Fig. 6, regenerative peripheral nerve interface (RPNI) using conductive polymers can be more reliable interface between living neural or muscle tissue and metal electrode64.

Fig. 4 (a) Stress-strain curves of free-standing PEDOT:PSS/STEC films. STEC is for stretchable and electrical conductivity enhancer.(b) Variation of the conductivity with strain for PEDOT:PSS with different STECs. (c) The chemical structures of STEC1, STEC2 and STEC3.Reproduced with permission from Ref. 57. Copyright 2017 American Association for the Advancement of Science.
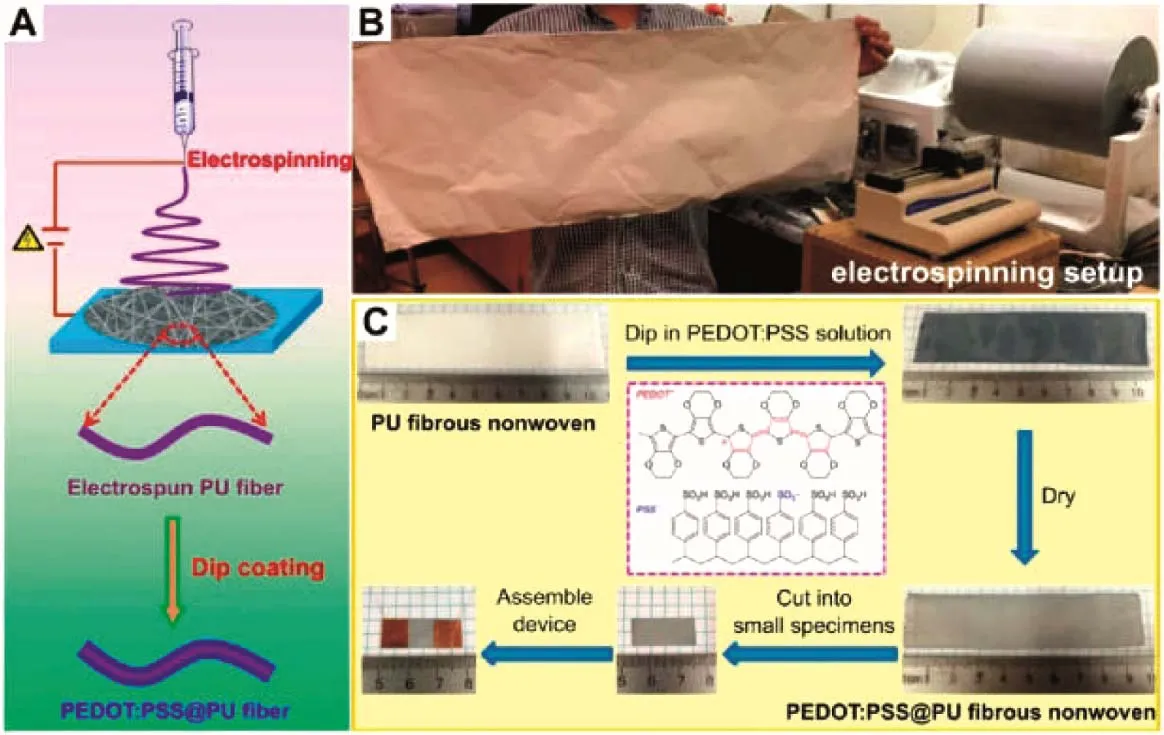
Fig. 5 Schematic fabrication of PEDOT:PSS/PU conductive textiles.(A) Electrospinning of PU and then dip-coating of PEDOT:PSS. (B) Photos of a piece of electrospun PU (~100 cm × 35 cm) and the electrospinning setup.(C) Dip-coating and sample preparation for electrical test. Reproduced with permission from Ref. 59. Copyright 2017 American Chemical Society.

Fig. 6 Schematic diagram of a neural interface.Reproduced with permission from Ref. 64. Copyright 2015 Cambridge University Press.
Conductive polymers including PANi, PPy and polythiophene have been investigated as the neural interface65-67. Recent research works focused more PEDOT:PSS and PEDOT derivatives arising from their good chemical stability and excelle nt charge transport properties. PEDOT has better stability than PPy. As shown in Fig. 7, PEDOT can dramatically lower the impedance by about two orders in magnitude at the biologically important temporal frequencies of 1 kHz and less68. This is associated with the open and fuzzy morphology and ionic conductivity of PEDOT. Hydrogels of conductive polymers were also investigated as the neural interface because hydrogels are biocompatible, ionically conductive and soft69.
4 Application of conductive polymers for energy conversion and storage
4.1 Thermoelectric application
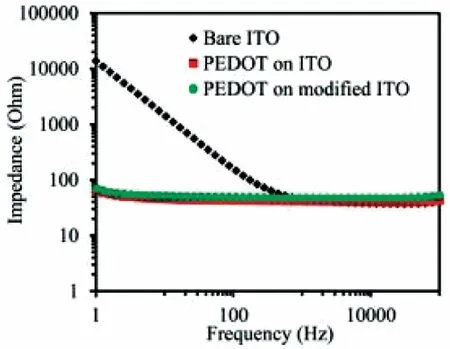
Fig. 7 Electrochemical impedance spectroscopy of bare ITO,unmodified, and modified ITO electrodeposited with PEDOT.Reproduced with permission from Ref. 68. Copyright 2015 American Chemical Society.
Thermoelectric generators can directly convert heat into electricity. They are important for the sustainable development because there is abundant of waste heat on earth. The conventional thermoelectric materials are inorganic semiconductors and semi-metals. But inorganic thermoelectric materials have problems of scarce elements, toxicity, high cost and poor mechanical flexibility. Recently, great progress was made on thermoelectric polymers. The thermoelectric conversion efficiency depends on the dimensionless figure of merit ZT value of the thermoelectric materials,with σ the electrical conductivity, S the Seebeck coefficient, T the absolute temperature and κ the thermal conductivity. σS2is called power factor. Power factor is important particularly for the thermoelectrical properties of polymers because polymers usually have low thermal conductivity. The Seebeck coefficient of thermoelectric polymers is usually much lower than the inorganic counterparts. Because intrinsically conductive polymers can have high electrical conductivity, they have been gaining more attention as thermoelectric materials. The thermoelectric systems with thermoelectric polymers can be flexible or even portable.

Fig. 8 Variation of the Seebeck coefficient (solid triangles), electrical conductivity (open triangles) and corresponding power factor (red squares) of PEDOT:TsO with oxidation level.Reproduced with permission from Ref. 72. Copyright 2011 Springer Nature.
Both p-type and n-type thermoelectric materials are required for highly efficient thermoelectric systems. The p-type thermoelectric polymers mainly include PEDOT and PANi70,71.Part of the reason is that both of them can be processed by solution processing techniques. The electrical conductivity and Seebeck coefficient of conductive polymers are interdependent.Lowering the doping level can increase the Seebeck coefficient but it decreases the electrical conductivity. Thus, there is an optimal doping level in terms of the power factor. Crispin’s lab dedoped PEDOT doped with tosylate (PEDOT:TsO) by using a chemical reductant, tetrakis(dimethylamino)ethylene (TDAE)72.As shown in Fig. 8, the Seebeck coefficient increases while the electrical conductivity increases with the decreasing doping level. The optimal power factor is 324 μW⋅m–1⋅K–2at the doping level of 0.22.
The combination of the secondary doping and subsequent reduction can give rise to conductive polymers with high electrical conductivity and high Seebeck coefficient72-74. Fan et al.73treated PEDOT:PSS films sequentially with H2SO4and NaOH solutions. The H2SO4treatment can enhance the conductivity of PEDOT:PSS to be higher than 3000 S⋅cm–1, and the NaOH treatment can remarkably enhance the Seebeck coefficient while does not lower the electrical conductivity too much. They observed a world-record power factor of 334 μW⋅m–1⋅K–2. As the thermal conductivity of PEDOT in the conductive state is about 0.37, the ZT values of these PEDOT:TsO and PEDOT:PSS samples are close to 0.3 at room temperature.
Good progress has been made in n-type thermoelectric polymers as well. n-type conductive polymers usually have poor stability because the electrons in the lowest unoccupied molecular orbital (LUMO) have high energy. Recently, a couple of n-type conductive polymers with good stability and high power factor were reported. The stable n-type conductive polymers usually contains metal elements75,76. Fig. 9 shows the temperature dependences of the electrical conductivity, Seebeck coefficient, thermal conductivity and ZT of poly(nickelethylenetetrathiolate). The highest ZT value is around 0.30 at 400 K.
4.2 Energy storage
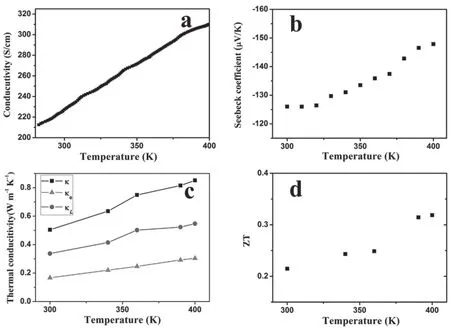
Fig. 9 Variations of (a) electrical conductivity, (b) Seebeck coefficient, (c) thermal conductivity and (d) ZT of poly(nickel-ethylenetetrathiolate) with temperature.Reproduced with permission from ref 75. Copyright 2016 John Wiley & Sons.

Fig. 10 Lithiation/delithiation capacity and Columbic efficiency (CE) of a Si nanoparticle-PANi electrode cycled at a current density of 6.0 A·g-1.Reproduced with permission from ref 91. Copyright 2013 Springer Nature.
Intrinsically conductive polymers can be oxidized and reduced electrochemically. They can thus have high capacity for energy storage77. PEDOT:PSS was used as the electrodes of transparent supercapacitors78. The supercapacitors can exhibit an areal capacitance of ~1 mF⋅cm–2. PEDOT:PSS fibers were also studied for supercapacitors79. The PEDOT:PSS fiber-based capacitors can have a areal capacitance of 119 mF⋅cm–2and areal energy density of 4.13 mWh⋅cm–2. Hydrogels of PANi were investigated as the electrode of supercapacitors as well, and the capacitance was 430 F⋅g–180. PEDOT on flexible cellulose paper was also used for supercapacitors81.
Composites of conductive polymers and oxides were demonstrated as the electrodes of supercapacitors82-85. For example, supercapacitors using composites of Ni―Mn―Co ternary oxide with PEDOT-PSS can exhibit a specific capacitance of 1234.5 F⋅g–1at a current density of 1 A⋅g–1and an energy density of 51.9 Wh⋅kg–1at a power density of 275 W·kg–182. PANi-RuO2with core-shell structure were studied as the electrodes for supercapacitors84. A thin layer of RuO2was grown on PANi nanofibers by atomic layer deposition (ALD).The RuO2layer can improve the stability and the energy density of the PANi supercapacitors.
Conductive polymers were used as additive of electrodes of batteries86–90. For example, PEDOT:PSS was added into LiFePO4electrodes of Li ion batteries87. PEDOT:PSS strongly influences the discharge capacity and potential of LiFePO4electrodes at high rates. Silicon as the anode material can give rise to high specific capacity for Li ion batteries. But it has a problem of poor cycling stability arising from its large volume changes during charging and discharging processes. Conductive polymers were used to improve the cycling stability91,92. As shown in Fig. 10, Wu et al.91coated PANi on Si nanoparticles for Li ion batteries and observed a cycle life of 5,000 cycles with over 90% capacity retention at current density of 6.0A⋅g–1.
5 Conclusions
Since the discovery more than 40 years ago, intrinsically conductive polymers have been one of the important research areas. The early conductive polymers have problems related to the electrical conductivity, stability and proessibility for their application. Great progress was made to overcome these problems. Electrical conductivities of higher than 1000 S⋅cm-1were observed for conductive polymers by controlling the synthesis conditions or secondary doping. Methods were developed to increase the stretchability of conductive polymers for their application as compliant electrode. Conductive polymers can be perfect materials for the neural interfaces. The thermoelectric properties of conductive polymers were recently increased dramatically. Conductive polymers can also have important application in energy storage systems including both supercapacitors and batteries.
- 物理化学学报的其它文章
- Wide Bandgap Random Terpolymers for High Efficiency Halogen-Free Solvent Processed Polymer Solar Cells
- Growing Carbon Quantum Dots for Optoelectronic Devices
- L-3,4-dihydroxyphenylalanine and Dimethyl Sulfoxide Codoped PEDOT:PSS as a Hole Transfer Layer: towards High-Performance Planar p-i-n Perovskite Solar Cells
- Asymmetric Quinoxaline-Based Polymer for High Efficiency Non-Fullerene Solar Cells
- Fluorination: An Effective Molecular Design Strategy for Efficient Photovoltaic Materials
- Improved Hole Injection Property of Solution-Processed MoO3 with UV-Ozone Treatment

