Wide Bandgap Random Terpolymers for High Efficiency Halogen-Free Solvent Processed Polymer Solar Cells
GUO Xia , FAN Qunping , CUI Chaohua , ZHANG Zhiguo , ZHANG Maojie ,* Laboratory of Advanced Optoelectronic Materials, College of Chemistry, Chemical Engineering and Materials Science,Soochow University, Suzhou 53, Jiangsu Province, P. R. China.
2 CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China.
Abstract: Over the past two decades, bulk heterojunction polymer solar cells(PSCs) have attracted significant attention owing to their potential applications in the mass fabrication of flexible device panels by roll-to-roll printing. To improve the photovoltaic performance of PSCs, much effort has been devoted to the optimization of properties of donor-acceptor (D-A) type polymer donor materials. Until now, the development of high-performance donor polymers is mainly dependent on the design and synthesis of binary polymers with a regular D/A alternating skeleton. Compared to binary polymers, random terpolymers with three different donor or acceptor monomer units possess synergetic effects of their inherent properties, such as optical absorption ability, energy levels, crystallinity, charge mobility, and morphological compatibility with the n-OS acceptors with suitable adjustment of the molar ratio of the three monomers. However, the irregularity in the polymer backbone of the random terpolymers may have an adverse effect on molecular packing, crystallinity, and charge mobility.Therefore, design and synthesis of high-performance terpolymers for PSCs is a challenging task. In this study, a series of wide bandgap random terpolymers PSBTZ-80, PSBTZ-60, and PSBTZ-40 based on alkylthiothienyl substituted benzodithiophene as the donor unit and two weak electron-deficient acceptor units of 5,6-difluorobenzotriazole (FBTz)and thiazolothiazole (TTz) were designed and synthesized for PSC applications. The optical, electrochemical, molecular packing, and photovoltaic properties of the polymers were effectively modulated by varying the FBTz:TTz molar ratio.Therefore, the PSC based on PSBTZ-60 as the donor material and narrow bandgap small molecule 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(4-hexyl-phenyl)-dithieno[2,3-d:2',3'-d']-s-indaceno[1,2-b:5,6-b']di thiophene) (ITIC) as the acceptor, processed using halogen-free solvents, exhibited high power conversion efficiency(PCE) of 10.3% with high open-circuit voltage (Voc) of 0.91 V, improved short-circuit current density (Jsc) of 18.0 mA·cm-2,and fill factor (FF) of 62.7%, which are superior to those of PSCs based on binary polymers PSBZ (a PCE of 8.1%, Voc of 0.89 V, Jsc of 14.7 mA·cm-2, and FF of 61.5%) and PSTZ (a PCE of 8.5%, Voc of 0.96 V, Jsc of 14.9 mA·cm-2, and FF of 59.1%). These results indicate that random terpolymerization is a simple and practical strategy for the development of high-performance polymer photovoltaic materials.
Key Words: Terpolymer; Polymer solar cells; Halogen-free solvent; Power conversion efficiency
1 Introduction
In the last four years, the polymer solar cells (PSCs) based on n-type organic semiconductor (n-OS) acceptor have emerged and made great progress1–6, and the corresponding power conversion efficiencies (PCEs) have exceeded 13% to date7–9. Recently, narrow bandgap (NBG) small molecule acceptors based on a fused-ring electron-donating core along with two strong electron-withdrawing end-groups have dominated high efficiency PSCs10–20, especially the most widely studied ITIC10. In comparison with the traditional fullerene derivatives (such as PC71BM and ICBA), the n-OS acceptor ITIC show wide absorption spectrum with small optical bandgap () of ca. 1.58 eV and high absorption coefficient of 105cm-1in the thin film processed with toluene,suitable molecular energy levels, and high electron mobility. So far, the PSCs based ITIC as acceptor have achieved the PCEs of more than 12%11.
The well-matched pair of polymer donor and n-OS acceptor plays a key role in achieving efficient PSCs. By now, the development of high-performance donor polymers is mainly dependent on the design and synthesis of binary polymers with a regular D/A alternating skeleton21–32. However, only a few polymer donor materials have been successfully applied to the PSCs and achieved high PCE values. For example, Hou et al.21reported a high performance wide bandgap (WBG) polymer PBDB-T with a skeleton of thienyl benzodithiophene (BDTT)-alt-benzodithiophene-4,8-dione (BDD). In comparison with the PBDB-T:PC71BM-based PSCs, the PSCs based on PBDB-T:ITIC showed a higher PCE of 11.21% due to the complementary absorption spectra and lower energy loss (Eloss,which is defined as- eVoc). Bin et al. also developed a series of high performance WBG polymers with a skeleton of BDTT-alt-benzotriazole (BTz)22–24, especially alkylthio and fluorine substituted 2D-conjugated polymer J6122. Combined with ITIC acceptor, the PSCs achieved a high PCE of 9.53%with a high short-circuit current density (Jsc) of 17.43 mA·cm-2due to the high absorption coefficient and strong crystallinity of polymer J61. Recently, our group reported a high performance polymer donor PSBZ25through the side-chain engineering on J61. Compared to J61, PSBZ showed stronger π–π interaction and smaller stacking spacing leading to higher absorption coefficient and hole mobility. As a result, the halogenated solvent processed PSCs based on PSBZ:ITIC gained a PCE of up to 10.5% with a high Jsc of 19.0 mA·cm-2. Very recently, our group designed a BDTT-alt-thiazolothiazole (TTz)-structured WBG polymer PSTZ29, and the PSTZ:ITIC-based PSCs still achieved a PCE of 8.13% when the open-circuit voltage (Voc)was up to 1.01 V.
Recently, a random terpolymerization strategy, in which three different donor or acceptor monomers unite together to polymerize, has been applied to achieve synergetic effects of their inherent properties, such as optical absorption ability,energy levels, charge mobility and morphological compatibility with the fullerene derivative acceptors33–36. However, the irregularity in the polymer backbone of the random terpolymers may have an adverse effect on molecular packing, crystallinity,and charge mobility. Moreover, highly efficient PSCs based on terpolymer are rarely reported37. Therefore, it is a challenging work to design and synthesized a high performance terpolymer for PSCs.
2 Results and discussion

Fig. 1 Synthetic routes and molecular structures of the binary polymers PSBZ and PSTZ, and random terpolymers PSBTZ-80,PSBTZ-60 and PSBTZ-40.
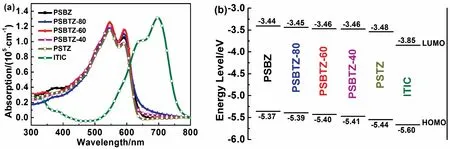
Fig. 2 (a) Absorption spectra and (b) molecular energy levels diagrams of the polymer donors and ITIC acceptor.
Synthetic routes of the binary polymers and random terpolymers were shown in Fig. 1. The monomers BDDT-S25,FBTz22and TTz29were synthesized in accordance with our previous methods. Polymers were obtained by Stille-coupling polymerization between BDDT-S, FBTz and TTz. Detailed synthesis procedures are described in Supporting Information(SI). The actual molecular composition of these polymers was determined by elemental analysis. The results showed that the BDDT-S/FBTz/TTz ratio in polymers were consistent with the molar feed ratio of BDDT-S/FBTz/TTz in polymerizations. The number/weight average molecular weights are 20.6/46.5,21.8/47.3, 21.3/48.9, 22.2/46.8, and 19.9/45.3 kDa with the polydispersity index of 2.26, 2.17, 2.29, 2.11, and 2.28 for polymers PSBZ, PSBTZ-80, PSBTZ-60, PSBTZ-40, and PSTZ, as estimated by gel permeation chromatography using high temperature (160 °C) 1,2,4-trichlorobenzene as the eluent.All polymers display good solubility in many common organic solvents such as toluene, chloroform, and chlorobenzene.
Fig. 2a shows the UV-Vis absorption spectra of polymer donors and ITIC acceptor in thin film prepared by the toluene solutions. All five polymers show the similar absorption spectra with a maximum absorption (λmax) peak at ca. 545 and an obvious absorption shoulder peak at ca. 594 nm, especially PSBTZ-60. The PSBTZ-60 film has a maximum absorption coefficient of 1.25 × 105cm-1, which is higher than those of binary polymers PSBZ (1.19 × 105cm-1) and PSTZ (1.15 × 105cm-1) as well as terpolymers PSBTZ-80 (1.19 × 105cm-1) and PSBTZ-40 (1.20 × 105cm-1). Moreover, polymer films have the similar absorption edges of ca. 637 nm and anof ca.1.95 eV, which is absorption complementary to ITIC acceptor(anof ca. 1.58 eV) in the Vis-NIR region.
The energy levels of the polymer donors and ITIC acceptor were summarized in Fig. 2b. With the increase in the proportion of TTz unit, the polymers show gradually lower energy levels.The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels are-5.37/-3.44, -5.39/-3.45, -5.40/-3.46, -5.41/-3.46, and-5.44/-3.48 eV for the PSBZ, PSBTZ-80, PSBTZ-60,PSBTZ-40, and PSTZ, respectively. The low HOMO level is beneficial for the related polymer donors to obtain higher Vocin PSCs38-40.
The PSCs with a device structure of ITO/ZnO/PFN/polymers: ITIC/MoO3/Al were fabricated to probe the photovoltaic properties of polymers, and the detailed preparation processes are recorded in SI. The current–voltage(J–V) characteristics of the PSCs are shown in Fig. S1 (SI) and detailed photovoltaic parameters are summarized in Table S1–S3 (SI)). Firstly, we use halogen-free toluene as solvent and a D/A weight ratio of 1/1 to select the optimal polymer for the device optimization. Compared to the other PSCs based on polymers:ITIC, the PSBTZ-60:ITIC-based PSCs achieved a higher PCE of 9.1% due to the relatively higher Jscof 16.5 mA·cm-2. Secondly, the D/A weight ratios of the PSBTZ-60:ITIC-based PSCs were optimized and found that the optimum ratio was 1/1 (see Fig. S1b and Table S2 in SI).Subsequently, the active layer morphology was optimized to improve the device performance by adding halogen-free diphenyl ether (DPE) as solvent additive. With the blend films processed with 1% DPE in volume, the PSCs showed an obviously increased Jsc from 16.5 to 18.0 mA·cm-2and significantly improved FF from 58.5% to 62.7%, while the Voc was slightly decreased to 0.91 V. As a result, the champion PSC achieves a high PCE of 10.3% (see Fig. S1c and Table S3 in SI).

Fig. 3 (a) The J–V curves and (b) EQE plots of the polymers:ITIC-based PSCs processed with 1% DPE. (c) The PCE versus FBTz contents in polymers. (d) The PCE versus annealing time of the PSBZT-60:ITIC-based PSCs under a temperature of 100 °C.
In order to facilitate comparison, the J–V and EQE curves of PSCs processed with 1% DPE was summarized in Fig. 3a and Fig. 3b, and the corresponding device performance date was summarized in Table 1. The active layer thicknesses of these devices are ca. 100 nm. Compared with the other PSCs based on ITIC as acceptor and binary polymer as donor, the PSCs based on PSBTZ-60:ITIC showed obviously improved Jsc from 14.7 and 14.9 to 18.0 mA·cm-2, slightly increased FF from 61.5% and 59.1% to 62.7%, and moderate Vocof 0.91 V.Moreover, the PSBTZ-60-based PSCs also displayed an obviously improved Jsccompared to the PSCs based on PSBTZ-80 or PSBTZ-40, which is consistent with PSBTZ-60 has a higher absorption coefficient. As shown in Fig. 3c, with the increase of FBTz acceptor content in the polymers, the PCE values of the related PSCs are first increased from 8.1% to 10.3%, and then decreased to 8.5%, while the highest PCE is gained for polymer PSBTZ-60 with an FBTz content of 60%.Moreover, the optimal device possesses a low Elossof ca. 0.57 eV, which is lower the empirical threshold of 0.6 eV.
In addition to high efficiency, excellent thermal stability is also important for the practical applications of PSCs in future.Herein, we have tested the thermal stability of the PSC devices,as shown in Fig. S2 and Table S4 in SI. The devices annealed at a typical high temperature of 100 °C for 120 min in the N2-filled glovebox still remained a high PCE of 9.4% due to the almost unchanged Voc, slightly improved FF, although Jscis decreased to some extent. As shown in Fig. 3d, with the high temperature annealing time extended to 300 min, the PSCs still achieved a PCE of 8.3%, which is ca. 81% of the original device efficiency. The above results indicate that the PSBTZ-60:ITIC-based PSCs possess high long-term thermal storage stability.
To confirm the high Jscof the PSCs, the EQE tests were carried out (Fig. 3b). Compared to the devices based on binary polymers, the devices based on terpolymers show higher EQE response values, especially the PSBTZ-60:ITIC-based device.Moreover, the PSBTZ-60:ITIC-based device possesses a higher maximum EQE value of ca. 81% at 554 nm in comparison with the devices based on polymer donors PSBZ (ca. 68% at 565 nm), PSBTZ-80 (ca. 78% at 550 nm), PSBTZ-40 (ca. 74% at 551 nm), and PSTZ (ca. 69% at 560 nm). The corresponding integral Jsc values from EQE are 14.2, 15.8, 17.3, 15.2, and 14.5 mA·cm-2, which are well agree with the measured Jscvalues.
The exciton dissociation and charge extraction processes areprobed by measuring the plots of photocurrent (Jph) versus effective voltage (Veff) of the devices (Fig. 4a)41–44. The parameter definitions and calculation methods are described in detail in SI. Under the maximum power output conditions, the exciton dissociation probabilities P(E, T) were calculated to be 78.8%, 84.2%, 88.8%, 83.7%, and 81.8% for the devices based on polymer donors PSBZ, PSBTZ-80, PSBTZ-60, PSBTZ-40,and PSTZ respectively, which implies that the PSC based on PSBTZ-60 possesses a more efficient exciton dissociation and charge extraction processes. To study the charge recombination mechanism in PSCs, the relationship of Vocwith light intensity(P) was probed (Fig. 4b)41,9. The slope of Vocversus lnP at kBT/q suggests that the device only has bimolecular recombination,while the slope of 2kBT/q means that the trap-assisted recombination dominates in the recombination mechanisms of device (where T, kBand q are the Kelvin temperature,Boltzmann constant and elementary charge, respectively). The device based on PSBTZ-60 (1.13kBT/q) displays a smaller slope compared to the devices based on polymer donors PSBZ(1.67kBT/q), PSBTZ-80 (1.35kBT/q), PSBTZ-40 (1.37kBT/q),and PSTZ (1.60kBT/q), which implies that the PSBTZ-60:ITIC-based device has fewer trap-assisted recombination. Moreover, the dependence of Jphunder different P was defined as Jph∝ PS, and studied to further probe the charge recombination process in PSCs (Fig. 4c). S values close to 1 indicate that the devices possess weak bimolecular recombination41,27. For the devices based on polymer donors PSBZ, PSBTZ-80, PSBTZ-60, PSBTZ-40, and PSTZ, the S values of the fitted lines in logarithmic coordinates are 0.94,0.97, 0.99, 0.97, and 0.95 respectively, which means that the PSBTZ-60-based device has less bimolecular recombination.

Table 1 Photovoltaic performance date of the PSCs based on polymers:ITIC processed with 1% DPE.

Fig. 4 (a) The Jph versus Veff, (b) the Voc and (c) the Jph versus light intensity of the polymers:ITIC-based PSCs processed with 1% DPE.
Photoluminescence (PL) quenching efficiencies of the polymers:ITIC blend films compared to their pure polymer or ITIC films were also measured to probe the exciton dissociation and charge transport processes (Fig. 5 and Fig. S3 in SI). At a excitation wavelength of 700 nm come from ITIC film, the PSBTZ-60:ITIC blend film shows a higher PL quenching efficiency of more than 95% in comparison with the other PSBZ:ITIC (86.3%), PSBTZ-80:ITIC (89.1%),PSBTZ-40:ITIC (89.7%), and PSTZ:ITIC (84.7%) blend films.Similar phenomena are also found at an excitation wavelength of 550 nm come from polymer donors (Fig. S3 in SI), implying that the devices based on PSBTZ-60:ITIC possess highly efficient photo-induced exciton dissociation and charge transport processes, which agrees with the higher EQE and Jscvalues of the related PSCs.
The hole/electron mobilities (μh/μe) are measured using the space charge limited current method (Fig. S4). The μh/μe were 2.84/0.65 × 10-4, 4.48/1.82 × 10-4, 6.27/3.65 × 10-4, 3.92/1.45 ×10-4, and 3.59/1.11 × 10-4cm2·V-1·s-1for the devices based on polymer donors PSBZ, PSBTZ-80, PSBTZ-60, PSBTZ-40, and PSTZ, respectively. The relatively high μh/μevalues and low μh/μeratio imply more effective and balanced charge transport in the PSBTZ-60:ITIC-based device, and thus a high PCE can be achieved for the PSCs.
The surface and bulk morphologies of polymer:ITIC blend films were also studied. For atomic force microscopy (AFM)images (5 μm × 5 μm) (Fig. 6a–e), all the blend films show clear polymer aggregation. Moreover, the PSBTZ-60:ITIC blend film showed a smoother surface with a smaller root-mean-square roughness (Rq) of 1.17 nm compared to the blend films of PSBZ:ITIC (2.23 nm), PSBTZ-80:ITIC (1.80 nm), PSBTZ-40:ITIC (1.77 nm), and PSTZ:ITIC (1.73 nm).For transmission electron microscopy (TEM) images (Fig.6f–j), all the blend films display the regular fibril texture,although only the PSBTZ-60:ITIC blend film has a relatively suitable phase separation. The small surface roughness and suitable phase separation with a fibril texture are beneficial to exciton separation and charge transport and hence improved the photovoltaic performance of PSCs.

Fig. 5 The PL spectra of ITIC and the corresponding blend films at an excitation wavelength of 700 nm.
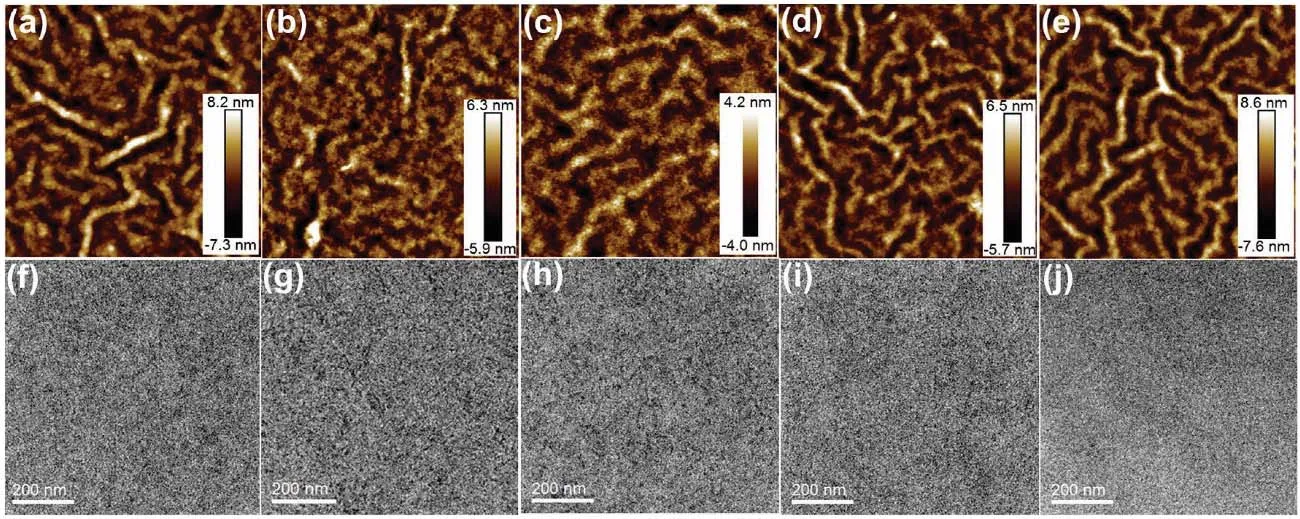
Fig. 6 (a–e) AFM and (f–j) TEM images.(a and f) PSBZ:ITIC, (b and g) PSBTZ-80:ITIC, (c and h) PSBTZ-60:ITIC, (d and i) PSBTZ-40:ITIC and (e and j) PSTZ:ITIC blend films.
3 Conclusions
A series of WBG random terpolymers based on BDTT-S as donor unit, FBTz and TTz as acceptor units were designed and synthesized for photovoltaic applications. By adjusting the molar ratio of two acceptor units in polymerization, the absorbance and energy levels of terpolymers can be effectively modulated. The terpolymer namely PSBTZ-60 showed the highest absorption coefficient and moderated energy levels. As a result, the PSCs based on PSBTZ-60:ITIC processed by the halogen-free solvents achieved a high PCE of 10.3% with a high Voc of 0.91 V, improved Jsc of 18.0 mA·cm-2and FF of 62.7%, which is obviously superior to the PSCs based on binary polymer donors PSBZ (8.1%) and PSTZ (8.5%). These results indicate that the random terpolymerization is a simple and practical strategy to design and optimize high-performance polymer photovoltaic materials.
Acknowledgment: This work is dedicated to Prof.Yongfang Li on the occasion of his 70th birthday.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- K+ Concentration-Dependent Conformational Change of Pb2+-Stabilized G-quadruplex
- Improved Hole Injection Property of Solution-Processed MoO3 with UV-Ozone Treatment
- Asymmetric Quinoxaline-Based Polymer for High Efficiency Non-Fullerene Solar Cells
- L-3,4-dihydroxyphenylalanine and Dimethyl Sulfoxide Codoped PEDOT:PSS as a Hole Transfer Layer: towards High-Performance Planar p-i-n Perovskite Solar Cells
- Growing Carbon Quantum Dots for Optoelectronic Devices
- Fluorination: An Effective Molecular Design Strategy for Efficient Photovoltaic Materials

