A network pharmacology approach to investigate the mechanisms of Si-Jun-Zi decoction in the treatment of gastric precancerous lesions
Liang-Jun Yang, Dao-Rui Hou, Ya Li, Zhi-Peng Hu, Yong Zhang*
1Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China. 2The First People's Hospital of Xiangtan City, Xiangtan, Hunan, China. 3Lin’an Hospital of Traditional Chinese Medicine, Lin’an, Zhejiang, China. 4Chengdu University of Traditional Chinese Medicine, Chengdu, China. 5Sichuan Second Hospital of Traditional Chinese Medicine,Chengdu, Sichuan China.
Background
Gastric cancer is one of the most common and aggressive malignant tumors around the world, with a 5-year overall survival rate less than 25% [1, 2]. And it is still the most prevalent cancer in Eastern Asia, such as China, Japan,and Korea [3]. Because of the high mortality, early detection and treatment is an important way to reduce the risk of death from gastric cancer. It is well known that gastric cancer is a continuous process starting from chronic superficial gastritis, atrophic gastritis, intestinal metaplasia, and ultimately to dysplasia and adenocarcinoma [4]. There are pieces of evidence that gastric precancerous lesions (GPL), which mainly includes intestinal metaplasia and dysplasia [5], plays a crucial role in the progression of gastric cancer. A cohort study showed that nearly 25% of patients with high-grade dysplasia received a diagnosis of gastric cancer within 1 year and 7% of patients with low-grade dysplasia progressed to invasive carcinoma [6]. Therefore, further researches on GPL is imperative to prevent the formation and development of gastric carcinoma.
Traditional Chinese medicine (TCM), which characterized by multiple compounds, has been used for preventing and treating cancers for centuries [7] and shows great potential in complex disease for its multi-targets manner [8]. Evidence has shown that TCM therapy offered a better survival benefit for patients with stage IV gastric adenocarcinoma compared with patients without any TCM treatment [9]. Si-Jun-Zi (SJZ)decoction, originated from Taiping Huimin Heji Jufang compiled by the bureau of peaceful benevolent dispensary in 1078 A.D. - 1085 A.D. (Song Dynasty of China), is a famous herbal formula for treating digestive diseases. The formula is composed of four herbs:Dang-Shen (Codonopsis Radix, DS), Fu-Ling (Poria Cocos Sc hw. W olf, FL), Bai-Zhu (Atractylodes Macrocephala Koidz, BZ) and Gan-Cao (licorice, GC).According to TCM theory, SJZ decoction is wildly used in spleen-deficiency syndrome for its efficacy in invigorating spleen and replenishing Qi. And pharmacological researches have demonstrated that SJZ decoction could promote the restoration of intestinal function [10, 11], modulate the immunity [12], alleviate the intestinal inflammatory response and improve the nutrition absorption [13]. Clinical study shows that SJZ decoction can reverse gastric pathological changes including chronic atrophic gastritis, intestinal metaplasia and gastric epithelial dysplasia, and favorably relieve clinical symptoms [14-16]. Although the therapeutic efficacy of SJZ decoction in the treatment of GPL has been verified, the active ingredients, cellular targets, and the molecular mechanisms of action are still unknown.
In this paper, we explore the underlying mechanisms of SJZ decoction by a network pharmacology approach,which is a powerful discipline incorporating systems biology, bioinformatics, and pharmacology [17]. As a new advanced analytical technique, network pharmacology analysis has been applied to TCM research to identify the effect and mechanism of action of medications used to treat complicated diseases [18, 19].The protocol of this work is presented above. Firstly, the active compounds of SJZ were selected via oral bioavailability (OB), drug-likeness (DL), Caco-2 cell permeability evaluation, and literature mining. Secondly,the targets were obtained by integrating the drug and the GPL targets. These predicted targets were mapped into a drug-target interaction and validated by GO (Gene ontology) and pathway enrichment. Since then, the targets were used to construct an integrated pathway for further analysis. Finally, an integrated “GPL pathway” was established to illuminating the molecular pathogenesis of SJZ on GPL, which provided a new approach for TCM modernization.
Material and methods
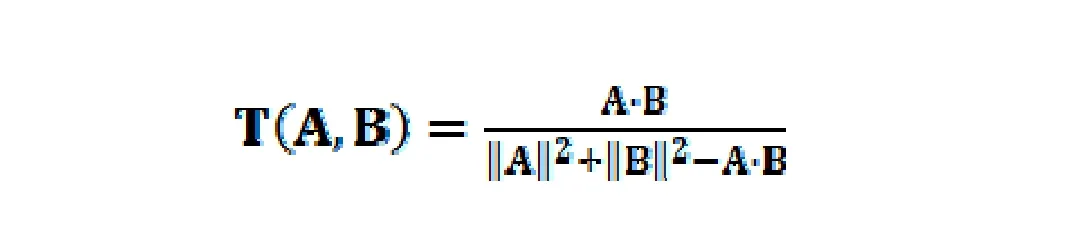
Active compounds screening
The compounds of SJZ were obtained from the database TCMSP (Traditional Chinese Medicines for Systems Pharmacology Database and Analysis Platform,http://lsp.nwsuaf.edu.cn/tcmsp.php), which is a platform of Chinese herbal medicines that captures the relationships between drugs, targets, and diseases [20].This database has collected 499 herbs with a total of 12144 chemicals based on precisely examined pharmacology and clinical knowledge. Simultaneously,literature search was conducted to find ingredients that have functions on humans. In this work, the ingredients of SJZ were put into TCMSP, a total of 491 molecules with 134 in DS, 34 in FL, 55 in BZ, 280 in GC were obtained. In order to get the ADME (absorption,distribution, metabolism, and excretion) properties of compounds, the molecular pharmacokinetic parameters including OB, DL, and caco-2 permeability were calculated to screen the potential chemical components.
(1) OB screening. OB reflects the capability of the orally administered drug be delivered to the circulatory system. It is one of the most important pharmacokinetics features in drug screening [21]. Compounds with a high OB values often have the potential to become therapeutic drugs [22]. In this work, the OB evaluation was calculated by a robust in silico model OBioavail 1.1 [21],which has advantages in accelerating the prediction of OB. Molecules with OB ≥ 30% were selected as the candidate compounds for further analysis [23-25].
(2) DL evaluation. DL is a qualitative concept used in drug design for estimating how “drug-like” a prospective compound is in line with the majority of known drugs[26]. It has been widely used to screen out chemicals with desired properties in pharmaceutical research [27]. Based on the molecular descriptors and Tanimoto coefficient[28], a database-dependent model is applied to calculate the DL index of each compound in SJZ. The DL calculation approach is shown as follows:
where A is the molecular properties of herbal ingredients,and B represents the average molecular properties of all molecules in the DrugBank database (http://www.drugbank.ca/) based on Dragon soft descriptors [29]. In this work, the ingredients with DL ≥ 0.18 were selected as candidate compounds for further research.
(3) Caco-2 cell evaluation. Caco-2 cell permeability is a valuable index for assessing oral absorption and is usually used as a model to evaluate the absorption of drugs from the intestinal epithelium barrier [30, 31]. In this work, a Caco-2 cell permeability prediction work platform preCaco2 was applied to calculate the drug absorption rate [32]. The threshold of Caco-2 cell permeability was set to 0 to screen possible candidate drugs presently.
Finally, the threshold values for the integrative screening system were OB ≥ 30%, DL ≥ 0.18, Caco-2 ≥ 0,and the ingredients meeting the conditions were selected as potential active compounds for further analysis.
Compound targets of SJZ
Target identification is crucial to illuminate the therapeutic mechanism of SJZ because of the multiple active components. The targets for these compounds were collected from TCMSP database, which used the SysDT model and the HIT (Herb ingredients’ targets) database[20]. Based on two powerful methods named Random Forest and SVM (Support Vector Machine), the SysDT shows outstanding in predicting drug-target interactions,with a concordance of 82.83%, a sensitivity of 81.33%,and a specificity of 93.62% [33].
The compounds with unknown targets were subjected to Swiss Target Prediction (http://www.swisstargetprediction.ch/), which is a database for target prediction of bioactive small molecules based on a combination of 2D and 3D similarity measures with known ligands [34]. The potential targets were carried out using the SMILES format of each constructed structure,and Homo sapiens was chosen as the source of the target.
Owing to the non-canonical description of the compound targets, the UniProt Knowledgebase(www.uniprot.org/) was used. The protein names were retrieved with the species limited to ?Homo sapiens? to obtain official symbols. Finally, the ingredient targets with gene symbol were obtained.
Disease targets of GPL
Information on GPL related target genes was screened by The Human Gene Database named Genecards(http://www.genecards.org/), which is a database containing information on all annotated and predicted human genes, proteins, and diseases [35]. Sources of Genecards come from 125 databases including genomic,transcriptomic, proteomic, genetic, clinical and functional information, and the information is considered reliable[36]. The database was searched using the keywords?gastric precancerous lesions? or ? precancerous lesion of gastric cancer?, and the genes which quality score ≥5 were selected as the GPL related genes.
GO and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment
In order to explore the underlying mechanisms of SJZ decoction that provides therapeutic effects in GPL, the GO and KEGG pathway enrichment were conducted. The GO defines the concepts relating to gene functions from three aspects: molecular function, cellular component,and biological process [37]. In this research, we performed GO analysis only for biological process.KEGG pathway database is a collection of comprehensive inferences for pathway mapping and is useful in predicting gene functions [38]. The enrichment analyses were performed by linking the targets to Enrichr(http://amp.pharm.mssm.edu/Enrichr/), which allows for the identification of associated pathways and ontology tools [39]. Enriched GO terms and pathways with adjusted P < 0.01 were obtained and analyzed for further research. And the terms which have no connection with the disease were detached.
Network construction
To decipher the complex therapeutic mechanisms of SJZ decoction in treating GPL, a compound-target network(C-T network) and target-pathway (T-P network) were constructed to uncover the relationship between candidate compounds and potential targets. (1) C-T network. A C-T network was constructed to illustrate the drug-target interactions of active ingredients in SJZ and their potential targets. (2) T-P network. Based on the previous work, a T-P network was built by mapping the target proteins to the enriched pathways.
The graphs were visualized and analyzed by Cytoscape version 3.2.1 [40], a powerful bioinformatics software for data visualization and integration. In the graphical networks, the compounds, targets, and pathways were represented by nodes, and the interaction between these nodes was represented by edges. Meanwhile, a vital topological parameter called degree was analyzed by the plugin Network Analyzer of Cytoscape [41]. The degree of a node is the number of edges associated with it and the nodes with a high degree can be regarded as the key nodes in the network.
GPL pathway construction and analysis
To explore the systematic mechanisms of SJZ decoction on GPL treatment, an incorporated “GPL pathway” was integrated based on the T-P network. Pathways related to GPL pathogenesis were picked out and assembled according to the pathological and clinical data.
Results
Active compounds identification
In total, 491 compounds were collected from 4 herbs of SJZ decoction. By screening three classical ADME parameters including OB, DL and Caco-2 permeability,123 potentially active compounds with 19 in DS, 13 in FL, 7 in BZ, 84 in GC were obtained. Additionally, 12 typical components of SJZ decoction were also reserved as the active components even though they do not meet the parameter. For instance, atractylenolide Ⅲ, a common ingredient in DS and BZ, has anti-inflammatory effects by suppressing the release of nitric oxide, prostaglandin E2, TNF-α (tumor necrosis factor-α) and IL-6(interleukin-6) related to the NF-κB and MAPK-signaling pathways [42]. Thus, a total of 135 active herbal ingredients were obtained in this work for further target prediction and network analysis (as showed in Table S1)
Target identification
Based on the target fishing approach introduced above, a total of 286 targets for the 135 compounds with 2170 connection between them were obtained. While, targets involving the pathogenesis of GPL, as well as the interaction mechanism, remained unknown. In order to find out the GPL related genes, the target genes related to GPL were searched in the GeneCards, which covers nearly 90% of human protein-coding genes in GeneCards[43]. Since then, 350 GPL related genes which known to be expressed were collected. Through a combination of the compound targets of SJZ decoction and the disease targets, 90 overlapping genes were selected as the key targets in the GPL treatment. And 111 compounds were saved as the active ingredients after deleting 24 candidate compounds which had no related targets. Finally, these 90 targets were considered to have effective therapeutic actions in the pathogenesis of GPL (Table S2).
To get the logical structure of the biological functions,GO enrichment and analysis were conducted. According to the results, these targets were significantly associated with 654 GO biological process terms, like the cellular response to cytokine stimulus (ID: 0071345, Count = 36),cytokine-mediated signaling pathway (ID: 0019221,Count = 37), regulation of apoptotic process (ID:0042981, Count = 37), negative regulation of apoptotic process (ID: 0043066, Count = 29), positive regulation of gene expression (ID: 0010628, Count = 33), etc. The top 20 significantly enriched terms were shown in Figure 1.
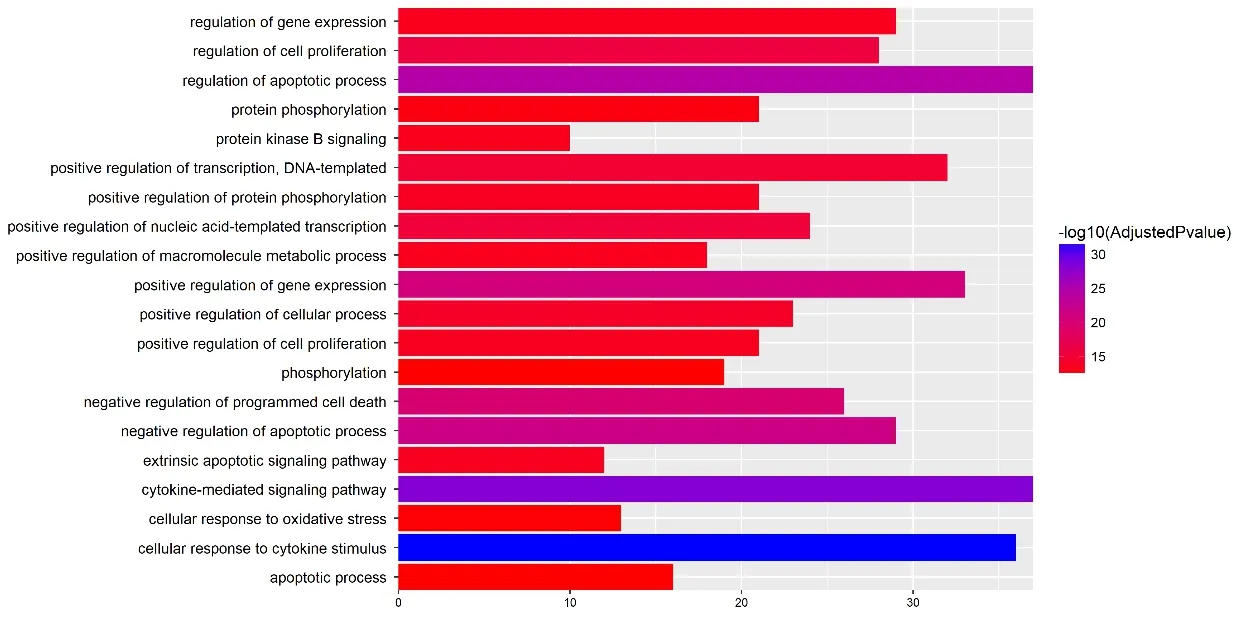
Figure 1 GO analysis of target genes
Network Construction
C-T network and analysis.TCM decoction exerts multiple biological and pharmacological effects through multiple compounds and targets. To illustrate the interrelationship between the key targets and their correlated herbal ingredients, a C-T network was built.The active compounds, targets, and interactions between them were showed in Figure 2, which have 201 nodes(111 compounds and 90 targets) and 944 edges. The C-T network analysis displayed that the average degree (the number of edges connecting to the nodes) per compound is 10.49, indicating that most compounds regulated multiple targets to exert therapeutic effects and the compounds with high degree may play crucial roles in the treatment of GPL. Typically, three compounds with degrees more than 20, such as quercetin, luteolin, and kaempferol become the critical ingredients for the SJZ because of their important positions in this network.
Additionally, lots of targets were connected with multiple compounds of different herbs, which exhibit an integrative function of SJZ decoction in the treatment of GPL. Among the candidate targets, COX-2(Cyclooxygenase-2), estrogen receptor 1, PPARG(peroxisome proliferative activated receptor) are targeted by 90, 79 and 66 compounds, which demonstrated the potential therapeutic targets of SJZ decoction.
T-P Network and analysis.To examine the signaling pathways of these target genes, KEGG pathway enrichment was conducted. After enrichment, 90 targets were mapped to 130 pathways according to the adjusted P-value. According to the pathogenesis of GPL and the gene counts related to these pathways, like hepatitis B(hsa05161, degree = 31), bladder cancer (hsa05219,degree = 19) which had no relationship with the GPL and degree less than 10 were detached. Finally, 21 remarkably pathways are likely to be the major pathways in the treatment of GPL (shown in Table 1). Taken together, 76 target genes were involved in these pathways, resulting in a T-P network (shown in Figure 3). It can be seen from the figure below that these targets were closely interact with pathways in cancer (hsa05200, degree = 46),PI3K-Akt signaling pathway (hsa04151, degree = 28),apoptosis (hsa04210, degree = 19), etc. These pathways could be regarded as the key pathways in the treatment of GPL.
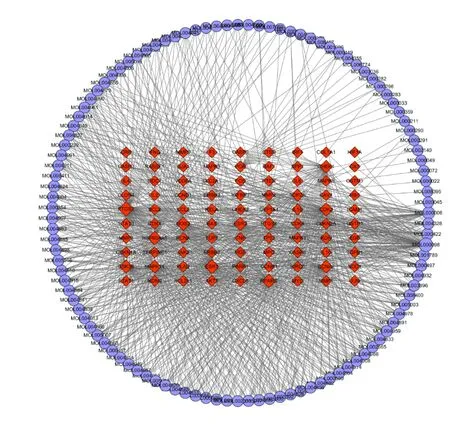
Figure 2 Compound-target network
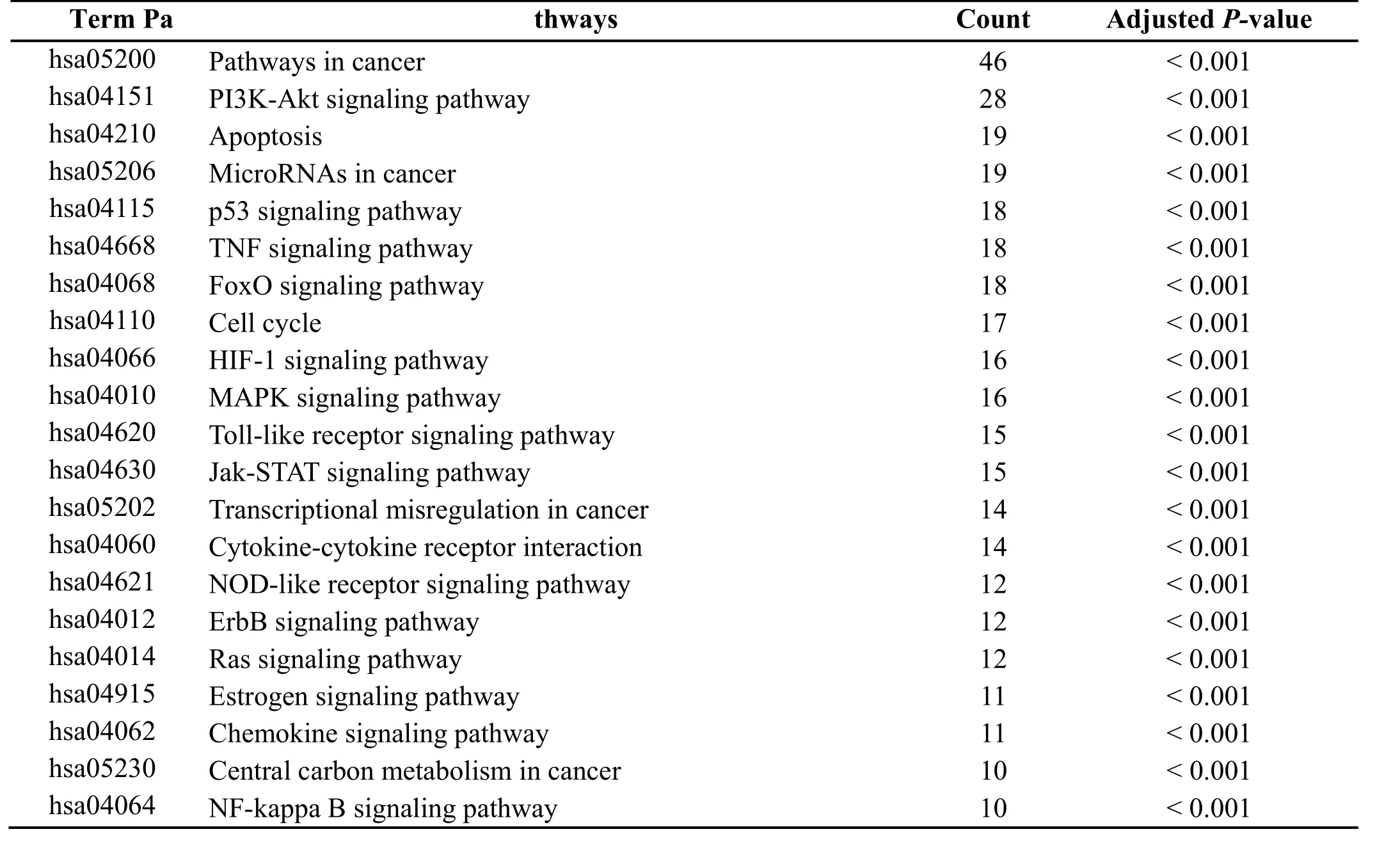
Table 1 The KEGG pathways of therapy target genes
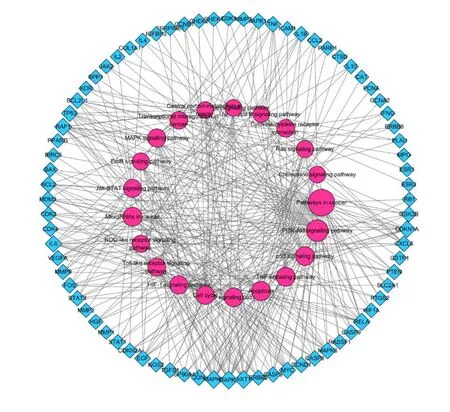
Figure 3 Target-pathway network
GPL pathway.In order to explore the integral regulation of SJZ for the treatment of GPL, an integrated GPL pathway was constructed through the T-P network analysis above. As showed in Figure 4, this GPL associated pathway can be separated into three therapeutic modules, including inflammation module,apoptosis module, and proliferation module, which reveal the underlying therapeutic effects of SJZ decoction.
Inflammation module.It is well known that gastric cancer is one of the best examples of inflammation-associated cancer in human beings. As a vital step in the gastric pathogenesis, gastric precancerous lesions triggered by environmental stimuli is closely connected with the deleterious effects of inflammation[44]. And chronic mucosal inflammation is associated with a great risk in the progression from chronic gastritis to gastric cancer [45]. From the figure above we can see that the inflammatory cytokines play crucial roles in the activation of the PI3K-Akt signaling pathway, the FoxO signaling pathway, and the NF-κB signaling pathway. For instance, H. pylori infection often induces the expression of the pro-inflammatory cytokine IL-8 (interleukin-8)through the activation of the NF-κB pathway [46]. And the modulation of these pathways is considered a useful approach to inhibit proinflammatory gene expression[47-49].
Apoptosis module.Apoptosis is the programmed cell death which keeps the survival and death balance in metazoan cells. Deficiency in cell apoptosis is related to the formation of some gastrointestinal malignancies [50],and exerts a positive influence on the morphogenesis of GPL [51]. As can be seen from the Figure 4, pathways including the PI3K-Akt signaling pathway, the p53 signaling pathway, the TNF signaling pathway, the FoxO signaling pathway, and the Ras signaling pathway are involving in the disorder of apoptosis. For example, p53,which participates in the PI3K-AKT signaling pathway and p53 signaling pathway, is playing a vital role in anti-tumor by inducing cell apoptosis [52]. And the C-T network shows that the p53 could be regulated by luteolin from DS and quercetin from GC. The results of the correlational analysis suggest that SJZ decoction may treat GPL by inducing cell apoptosis in gastric mucosa.
Proliferation module.It is well known that tumor cell growth is closely associated with the imbalance between cell proliferation and apoptosis [53]. And the acquisition of cell proliferation allows the progression of gastric precancerous lesions, invasion, and metastasis [54]. The figure above indicates that the PI3K-Akt signaling pathway, p53 signaling pathway, MAPK signaling pathway, and FoxO signaling pathway have a tight relationship with the disorder of cell proliferation. For instance, the PI3K-Akt pathway is an important signaling pathway that is activated in a large variety of tumors and the phosphorylation of AKT (Protein kinase B) could promote cell cycle progression and cell proliferation [55].As a vital molecule, some ingredients like luteolin,kaempferol, naringenin, and quercetin which connected to the Akt, may exert some influences on this target. It has been reported that kaempferol could induce G2/M cell cycle arrest by suppressing AKT activation, which suggested that kaempferol may be useful in cancer prevention [56]. Thus, this work showed that SJZ decoction might integrate diverse signaling pathways to modulate cell proliferation to prevent cancer.
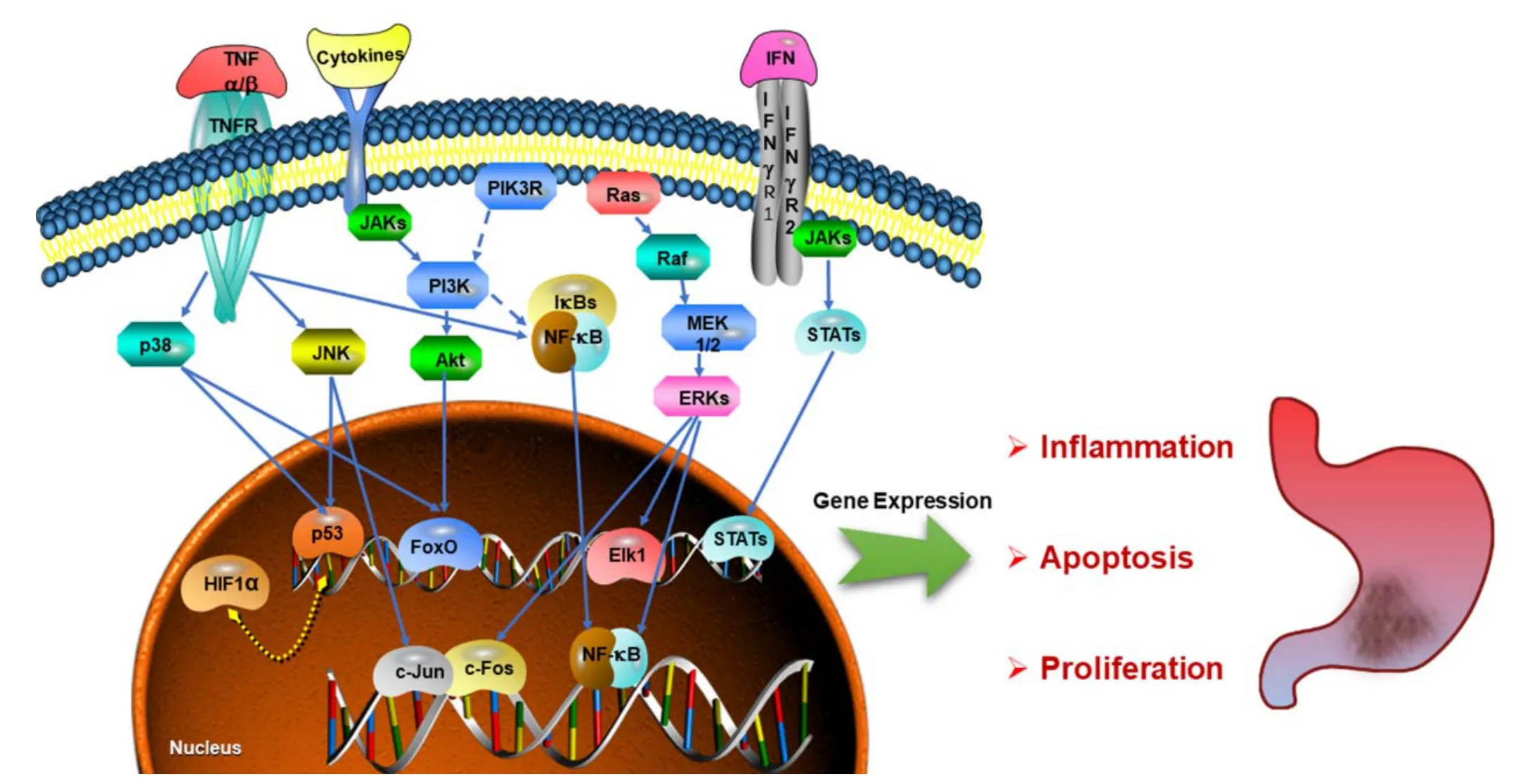
Figure 4 Gastric precancerous lesions associated pathway
Discussion
Gastric cancer is one of the most common types of cancer and it remains the second leading cause of cancer death worldwide [57]. Although the incidence of gastric cancer has decreased, the mortality rate has markedly improved[58] and there is still a long way to go before complete conquest of this disease [59]. Therefore, early diagnosis and treatment of GPL are crucial to reduce the morbidity and mortality of gastric cancer [59, 60]. As a useful alternative medicine with a long history, TCM is attracting more and more attention across the world for its remarkable effect in clinical practice. It is well acknowledged that spleen deficiency is the key pathogenesis of GPL [61]. SJZ decoction has been applied to treat GPL owing to its ability to fortify the spleen and replenish the Qi. Thus, it is imperative to use the network pharmacology approach to explore the therapeutic mechanism of SJZ decoction in the treatment of GPL.
In this study, there are 111 compounds collected from SJZ decoction and 90 targets considered to have therapeutic effects in treating GPL. Among the 111 compounds linked to the C-T network, quercetin, luteolin,kaempferol, stigmasterol, atractylenolide I are known bioactive compounds and have been shown to have anti-cancer effects [62-66]. For instance, quercetin, a vital member belonging to the flavonoid family, is one of the most prominent dietary antioxidants and famous for its anticarcinogenic potential. It can regulate the balance of gastric cell proliferation and apoptosis to protect against gastritis and induce the apoptosis of human gastric carcinoma cells by stimulating the activity of caspase-3 and decreasing the rate of Bcl-2/Bax [67, 68]. According to a clinical research in Sweden, high dietary quercetin intake is inversely related to the risk of gastric cancer,especially for women exposed to oxidative stress [69].Luteolin is a natural flavonoid present in several herbs.Researches indicated that luteolin can prevent cancer by arresting the cell cycle, inducing apoptosis [70, 71]. And it exerts a significant anti-tumor effect in gastric cancer through decreasing the expression and phosphorylation of c-Met (tyrosine-protein kinase Met), and downstream phosphorylation of AKT and ERK (extracellular signal-regulated kinase) [72]. Kaempferol is a natural flavonoid found in many herbs [73]. Epidemiologic studies have demonstrated that dietary Kaempferol intake could reduce the risk of cancer, especially gastric cancer[74], and induce apoptosis in human stomach carcinoma cells [75]. All in all, these findings suggest that the active components in SJZ decoction are effective in treating the disease.
On the other hand, lots of targets were found to be linked to multiple compounds in the C-T network. Among these targets, COX-2, TNF, and NOS2 (Nitric Oxide Synthase 2) are inflammation-associated enzymes with oncogenic function in tumor progression [76, 77]. Such as COX-2, evidence shows that COX-2 over expression is related to gastric premalignant changes and subsequent tumor occurrence [78], and the decrease of COX-2 is related to the regression of precancerous lesions [79].CDK2 (Cyclin-dependent kinase 2), GSK3B (Glycogen synthase kinase 3 beta), Bax, and Bcl-2, which involved in cell proliferation or apoptosis processes, are playing important roles in the tumorigenesis [80-83]. Certain compounds in SJZ decoction like quercetin, whose targets are Bcl-2 and Bax in the C-T network, have been shown to be effective in deregulating the expression of Bcl-2 and Bax [84]. By analyzing the targets, the work revealed that this formula may treat GPL at numerous levels.
According to the GO analysis and the KEGG pathway enrichment, the results demonstrated that SJZ decoction may treat GPL by modulating gastric mucosal inflammation, cell apoptosis, and proliferation. For example, cellular response to cytokine stimulus (GO:0071345) involves in inflammation, regulation of apoptotic process (GO: 0042981) and negative regulation of apoptotic process (GO: 0043066) involve in cell apoptosis, and regulation of cell proliferation (GO:0042127) involves in cell proliferation, respectively. It is well known that chronic inflammation is an established risk factor during the progression from GPL to gastric cancer [45]. And the disruption in the balance between gastric epithelial cell proliferation and cell death is linked to the progression from chronic gastritis, atrophy,intestinal metaplasia, dysplasia and ultimately to cancer[85]. KEGG pathway analysis indicated that SJZ decoction could target 21 pathways which involved in these biological processes to intervene in the pathogenesis of GPL. For instance, BZ, a widely used traditional herbal medicine for gastrointestinal system diseases, has been shown to be effective in down regulating the production and mRNA expression of inflammatory cytokines like TNF-α, IL-6, and IL-8 through inhibition of the NF-κB signal pathway [86].Studies have demonstrated that Atractylenolide III,obtained from DS and BZ, is able to decrease the TNF level in a dose-dependent manner [87]. All these data indicate that these pathways might interact to exert their combined effects against GPL, which could reveal the potential effects of SJZ decoction.
Although numerous studies have demonstrated that TCM formulations are effective and safe in treating GPL[88], and could inhibit the carcinogenesis of gastric mucosa [89], the mechanisms of action are still unknown.The network pharmacology approach applied in this work provided a novel approach for exploring the molecular mechanisms underlying the therapeutic effects of SJZ decoction and improved our understanding of herbal medicines. The results indicated that the pharmacological mechanisms of SJZ decoction on GPL might be strongly associated with its synergic modulation on inflammation,cell apoptosis, and proliferation. However, there was no experimental verification in this work, such as quantitative real-time PCR or western blot analysis to estimate the predictions. More works are required to be done to verify the result and to find the key mechanisms.
Conclusion
In this study, we utilized a network pharmacology approach by integrating active compounds screening,targets prediction, network analysis, and pathway analysis to explore the therapeutic mechanism of SJZ decoction on GPL. The result revealed that SJZ decoction exerts a therapeutic effect by intervening in the mucosal inflammation, cell apoptotic process, and cell proliferation. The approach applied in this work offers a new approach to explore TCM formulations and promotes the therapeutic application of traditional medicines in modern medicine.
1. Cunningham SC, Kamangar F, Kim MP, et al.Survival after gastric adenocarcinoma resection:eighteen-year experience at a single institution. J Gastrointest Surg 2005, 9: 718-725.
2. Yamaoka Y. How to eliminate gastric cancer-related death worldwide. Nat Rev Clin Oncol 2018, 15:407-408
3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources,methods and major patterns in GLOBOCAN 2012.Int J Cancer 2015, 136: E359-386.
4. Correa P. A human model of gastric carcinogenesis.Cancer Res 1988, 48: 3554-3560.
5. Marques-Silva L, Areia M, Elvas L, et al. Prevalence of gastric precancerous conditions: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2014, 26: 378-387.
6. de Vries AC, van Grieken NC, Looman CW, et al.Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 2008, 134: 945-952.
7. Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends 2010, 4:297-307.
8. Wang X, Zhang A, Sun H. Future perspectives of Chinese medical formulae: chinmedomics as an effector. OMICS 2012, 16: 414-421
9. Gao L, Hao J, Niu YY, et al. Network pharmacology dissection of multiscale mechanisms of herbal medicines in stage IV gastric adenocarcinoma treatment. Medicine (Baltimore) 2016, 95: e4389.
10. Yu X, Cui Z, Zhou Z, et al. Si-jun-zi decoction treatment promotes the restoration of intestinal function after obstruction by regulating intestinal homeostasis. Evid Based Complement Alternat Med 2014, 2014: 928579.
11. Liu L, Han L, Wong DY, et al. Effects of Si-Jun-Zi decoction polysaccharides on cell migration and gene expression in wounded rat intestinal epithelial cells. Br J Nutr 2005, 93: 21-29.
12. Chang CH, Yu B, Su CH, et al. Coptidis rhizome and Si Jun Zi Tang can prevent Salmonella enterica serovar Typhimurium infection in mice. PLoS One 2014, 9: e105362.
13. Li L, Zou C, Zhou Z, et al. Effects of herbal medicine Sijunzi decoction on rabbits after relieving intestinal obstruction. Braz J Med Biol Res 2017, 50:e6331.
14. Song FL, Lin YF, Li HR. Effect of Jiawei Sijunzi decoction based triple therapy on atrophic gastritis and expression of PCNA. China J Trad Chin Med Pharm 2009, 4: 367-369.
15. Ss ZN, Wang ZF, Luo XM. Meta-analysis of Modified Sijunzi Tang for Treating Chronic Atrophic Gastritis. Chin J Exp Tradi Med Formulae 2015, 21:204-208.
16. Tang RY, Lin J. Status of clinical studies on treating precancerous lesions of gastric cancer with Chinese herbs. Trad Chin Med 2014, 3: 79-84.
17. Hao DC, Xiao PG. Network pharmacology: a rosetta stone for traditional Chinese medicine. Drug Dev Res 2014, 75: 299-312.
18. Chandran U, Patwardhan B. Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera.J Ethnopharmacol 2017, 197: 250-256.
19. Zuo H, Zhang Q, Su S, et al. A network pharmacology-based approach to analyse potential targets of traditional herbal formulas: An example of Yu Ping Feng decoction. Sci Rep 2018, 8: 11418.
20. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014, 6: 13.
21. Xu X, Zhang W, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci 2012, 13:6964-6982.
22. Alam MA, Al-Jenoobi FI, Al-Mohizea AM, et al.Understanding and managing oral bioavailability:physiological concepts and patents. Recent Pat Anticancer Drug Discov 2015, 10: 87-96.
23. Deng X, Xing X, Sun G, et al. Guanxin Danshen formulation protects against myocardial ischemia reperfusion injury-Induced left ventricular remodeling by upregulating estrogen receptor β.Front Pharmacol 2017, 8: 777.
24. Zheng C, Wang J, Liu J, et al. System-level multi-target drug discovery from natural products with applications to cardiovascular diseases. Mol Divers 2014, 18: 621-635.
25. Li J, Zhao P, Li Y, et al. Systems pharmacology-based dissection of mechanisms of Chinese medicinal formula Bufei Yishen as an effective treatment for chronic obstructive pulmonary disease. Sci Rep 2015, 5: 15290.
26. Walters WP, Murcko MA. Prediction of'drug-likeness'. Adv Drug Deliv Rev 2002, 54:255-271.
27. Tian S, Wang J, Li Y, et al. The application of in silica drug-likeness predictions in pharmaceutical research. Adv Drug Deliv Rev 2015, 86: 2-10.
28. Yamanishi Y, Kotera M, Kanehisa M, et al.Drug-target interaction prediction from chemical,genomic and pharmacological data in an integrated framework. Bioinformatics 2010, 26: i246-254.
29. Mauri A, Consonni V, Pavan M, et al. DRAGON software: An easy approach to molecular descriptor calculations. Match Commun Math Comput Chem 2006, 56: 237-248.
30. Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 1991, 175: 880-885.
31. M GL, C YY. Predicting Caco-2 permeability using support vector machine and chemistry development kit. J Pharm Pharm Sci 2006, 9: 210-221.
32. Li LY, Li Y, Wang YH, et al. Prediction of human intestinal absorption based on molecular indices. J Mol Sci 2007, 23: 286-291.
33. Yu H, Chen J, Xu X, et al. A systematic prediction of multiple drug-target interactions from chemical,genomic, and pharmacological data. PLoS One 2012,7: e37608.
34. Gfeller D, Grosdidier A, Wirth M, et al.SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014. 42 (Web Server issue): W32-38.
35. Rebhan M, Chalifa-Caspi V, Prilusky J, et al.GeneCards: integrating information about genes,proteins and diseases. Trends Genet 1997, 13: 163.
36. Safran M, Dalah I, Alexander J, et al. GeneCards Version 3: the human gene integrator. Database(Oxford). 2010, 2010: baq020.
37. Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000, 25:25-29.
38. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000, 28:27-30.
39. Chen EY, Tan CM, Kou Y, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform 2013, 14: 128.
40. Su G, Morris JH, Demchak B, et al. Biological network exploration with Cytoscape 3. Curr Protoc Bioinform 2014, 47: 1-24.
41. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003, 13: 2498-2504.
42. Ji GQ, Chen RQ, Wang L. Anti-inflammatory activity of atractylenolide III through inhibition of nuclear factor-κB and mitogen-activated protein kinase pathways in mouse macrophages.Immunopharmacol Immunotoxicol 2016, 38:98-102.
43. Fishilevich S, Zimmerman S, Kohn A, et al. Genic insights from integrated human proteomics in GeneCards. Database (Oxford) 2016, 2016. pii:baw030.
44. Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer 2017, 17: 594-604.
45. Menheniott TR, O'Connor L, Chionh YT, et al. Loss of gastrokine-2 drives premalignant gastric inflammation and tumor progression. J Clin Invest 2016, 126: 1383-1400.
46. Sharma SA, Tummuru MK, Blaser MJ, et al.Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol 1998, 160: 2401-2407.
47. Cianciulli A, Calvello R, Porro C, et al. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int Immunopharmacol 2016, 36: 282-290.
48. Li Z, He Q, Zhai X, et al. Foxo1-mediated inflammatory response after cerebral hemorrhage in rats. Neurosci Lett 2016, 629: 131-136.
49. He YQ, Yang H, Shen Y, et al. Monotropein attenuates ovariectomy and LPS-induced bone loss in mice and decreases inflammatory impairment on osteoblast through blocking activation of NF-κB pathway. Chem Biol Interact 2018, 291: 128-136.
50. Que FG, Gores GJ. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterol 1996, 110:1238-1243.
51. Ishida M, Gomyo Y, Tatebe S, et al. Apoptosis in human gastric mucosa, chronic gastritis, dysplasia and carcinoma: analysis by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling. Virchows Arch 1996, 428:229-235.
52. Pujals A, Favre L, Pioche-Durieu C, et al.Constitutive autophagy contributes to resistance to TP53-mediated apoptosis in Epstein-Barr virus-positive latency III B-cell lymphoproliferations. Autophagy 2015, 11:2275-2287.
53. Fan YZ, Zhao ZM, Fu JY, et al. Norcantharidin inhibits growth of human gallbladder carcinoma xenografted tumors in nude mice by inducing apoptosis and blocking the cell cycle in vivo.Hepatobiliary Pancreat Dis Int 2010, 9: 414-422.
54. Díaz P, Valenzuela VM, Bravo J, et al. Helicobacter pylori and gastric cancer: adaptive cellular mechanisms involved in disease progression. Front Microbiol 2018, 9: 5.
55. Cappellini A, Tabellini G, Zweyer M, et al. The phosphoinositide 3-kinase/Akt pathway regulates cell cycle progression of HL60 human leukemia cells through cytoplasmic relocalization of the cyclin-dependent kinase inhibitor p27(Kip1) and control of cyclin D1 expression. Leukemia 2003, 17:2157-2167.
56. Huang WW, Tsai SC, Peng SF, et al. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int J Oncol 2013, 42:2069-2077.
57. Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol 2014, 20: 13767-13774.
58. Choi YJ, Kim N. Gastric cancer and family history.Korean J Intern Med 2016, 31: 1042-53.
59. Joo MK, Park JJ, Chun HJ. Recent updates of precision therapy for gastric cancer: Towards optimal tailored management. World J Gastroenterol 2016, 22: 4638-4650.
60. Pasechnikov V, Chukov S, Fedorov E, et al. Gastric cancer: prevention, screening and early diagnosis.World J Gastroenterol 2014, 20: 13842-13862.
61. Chen J, Zhao AG, Cao ND, et al. Advances in Research of Spleen Deficiency in Gastric Precancerous Conditions, Precancerous Lesions of Gastric cancer and Gastric cancer. Chin Arch Trad Chin Med 2013, 31: 1654-1657.
62. Khan F, Niaz K, Maqbool F, et al. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8: E529.
63. Tuorkey MJ. Molecular targets of luteolin in cancer.Eur J Cancer Prev 2016, 25: 65-76.
64. Kangsamaksin T, Chaithongyot S,Wootthichairangsan C, et al. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS One 2017, 12: e0189628.
65. Qin Y, Cui W, Yang X, et al. Kaempferol inhibits the growth and metastasis of cholangiocarcinoma in vitro and in vivo. Acta Biochim Biophys Sin(Shanghai) 2016: 48: 238-245.
66. Huang JM, Zhang GN, Shi Y, et al. Atractylenolide-I sensitizes human ovarian cancer cells to paclitaxel by blocking activation of TLR4/MyD88-dependent pathway. Sci Rep 2014, 4: 3840.
67. Wang P, Zhang K, Zhang Q, et al. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol In Vitro 2012, 26: 221-228.
68. Zhang S, Huang J, Xie X, et al. Quercetin from Polygonum capitatum Protects against Gastric Inflammation and Apoptosis Associated with Helicobacter pylori Infection by Affecting the Levels of p38MAPK, BCL-2 and BAX. Molecules 2017, 22:pii: E744.
69. Ekström AM, Serafini M, Nyrén O, et al. Dietary quercetin intake and risk of gastric cancer: results from a population-based study in Sweden. Ann Oncol 2011, 22: 438-443.
70. Wang F, Gao F, Pan S, et al. Luteolin induces apoptosis, G0/G1 cell cycle growth arrest and mitochondrial membrane potential loss in neuroblastoma brain tumor cells. Drug Res (Stuttg)2015, 65: 91-95.
71. Ju W, Wang X, Shi H, et al. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol 2007,71: 1381-1388.
72. Lu J, Li G, He K, et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J Transl Med 2015, 13: 42.
73. Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem 2013, 138:2099-2107.
74. Garcia-Closas R, Gonzalez CA, Agudo A, et al.Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control 1999, 10: 71-75.
75. Liao W, Chen L, Ma X, et al, Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur J Med Chem 2016, 114: 24-32.
76. Basudhar D, Glynn SA, Greer M, et al.Coexpression of NOS2 and COX2 accelerates tumor growth and reduces survival in estrogen receptor-negative breast cancer. Proc Natl Acad Sci USA 2017, 114: 13030-13035.
77. Chung SS, Wu Y, Okobi Q, et al. Proinflammatory cytokines IL-6 and TNF-α increased telomerase activity through NF-κB/STAT1/STAT3 activation,and withaferin A inhibited the signaling in colorectal cancer cells. Mediators Inflamm 2017, 2017:5958429.
78. Badary DM, MZAA R, Ashmawy AM, et al. H.pylori infection increases gastric mucosal COX2 and mTOR expression in chronic gastritis: Implications for cancer progression. Pathophysiol 2017, 24:205-211.
79. Zhang Y, Pan KF, Zhang L, et al. Helicobacter pylori,cyclooxygenase-2 and evolution of gastric lesions:results from an intervention trial in China.Carcinogenesis 2015, 36: 1572-1579.
80. Gürsel DB, Banu MA, Berry N, et al. Tight regulation between cell survival and programmed cell death in GBM stem-like cells by EGFR/GSK3b/PP2A signaling. J Neurooncol 2015,121: 19-29.
81. Ling H, Samarasinghe S, Kulasiri D. Computational experiments reveal the efficacy of targeting CDK2 and CKIs for significantly lowering cellular senescence bar for potential cancer treatment.Biosystems 2013, 111: 71-82.
82. Rubenstein M, Hollowell CM, Guinan P.Suppression of BCL2 by antisense oligonucleotides and compensation by non-targeted genes may enhance tumor proliferation. In Vivo 2015, 29:687-693.
83. Liu Z, Ding Y, Ye N, et al. Direct activation of bax protein for cancer therapy. Med Res Rev 2016, 36:313-341.
84. Liu Y, Tang ZG, Lin Y, et al. Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed Pharmacother 2017, 92: 33-38.85. Leung WK, Yu J, To KF, et al. Apoptosis and proliferation in Helicobacter pylori-associated gastric intestinal metaplasia. Aliment Pharmacolther 2001, 15: 1467-1472.
86. Hong MH, Kim JH, Bae H, et al. Atractylodes japonica Koidzumi inhibits the production of proinflammatory cytokines through inhibition of the NF-kappaB/IkappaB signal pathway in HMC-1 human mast cells. Arch Pharm Res 2010, 33:843-851.
87. Li CQ, He LC, Jin JQ. Atractylenolide I and atractylenolide III inhibit Lipopolysaccharideinduced TNF-alpha and NO production in macrophages. Phytother Res 2007, 21: 347-353.
88. Mo XJ, Wei CH, Cheng GZ. Efficacy of Chinese herbal medicine in chronic atrophic gastritis: a systematic review. Liaoning J Trad Chin Med 2013,40: 840-846.
89. Liu NN, Deng WL, Wu CJ, et al. Effect of Jianpi Jiedu Recipe on angiogenesis and the PTEN/PI3K/AKT signaling. Trad Med Res 2018, 3:29-39.
 Traditional Medicine Research2018年6期
Traditional Medicine Research2018年6期
- Traditional Medicine Research的其它文章
- The role of Si-Jun-Zi decoction in the management of gastric precancerous lesions and its mechanism
- Recent advances in network pharmacology applications in Chinese herbal medicine
- Analysis of the action mechanism of Fang Ji Huang Qi decoction in treating rheumatoid arthritis by network pharmacology
- Network pharmacology-based approach to investigate the mechanisms of Yiyi Fuzi Baijiang Powder in the treatment of malignant tumors
- Camel milk could be helpful in the treatment of asthma
