Extraction of Lignin from Tobacco Stem using Ionic Liquid
Ming-hui FnSho-lin GeZho ZhngYing-song XieQun-xin Li
a.Anhui Key Laboratory of Tobacco Chemistry,China Tobacco Anhui Industrial Co.,LTD,Hefei 230088,China
b.Department of Chemical Physics,Key Laboratory of Urban Pollutant Conversion,Chinese Academy of Sciences,Anhui Key Laboratory of Biomass Clean Energy,University of Science and Technology of China,Hefei 230026,China
Key words:Tobacco stem,Pretreatment,Lignin,Ionic liquid∗Author to whom correspondence should be addressed. E-mail:liqx@ustc.edu.cn,Tel:+86-551-63601118,FAX:+86-551-63606689
I.INTRODUCTION
Stem roughly accounts for 25%of the total mass and usually the cut stem is added into the cigarette to a certain proportion[1].The advantages of this approach are obvious,such as lowering the cost of production and the tar content,improving cigarette combustion property and filling capacity.Lignin is polymerized through three primary phenyl propane monomers-coniferyl alcohol(G),sinapyl alcohol(S),and coumaryl alcohol(G)[2].In the plant cell walls,lignin serves to maintain the intergrity of the cellulose/hemicellulose/pectin matrix.Stem lignin has relatively high molecular weight of about 4.8×105and in tobacco stem lignin has a moderate content at about 6%,but it in fluences the combustion characteristics of cigarette products significantly. During the process of cigarette production,lignin is relatively stable while some harmful substances such as aromatic amines,catechol and phenol in the tobacco tar may be mainly caused by lignin because its macromolecule contains a variety of benzene rings,thus limiting the addition of stem into the cigarette products.Traditional researches of tobacco lignin focus on the determination of lignin in tobacco and the main method of lignin separation used in tobacco industry is Van Soest method which uses chexadecyl trimethylamine bromide(CTMAB)and sulphuric acid as acid detergent.This method results in severely structural destruction of lignin.
We expect a modified method which can maximally maintain the original lignin structure.Recently different kinds of ionic liquids(ILs)are used for timber treatment such as birch,spruce,and pine[3–6].Leeet al.found that 1-allyl-3-methylimidazolium chloride([Amim][Cl])and 1-butyl-3-methylimidazolium acetate([Bmim][OAc])can dissolve maple at 110◦C[7].Zalzeskiet al.showed that lignin can dissolve in 1-ethyl-3-methylimidazolium diethylphosphate([Emim][DEP])[8].Kimet al.directly extracted lignin from birch using 1-ethyll-3-methylimidazolium chloride([Emim][OAc])and named the lignin as ionic liquid lignin(ILL)[9].Sunet al.showed that lignin was degraded partially in[Emim][OAc]and explained the model of the bond break ofβ-O-4 of lignin in acidic ionic liquidis was proposed[10].The mechanism of lignin dissolution and regeneration is the destruction and reorganization of hydrogen bond,so the mixture of acetone and water can be used for lignin regeneration[11].In general,lignin can dissolve in imidazolium ionic liquids well because ofπ-πconjugation formed byπelectrons in imidazole ring and aromatic rings in lignin[12].
II.MATERIALS AND METHODS
A.Feedstocks
Thetobaccostem wascollectedfrom Yunnan Province and was grinded to fine powder before use.The chemical compositions(mass ratio)of the stem are: 35.52%carbon,43.18%oxygen,5.17%hydrogen,2.36%nitrogen;and the lignin content is about 7.52%on dry basis.The model lignin we chose was manufactured from wheat straw and purchased from Lanxu Biotechnology Co.Ltd.(Hefei,China).The chemical compositions(mass ratio)of model lignin mainly consist of 62.55%carbon,29.91%oxygen,5.89%hydrogen,1.65%nitrogen.All the data mentioned above are measured by elemental analyzer(Vario EL-III,Elementar,Germany). Four typical imidazolium-based ionic liquids(IBILs),1-ethyl-3-methylimidazolium diethylphosphate([Emim][DEP]),1-butyl-3-methylimidazolium acetate([Bmim][OAc]),1-allyl-3-methylimidazolium chloride([Amim][Cl])and 1-ethyll-3-methylimidazolium acetate([Emim][OAc]),were purchased from Lanzhou Institute of Chemical Physics.Other reagents including acetone,potassium permanganate,sulfuricacid werepurchased from Sinopharm Chemical Reagent Company Limited in China(Shanghai,China).
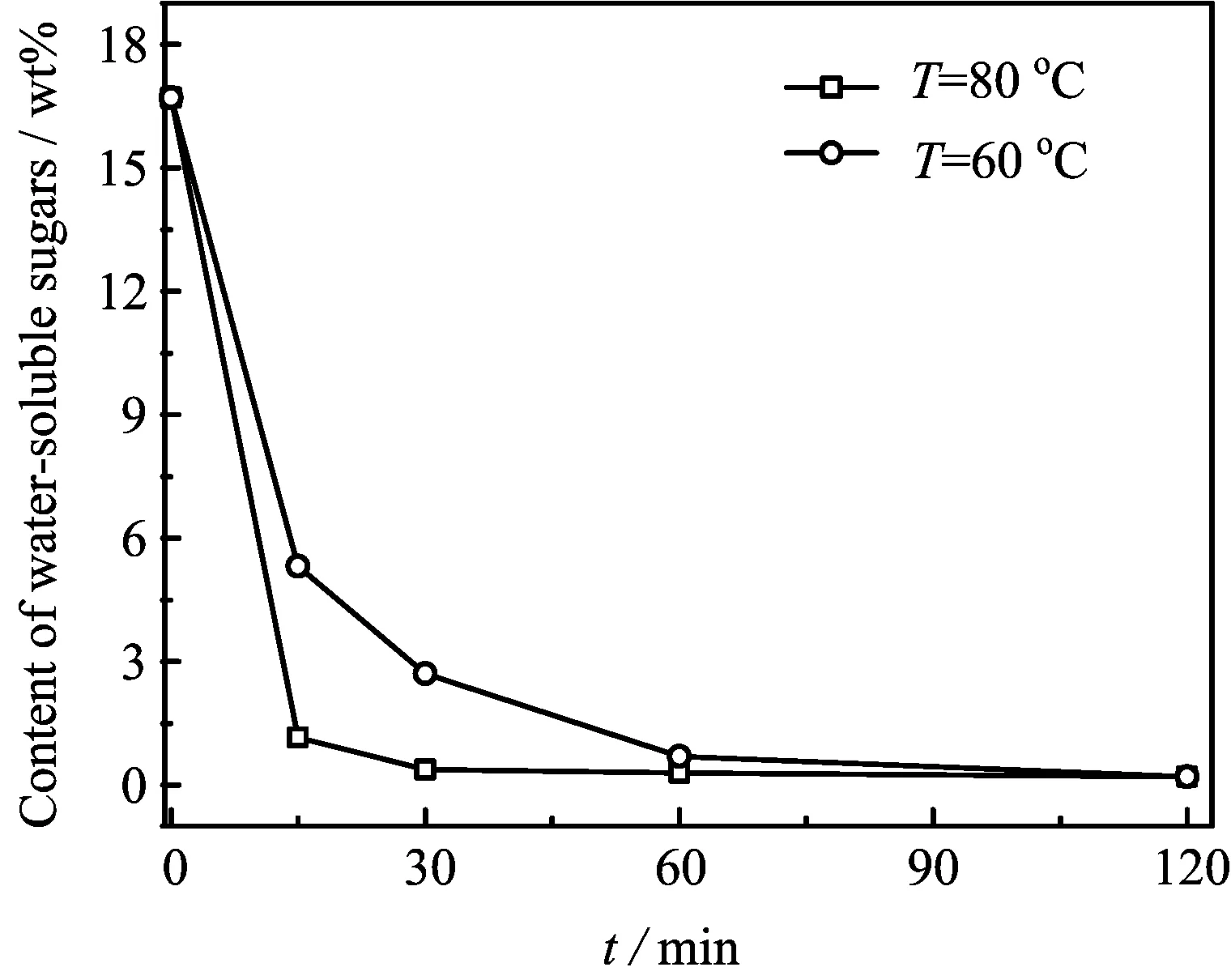
FIG.1 Effects of temperature and time on the removal of water-soluble sugars processing of tobacco stem.Reaction conditions:stem/water of 1:10.
E.Characteristic of samples
B.Optimum ionic liquid for lignin dissolution and regeneration
Prior to lignin extraction from tobacco stem,we tested four typical imidazolium-based ionic liquids for lignin dissolution and regeneration.In each test,3 g dry model lignin powder was added into a certain kind of imidazolium-based ionic liquid(60 g)in a boiling flask at a given temperature with stirring.Then cool down the system to room temperature after reaction and add acetone/water(in volume ratio of 1:1)mixture dropwise to precipitate lignin.Filter the mixture and wash the regeneration lignin with 80◦C hot water to remove ionic liquid residue.
C.Pretreatment of tobacco stem
The stem was smashed to fine powder and was sieved using 200-mesh sieve prior to use.Water content of tobacco stem powder is 6.46%based on oven method.Then the sieved powder was washed with distilled water under different conditions to remove soluble sugar and nicotine.The residue was then dried in an oven at 90◦C for 12 h after water-treated procedure.
D.Retreatment of tobacco stem
The selected ionic liquid was added into a flask and heated to the given temperature without oxygen.Then the ionic liquid was added to the retreated tobacco stem powder(ionic liquid/pretreated stem powder in weight ratio of 20:1)with stirring.The organic liquid was separated from the residue by filtration.Then aceton/deionized water(in volume ratio of 1:1)was added to precipitate the stem lignin,and the lignin was washed carefully with hot water three times then dried in oven at 100◦C overnight to obtain tawny powder.
The total saccharides content of stem samples was measured using an AutoAnalyzer 3 equipment and lignin content in the tobacco samples before and after delignifications were determined using a UV-Vis Spectrophotometry(UV-2550,Shimadzu)according to an US Patent[13].Fourier transforms infrared(FT-IR)spectra of the stem samples were recorded on a Bruker vector-2 spectrophotometer using KBr disc as carrier.Phenom SEM(Phenom Pro,China)was used for the space resolved analysis.
III.RESULTS AND DISCUSSION
A.Pretreatment of stem
Lignin and saccharides are connected via covalent bonds to form lignin-carbohydrate compound and tobacco stem contains 16.71%of carbohydrate on a dry weight basis(continuous flow method).Water can easily remove most of the water-soluble sugars such as glucose,fructose,and sucrose as shown in FIG.1.Temperature has a significant effect on the removal of watersoluble sugars progress and we can see from FIG.1(a)that the residual water-soluble removal rate arrived at 97.64%in 30 min at 80◦C.When the temperature dropped to 60◦C,it took 60 min to promote the residual water-soluble removal rate to 95.65%as shown in FIG.1(b).The yield of washed stem powder is about 46.25%after washing 30 min at 80◦C.
SEM is now widely used to study the structural change of biomass during various pretreatments and FIG.2 shows the SEM micrographs of the stem powder samples after different treatments.FIG.2(a)is the untreated stem and it can be seen that the microstructure in raw stem is tighter than that in water-treated stem powder.It revealed that rising washing temperature or prolonging washing time leads to the relaxation of the microstructure in stem.FIG.2(f)gives fine image that the microstructure in stem becomes looser after the removal of water-soluble sugars progress was carried out at 80◦C for 30 min,which is bene ficial for subsequent removal of lignin.
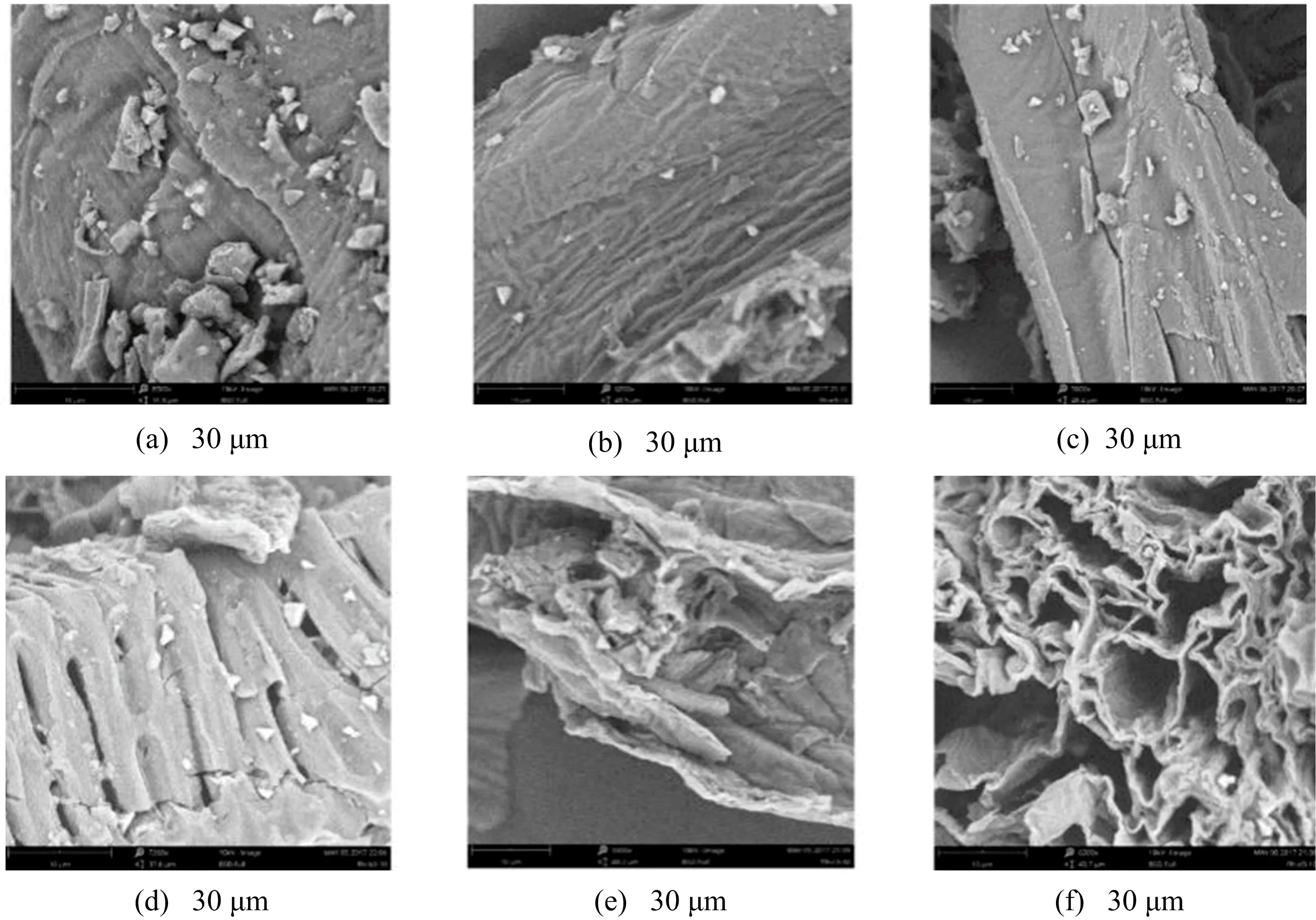
FIG.2 SEM photograph of tobacco stems of(a)raw stem powder and SEM photograph of tobacco stems after removal of water-soluble sugars progress at different temperatures and times:(b)T=20 ◦C,t=15 min;(c)T=40 ◦C,t=15 min;(d)T=60 ◦C,t=15 min;(e)T=80 ◦C,t=15 min;(f)T=80 ◦C,t=30 min.
B.Imidazolium-based ionic liquids(IBILs)screening for lignin regeneration
Model lignin is insoluble in water but is soluble in all these four different IBILs.Comparative tests of lignin regeneration were carried out at 150◦C for 4 h(ionic liquid/model lignin in weight ratio of 20:1)and the regeneration yieldYof lignin was calculated based on Eq.(1):
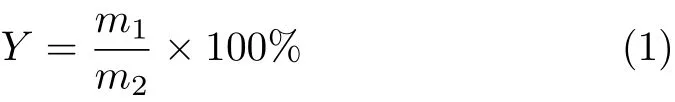
wherem1is the the mass of precipitate lignin,andm2is the mass of original lignin. The resultsshow thattheregeneration yield oflignin in different IBILs decereased in the following order: [Emim][DEP](82.63%)>[Bmim][OAc](73.05%)>[Emim][OAc](69.82%)>[Amim][Cl](56.41%). To explain the difference,we used pyridine as a probe for the analysis of acid site in imidazolium-based ionic liquids.The electron pair provided by N atom in pyridine can form PyH+with H proton donated by br¨onsted acid.FIG.3(a)is a typical FT-IR spectrum of pure pyridine(blank test)and the peaks at 1438,1480,1578 cm−1are characteristic absorption peaks of pyridine.The absorption peak at 1586 cm−1is related to the br¨onsted acid site which demonstrates the existence of br¨onsted acid in all four ionic liquid types.The peak intensity of br¨onsted acid site in[Emim][DEP]is a little higher than in[Amim][Cl]but the existence of Cl−greatly enhances the hydrogen acceptor of[Amim][Cl]and lowers the regeneration yield of lignin.[Emim][DEP]provides good solubility and regeneration yield of lignin.Meanwhile,the double bond in side chain of[Emim][DEP]reduces the solubility of cellulose,which is in favor of the lignin extraction from stem.
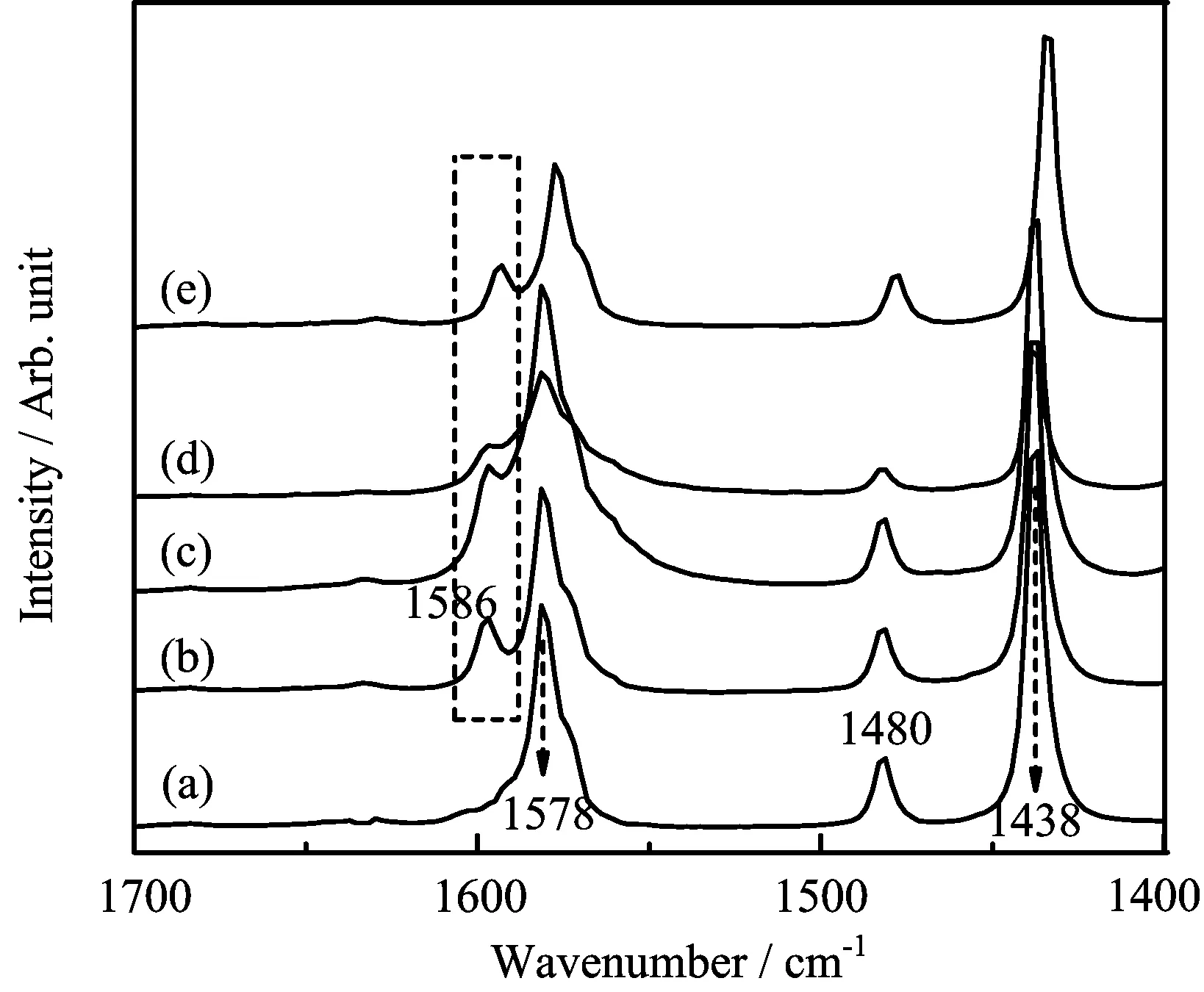
FIG.3 FT-IR spectra of(a)pure pyridine,(b)pyridine/[Emim][DEP]solution,(c)pyridine/[Bmim][OAc]solution, (d) pyridine/[Emim][OAc]solution, (e) pyridine/[Amim][Cl]solution.The solutions of(b)−(e)are all in volume ratio of 5:1.
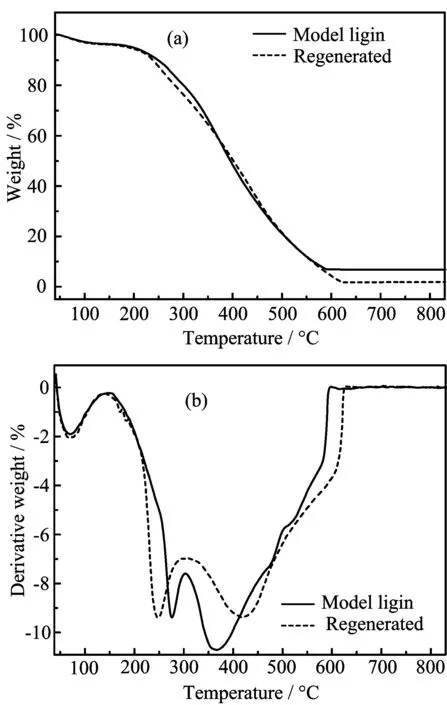
FIG.4(a)TGA and(b)DTG analysis for model lignin and regenerated model lignin.

FIG.5(a)UV-Vis absorption spectrum of model lignin dissolved in 0.3mg/mL dioxane/water mixture(in volume ratio of 4:1),(b)The dependence of the UV-Vis absorbance on the lignin concentration.
C.Thermo gravimetric analysis
Lignin hascomplex amorphousstructuresand thermo gravimetric analysis(TGA)is an important method to detect the physical and chemical properties of polymeric compound.FIG.4 shows the TG and DTG results of model lignin before and after[Emim][DEP]treatment.Comparing the thermal weight loss of the two lignin samples,we found that they almost had the same weight loss ratio of 5%at the initial heating stage(40−145◦C).It is mainly because free water in lignin macromolecule is evaporated at this stage.The main depolymerization stages of the two lignin samples occur at 200−600◦C.The initial degradation temperatures of model lignin and regenerated model lignin are 142 and 137◦C respectively,which means the thermostability of lignin almost remains unchanged after[Emim][DEP]treatment.TGA shows that the temperature of 10%weight loss of model lignin and regenerated model lignin are 248 and 238◦C respectively.The temperature of 50%weight loss of model lignin and regenerated model lignin are 395 and 401◦C respectively.Macromolecule structure in lignin was depolymerized to various aromatic compounds and gaseous products including CO,CO2and light ole fins in the fast weight loss temperatures region[14].When temperature rises above 622◦C,
the regenerated model lignin has a lower residue ratio of 1.76%while the reissue ratio of model lignin reached 6.63%at 590◦C.This is because the lignin experienced condensation polymerization at this temperature and formed cross-linked structure compounds with high stability.The temperature of maximum weight loss rate of model lignin occurs at 419◦C,which is higher than that of regenerated model lignin(367◦C).As we can see from the FT-IR data in FIG.6,the content of D-xylose in regenerated model is a little higher than in untreated model lignin and the increased content of carbohydrate led to a slight drop in thermostability[15].
D.Effect of temperature and reaction time on lignin extraction
The purity of lignin we extracted from stem was determined using UV-Vis Spectrophotometry(UV-2550,Shimadzu).The experiments were performed as the following steps:
(i)Dissolve 30 mg of model lignin in 100 mL dioxane/water(in volume ratio of 4:1)mixture,then the wavelength of maximum absorbency is determined on the basis of full wave scanningas shown in FIG.5(a).The characteristic absorption peak of lignin in UV range is 231 nm which is related to the conjugated doublebond in lignin macromolecule[16].
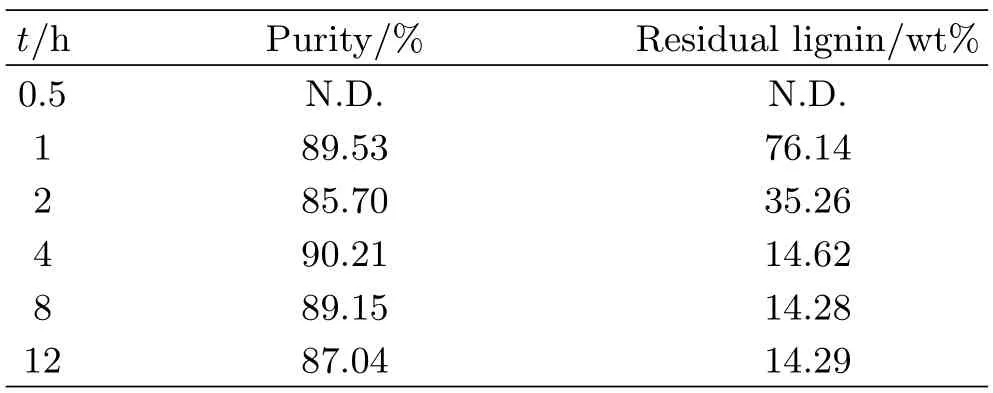
TABLE I Effect of reaction time on the extraction of lignin from tobacco stem using[Emim][DEP]at 150◦C.
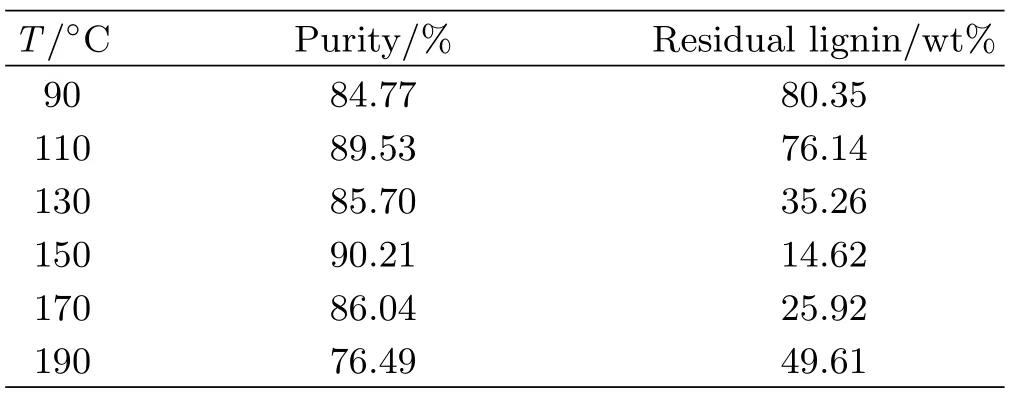
TABLE II Effect of temperature on the extraction of lignin from tobacco stem using[Emim][DEP]for 4 h.
(ii)Prepare lignin/dioxane/water solutions with different concentration,and detect the absorbance of different solutions at 231 nm respectively.FIG.5(b)represents the relationship between absorbance and lignin concentration.After linear fitting,the following equation is obtained:

whereAis the absorbance of solutions at 231 nm,cis the concentration of the solutions.The linear relationship in the range of 0.02−0.07 mg/mL is good and the coefficient of determinationR2is 0.9868,so this equation is used as a standard line.
(iii)Dissolve a certain amount of stem lignin in dioxane/water(in volume ratio of 4:1)mixture.The concentration of this solution was labeled asc1,then the absorbance ofc1was tested at 231 nm.The real concentration(labeled asc2)of stem lignin can be obtained using standard line mentioned above.And the purity of stem ligninP(%)was calculated based on Eq.(3):

whereA1is the absorbance ofc1,and theA2is the absorbance ofc2.
(iv)The residual lignin in stem was calculated according to the method in a US patent[13].

FIG.6 FT-IR spectra of(a)model lignin under different treatments,(b)tobacco stem powder before and after the delignification using[Emim][DEP]at 150◦C for 4 h.
Temperature has an important effect on lignin extraction from stem with the selected[Emim][DEP]as shown in Table I.The content of residual lignin in stem decreases from 89.61%to 25.92%when reaction temperature increases from 90◦C to 190◦C.That is because the apparent viscosity of ionic liquid decreases with the temperature increasing,which is favorable to mass transfer.The extraction ratio of lignin reaches its maximum value at 150◦C which is close to the glass transition temperature of lignin.But high temperatures over 150◦C will lead to the cracking of[Emim][DEP],so the content of residual lignin is obviously elevated.We can conclude from Table II that prolonging reaction time can effectively reduce the residual lignin ratio.If the reaction time is too short,stem cannot completely dissolve in[Emim][DEP].However,the reaction time should not last too long,as excessive reaction time not only wastes energy but also aggravates the degradation of lignin.
E.FT-IR analysis of stem lignin
FT-IR spectroscopy is now widely used to obtain direct information on chemical changes of lignin after treatments.We use model lignin to investigate the influence of washing process and[Emim][DEP]treatment.FIG.6(a)presents the FT-IR spectra of untreated model lignin,water treated model lignin and regener-ated model lignin using[Emim][DEP].The peaks at 1116 and 1270 cm−1are characteristic absorptions of syringyl unit and guaiacyl unit respectively. There is barely no obvious absorbance at 1166 cm−1which means model lignin contains few hydroxyl phenyl unit.The regenerated model lignin shows a shoulder peak at 948 cm−1which is due to the C–H bending ofβ-D-xylose.Each spectrum gives a peak at 1046 cm−1,which is attributed to the xylan.The absorbance at 1215 cm−1is arised from C–O stretching while the absorbance at 1461 cm−1is assigned to the asymmetric C–H stretching vibration.The peaks at 1426 and 1514 cm−1are related to the skeleton vibration of aromatic rings.The absorption band at 1611 cm−1is related to the stretching of conjugated C=O and the band at 1705 cm−1is related to the stretching of unconjugated C=O.The peak at 2852 cm−1is due to the C–H vibration of alkyl group while the peak at 2929 cm−1is assigned to the C–H stretching of aromatic rings.The infrared spectra of untreated and washed model lignin are very similar,which means water processing procedure barely changes the chemical constructions of lignin.The relative absorption intensities at 1426 and 1514 cm−1are almost unchanged after regeneration process.FIG.6(b)shows the FT-IR spectra of tobacco stem powder before and after delignification process using[Emim][DEP]at 150◦C.And prior to extraction,stem powder was washed by distilled water at 80◦C for 30 min to remove almost all of saccharides and loose microstructure of stem.For delignification stem,the intensity of absorption peaks at 1118 and 1272 cm−1are decreased significantly,indicating that delignification process using[Emim][DEP]can remove most of the lignin.
IV.CONCLUSION
Lignin dissolution and regeneration experiments were carried out using different kinds of imidazolide ionic liquids and the results show that[Emim][DEP]provides good solubility and regeneration yield of lignin.Water can easily remove most of the water-soluble sugars in stem and loose the microstructure which is bene ficial for subsequent removal of lignin.The extraction of lignin from tobacco stem is successfully realized using[Emim][DEP].Under the optimal conditions(T=550◦C,t=4 h),the highest extraction yield and the purity of lignin reached 85.38%and 90.21%.The present research potentially provides a foundation for future study of the pyrolysis products of lignin after cigarette combustion.
V.ACKNOWLEDGEMENTS
This work is supported by the China Postdoctoral Science Foundation(BH2060000062)and China Tobacco Anhui Industrial Co.,LTD.
[1]K.B.Ji,Ph.D Dissertion,Guangzhou:South China University of Technology,(2013).
[2]F.G.Calvo-Flores and J.A.Dobado,ChemSusChem.3,1227(2010).
[3]D.A.Fort,R.C.Remsing,R.P.Swatloski,P.Moyna,G.Moyna,and R.D.Rogers,Green Chem.9,63(2007).
[4]N.Jiang,Y.Q.Pu,R.Samuel,and A.J.Ragauskas,Green Chem.11,1762(2009).
[5]I.Kilpel¨ainen,H.B.Xie,A.King,M.Granstrom,S.Heikkinen,and D.S.Argyropoulos,J.Agric.Food Chem.55,9142(2007).
[6]B.Li,J.Asikkala,I.Filpponen,and D.S.Argyropulos,Ind.Eng.Chem.Res.49,2477(2010).
[7]S.H.Lee,T.V.Doherty,R.J.Linhardt,and J.S.Dordick,Biotechnol.Bioeng.102,1368(2009).
[8]J.Zakzeski,P.C.A.Bruijnincx,A.L.Jongerious,and B.M.Weckhuysen,Chem.Rev.110,3552(2010).
[9]J.Y.Kim,E.J.Shen,I.Y.Eom,K.Won,Y.H.Kim,D.Choi,I.G.Choi,and J.W.Choi,Bioresour.Technol.102,9020(2011).
[10]Y.C.Sun,J.K.Xu,F.Xu,R.C.Sun,and G.L.Jones,RSC Adv.4,2743(2014).
[11]W.Y.Li,N.Sun,B.Stoner,X.Y.Jiang,X.M.Lu,and R.D.Rogers,Green Chem.13,2038(2011).
[12]M.Zavrel,D.Bross,M.Funke,J.Bchs,and A.C.Spiess,Bioresour.Technol.100,2580(2009).
[13]X.S.Chai and J.Y.Zhu,Method for Rapidly Determining a Pulp Kappa Number Using Spectrophotometry,US 6475339 B1(2002).
[14]J.C.Domnguez,M.Oliet,M.V.Alonso,M.A.Gilarranz,and F.Rodrguez,Ind.Crops Prod.27,150(2008).
[15]H.P.Yang,R.Yan,H.P.Chen,C.G.Zheng,D.H.Lee,and D.T.Liang,Energy Fuels 20,388(2006).
[16]A.Barros,A.Dhanabalan,C.J.L.Constantino,D.T.Balogh,and O.N.Oliveira.Jr.,Thin Solid Films 354,215(1999).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- A Simple,Compact and Rigid Scanning Tunneling Microscope
- Gd Doped Hollow Nanoscale Coordination Polymers as Multimodal Imaging Agents and a Potential Drug Delivery Carriers
- 3D Macro-Micro-Mesoporous FeC2O4/Graphene Hydrogel Electrode for High-Performance 2.5 V Aqueous Asymmetric Supercapacitors
- Gamma Ray Radiation Effect on Bi2WO6Photocatalyst
- Ag-Cu Nanoparticles Supported on N-Doped TiO2Nanowire Arrays for Efficient Photocatalytic CO2Reduction
- UV Laser Regulation of Surface Oxygen Vacancy of CoFe2O4for Enhanced Oxygen Evolution Reaction
