3D Macro-Micro-Mesoporous FeC2O4/Graphene Hydrogel Electrode for High-Performance 2.5 V Aqueous Asymmetric Supercapacitors
Wei-shuai LiuYu-qing SongHeng WangHong-fei WangLi-feng Yan
Department of Chemical Physics,iCHEM,University of Science and Technology of China,Hefei 230026,China
Key words:Asymmetric supercapacitors,High-voltage supercapacitors,3D Macro-micromesoporous,FeC2O4/rGO hydrogel
I.INTRODUCTION
Supercapacitors,also known as electrochemical capacitors,belong to a new type of electrochemical energy storage device.The power density of supercapacitors is much larger than that of the conventional battery,the charge and discharge process is more rapid[1,2].In view of the above advantages,supercapacitors have broad application prospects in autonomous electric vehicles,rail transportation,wind power pitch,and highenergy weapons[3,4].However,the current low energy density hinders its wider application[5,6].
Typical supercapacitor comprises six components,theelectrodematerial,currentcollector,thediaphragm,electrolyte,battery post and shell.The electrode material is the key factor in determining the electrochemical properties of supercapacitors.There are three main types of supercapacitor electrode materials,including carbon materials,conductive polymers and transition metal oxides/hydroxides[7].Generally metal oxides are considered to be important electrode materials for supercapacitors,which are conducive to break through the voltage limit for aqueous asymmetric supercapacitors and acquire a remarkable energy density[8].When metal oxides are used as supercapacitor electrodes,pseudocapacitive behaviors can be revealed sufficiently and high specific capacitance can be con firmed,such as SnO2,MnO2,RuO2,Co3O4,Nb2O5,etc.[4,9−15].
However,most of metal oxides are limited due to cost,toxicity and availability.Therefore,Fe2O3and Fe3O4have been intensively investigated in the application of supercapacitors by virtue of their low cost,corrosion resistance,environmental friendliness,natural abundance and low toxicity for the past few years.However,their shortcomings are also obvious compared to other metal oxides used as supercapacitors.Their poor electrical conductivity and low surface area lead to an unsatisfactory cycling stability and poor rate capability[16,17].In order to solve these problems,depositing them onto the surface of porous carbon materials is usually employed,such as 3D graphene network[18,19].
In order to increase the specific surface area of the carbon materials,graphene hydrogel with porous structure has been prepared by various methods.However,the pore of the hydrogel is generally in size of several hundreds of nanometers or micrometers,and it is still a challenge to prepare closed stacking 3D graphene materials to increase the surface area with retaining of ion channels.On the other hand,porous metal oxides or salts provide possibility to enhance the surface area,and the combination of macroporous rGO hydrogel with micro-mesoporous metal oxides or salts may lead to a multilevel macro-micro-mesoporous structure,which makes the materials attracting active materials for electrodes in supercapacitors.
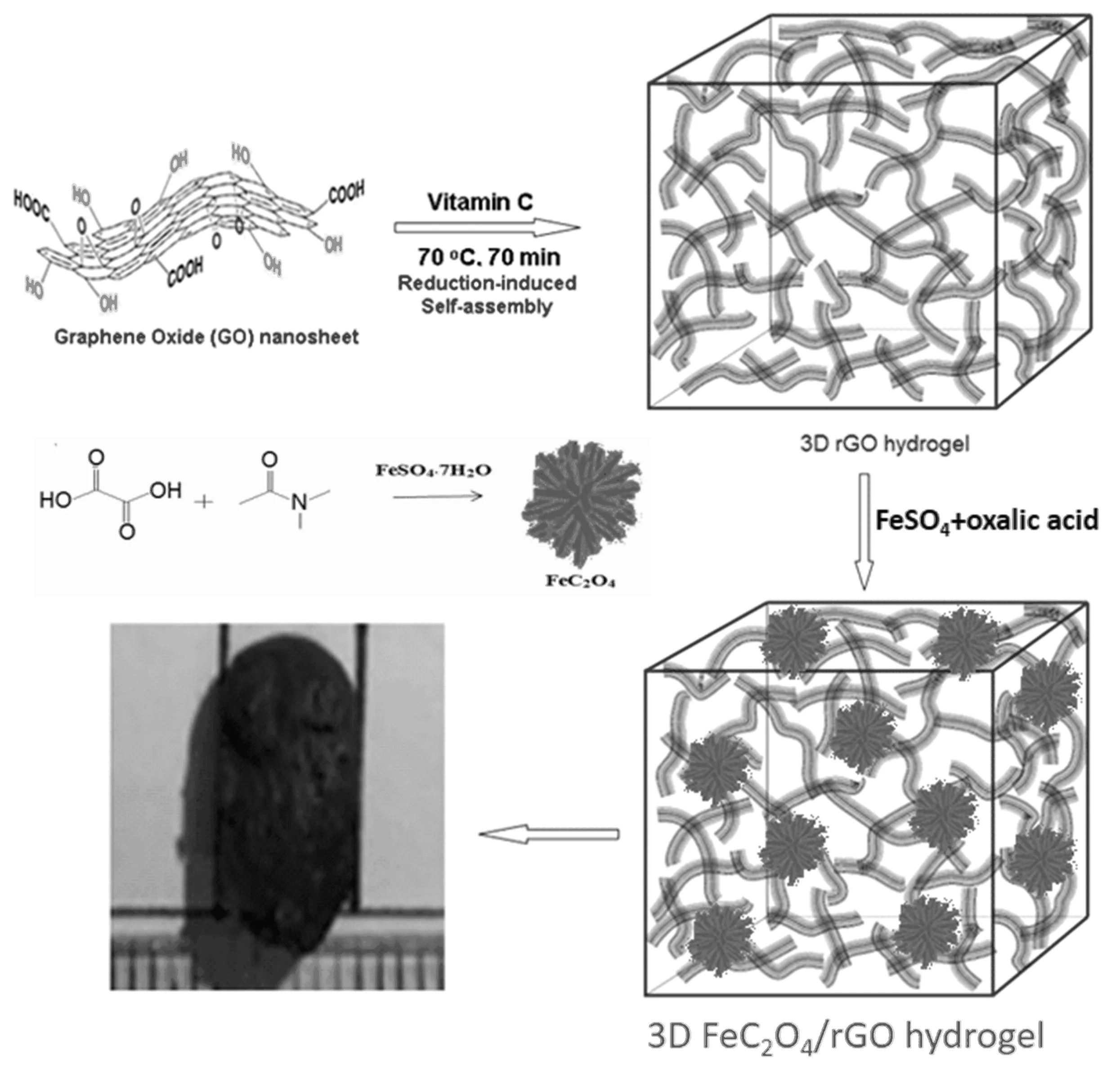
Scheme 1 An illustration of the preparation of micro-mesoporous FeC2O4and the macro-micro-mesoporous FeC2O4/rGO hydrogel.
It has been reported that ferrous oxalate with microporous and mesoporous structure has been prepared,and the porous structure brings a relatively large specific surface area[20].At present,it is rarely reported about the application of micro-mesoporous ferrous oxalate to supercapacitors while ironic oxide is well known active materials.On the other hand,graphene can be introduced to expand specific surface area and enhance conductivity.Actually graphene has been actively investigated for supercapacitor applications due to its high surface area,excellent electrical conductivity and chemical stability[21].In our previous study,we developed a simple reduction-inducedin situself-assembly route to prepare 3D graphene hydrogel or nanocomposite hydrogel with interconnected pores.After the addition of reductant,graphene oxide was reduced to graphene in the solution.Due to the steric hindrance between the graphene sheets and the presence ofπ-πmutual attraction,the graphene sheets were stabilized according to a certain orientation.Part of the graphene layers would be stacked on a parallel surface and part of the graphene layers would be staggered at a certain angle,eventually the graphene sheets were cross-linked together to form a closely packed three-dimensional structure of the graphene hydrogel[22].The prepared graphene hydrogel was not easily loosened.3D architectures composite hydrogel of graphene/metal oxides had been fabricated for energy storage applications[21,23].So the combination of 3D graphene hydrogel with micro-mesoporous ferrous oxalate may be a change to prepare novel monolithic active materials with macromicro-mesoporous structure,and can be directly used as binder-free electrodes for supercapacitors(Scheme 1).
The current research progress shows the voltage window of asymmetric supercapacitors where negative electrode and positive electrode work in separate potential windows is nearly twice that of symmetric supercapacitors[24].In general,the former mainly ranges from 1.4 V to 2.0 V while the latter is just about 1 V[25].It has been proven that the structure of asymmetric supercapacitors provides an opportunity to break through the thermodynamic limit of the cell voltage of aqueous asymmetric.For the sake of improving the cell voltage of aqueous asymmetric supercapacitor beyond 2 V,the exploration of suitable positive electrode materials with large oxygen evolution overpotential and negative electrode materials with large hydrogen evolution overpotential has never stopped.The pore structure of rGO hydrogel can provide abundant adsorption active sites for ion adsorption,while mesopores and macropores can serve as fast transport channels for ion diffusion to micropore surface[26].In addition,compared with acidic and alkaline aqueous electrolytes,neutral aqueous electrolytes have lower hydrogen ion and hydroxide ion concentration and therefore the surface hydrogen evolution and oxygen evolution overpotential are higher,and carbon-based supercapacitors obtain higher specific capacitance and working voltage.The 3D micro-mesoporous FeC2O4/graphene hydrogel can significantly help us improve the cell voltage from 1.23 V to 2.5 V.According to the equation of energy densityE=1/2CV2,we can increase the specific energy(E)of supercapacitors by increasing the cell voltage(V)or by increasing the specific capacitance(C)[27−29].When the energy stored at the positive pole is equal to the energy stored by the negative electrode,the voltage range of the supercapacitor is fully utilized,which is related to the quality of positive and negative electrode materials.The mass ratio(R)between the positive and the negative electrode has been adjusted taking into account formula which expresses that the same charge is passed through both electrodes[30]:

whereM+andM−are the mass,C+andC−are the specific capacitance,and∆E+and∆E−are the potential window for the positive and negative electrodes,respectively.Ultimately,energy density is significantly improved when the cell voltage of aqueous asymmetric is promoted without sacrificing power density.The electrode materials are easy to prepare since no conductive agent and binder are added.We are looking forward to the follow-up application of the asymmetric supercapacitor whose negative electrode bases on FeC2O4/rGO hydrogel and positive electrode bases on pure rGO hydrogel.
II.EXPERIMENTS
A.Materials
Graphite powder was purchased from Alfa Aesar.Sodium hydroxide,potassium hydroxide,potassium permanganate,sodium sulfate,hydrogen peroxide,sulfuric acid,N,N-dimethyl acetamide(DMAc),iron(II)sulfate heptahydrate,L(+)-ascorbic acid,hydrochloric acid,and oxalic acid dehydrate were analytical grade and purchased from Shanghai Chemical Reagents Company.All chemicals were used without further purification and all experiments were carried out with deionized water.
B.Preparation of 3D reduced graphene oxide(rGO)hydrogel
GO was prepared by a modified Hummer’s method[9].Suspensions of GO was formulated by means of dissolving 0.18 g of GO into 20 mL of deionized water.In order to be uniformly dispersed,suspensions of GO was stirred for 0.5 h and ultrasound for 0.5 h.Meanwhile,2 mmol of oxalic acid was dissolved in 20 mL of DMA.Then mixed them up and added 0.55 g of ascorbic acid and 0.12 g of sodium hydroxide.The mixed suspension was poured into the test tube and heated at 76◦C for 1 h.In order to remove the impurities,the as-prepared rGO hydrogel should be dialyzed in water for 3 days.
C.Synthesis of macro-micro-mesoporous FeC2O4/graphene hydrogel
Similar to the preparation of rGO hydrogel,0.09 g of GO was dissolved in 10 mL of deionized water.0.7 mmol of iron(II)sulfate heptahydrate was dissolved in 10 mL of deionized water.Next mixing them under stirring and ultrasound for 0.5 h.1.4 mmol of oxalic acid was added to 10 mL of DMA,then 0.27 g of ascorbic acid and 0.06 g of sodium hydroxide were added.The whole process was accompanied by stirring.Finally,the mixture was heated for 1 h at 76◦C water bath.The asprepared micro-mesoporous FeC2O4/graphene hydrogel needed to be dialyzed in water for 3 days.Datta and co-workers reported the micro-mesoporous FeC2O4can be synthesized by means of the reaction of ferrous ion and oxalic acid in a mixed solution of water and DMA.In our experiments,the huge specific surface area of graphene on one hand provided large amount of ferrous ion attachment site,on the other hand it hindered the aggregation of rod-like ferrous oxalate.The rod-like ferrous oxalate retained the microporous mesoporous structure.
D.Construction of an asymmetric supercapacitor
As mentioned before,we prepared an aqueous asymmetric supercapacitor without any addition of conductive agent and binder.All we need to do is just to cut the FeC2O4/graphene hydrogel and rGO hydrogel into pieces,then they were directly assembled into an asymmetrical supercapacitor in the form of electrodes.The FeC2O4/graphene hydrogel was used as the positive electrode and the rGO hydrogel was used as the negative electrode.Before the experiment,the two electrodes should be immersed in the electrolyte for 12 h,respectively.The power density(P)and energy density(E)of the supercapacitor cells were calculated by the equation ofP=E/∆tandE=Ct∆V2/2,where ∆tstands for discharging time,∆Vrepresents voltage drop upon discharging,Ctstands for specific capacitance of the supercapacitor cells which was calculated by the equation ofCt=I∆t/m∆V,whereIis the constant discharge current,mis the mass of active materials in the electrode[20].Moreover we compared the different electrolytes,including aqueous Na2SO4solution(1 mol/L)and aqueous KOH solution(1 mol/L).
E.Characterization of electrode materials
The morphology and structure of the as-prepared materials were characterized and analyzed by scanning electron microscopy(SEM,Superscan SSX-550,Shimazu),the prepared hydrogel was cleaned,and the aerogels were obtained after freeze-drying,which were tested.Transmission electron microscopy(TEM,Hitachi H-600),a small amount of sample was dissolved in absolute ethanol and made ultrasonic process for 20 min,it was dispersed to copper network and dried,then could be tested.To research the thermal stability of the materials,with the heating rate of 10◦C/min in air from room temperature to 800◦C,thermogravimetric analysis was conducted(TGA,DTA-50,Shimazu).The crystal structures of the as-prepared materials were carried out by X-ray diffraction(D/MAX-1200,Rigaku Denki Co.Ltd.,Japan)with Cu Kαradiation.The pore size distribution and specific surface area of the as-prepared material were evaluated using an automatic microporous physical chemical adsorption instrument(ASAP 2020 M+C,Micromeritics).The nitrogen adsorption-desorption was carried out at 77.35 K.All electrochemical measurements of the materials were conducted through a CHI 660C electrochemical workstation.
III.RESULTS AND DISCUSSION
The structure and diffraction patterns of the FeC2O4nanoparticles and FeC2O4/graphene composites was first characterized by X-ray diffraction and presented in FIG.1(a).For the relatively low peaks of rGO,the emergence of a broad peak at about 24.5◦corresponds to the self-assembly and accumulation of graphene-like layered structure after the reduction of grapheme,during this process ferrous oxalate can be wrapped.At the same time,the characteristic peaks around 10◦of oxidized graphene disappeared,indicating a high degree of reduction and the formation of the hydrogel.For the XRD pattern of FeC2O4nanoparticles,all diffraction peaks correspond with the phase of iron oxalate hydrate(JCPDS 22-0635)that demonstrated the formation of FeC2O4on the graphene sheets.Generally,the amorphous and poor crystallization nature of the nanoparticles exhibit broad peaks and are affected by a variety of factors.For the dried FeC2O4/rGO hydrogel,the diffraction peaks appearing bifurcation correspond to two ferrous oxalate(α-FeC2O4,β-FeC2O4)which were mainly affected by the temperature of synthetic materials[31].As shown in FIG.1(a),in the dried FeC2O4/rGO hydrogel,no graphene sheets diffraction peaks were detected owing to the relatively low diffraction intensity of rGO.
TGA experiments were conducted in air for the sake of con firming the loading amount of FeC2O4nanoparticles in the FeC2O4/rGO composites and exploring the

FIG.1(a)XRD patterns of FeC2O4nanoparticles,dried rGO,and FeC2O4/rGO hydrogel,(b)TGA curves of dried FeC2O4/rGO and rGO hydrogel.
thermal stability of the FeC2O4/rGO composites and FeC2O4nanoparticles.For the FeC2O4/rGO composites,the oxidation of carbon and removal of oxygen radicals in graphene and the decomposition of ferrous oxalate proceed simultaneously during the heating process.In fact,the as-prepared of ferrous oxalate contains two crystal water,just abbreviated FeC2O4·2H2O to FeC2O4.As shown in FIG.1(b),the TGA curves of FeC2O4consisted of two stages.The mass loss was about 20.33%at approximately 200◦C at the first stage,which corresponded to the decomposition of ferrous oxalate and loss of two crystal water in air[32,33].The mass loss was about 34.20%at around 270◦C in the second stage attributing to the decomposition of ferrous oxalate into ferric oxide,carbon monoxide and carbon dioxide in air.There was no quality loss above 270◦C.The carbon content is higher than that of ferrous oxalate.Due to the oxidation of carbon and removal of oxygen radicals in graphene,the two stages of decomposition of FeC2O4were obscured.There was no difference between the thermal decomposition of FeC2O4on rGO matrix and the thermal decomposition of FeC2O4.The TGA curves of FeC2O4/rGO composites show the major mass loss was mainly from 350◦C to 570◦C,which could be ascribed to the combustion of rGO.The mass loss was about 65.79%and remained stable afterwards.In the end,content of FeC2O4in FeC2O4/rGO composites could be calculated.

FIG.2(a)Photograph of the as-prepared dried rGO and FeC2O4/rGO hydrogels,(b,c)SEM images of the freeze-dried rGO hydrogel,and(d,e)the freeze-dried FeC2O4/rGO hydrogel.(f)EDS elemental composition of Fe,O,and C correspond the selective specific area.
The morphology of the as-prepared materials were studied by means of TEM and SEM,respectively.As shown in FIG.2(a),the as-prepared rGO and FeC2O4/rGO hydrogels are black elastic cylinders.The overall shape of the hydrogels can be preserved when they were freeze-dried,and the prepared porous properties of the materials could be demonstrated due to the loss of moisture inside and outside the surface.As shown in FIG.2(b),the freeze-dried rGO hydrogel contained many drapes and accompanied by many large holes(>500 nm)in the cross-section(FIG.S1 in supplementary materials).The SEM image completely exhibited the cross-linked channels of 3D architecture.In FIG.2(c),the outer surface seemed smooth and no other impurities existed.The presence of elements of carbon and oxygen in the rGO hydrogel indicated that the graphene oxide was not reduced thoroughly and still contained oxygen-containing group.In fact,completely removing oxygen-containing groups required a high temperature of 1100◦C.FIG.2(d)shows the crosssection image of the composite hydrogel,and plenty of rod-like materials can be found,part of them are petallike structure,and the distribution of the nanoparticles is homogeneous.FIG.2(e)shows the enlarged image of the structure of freeze-dried FeC2O4/rGO hydrogel distinctly.The presence of iron element was verified by the EDS measurement(FIG.2(f)),indicating the formation of FeC2O4inside the rGO hydrogel.

FIG.3(a)Photograph of the as-prepared wet rGO and FeC2O4/rGO hydrogels,(b)TEM images of the rGO hydrogel sheets and(c,d)the porous FeC2O4nanorods.
FIG.3(a)shows the as-prepared wet rGO and FeC2O4/rGO hydrogel which contain a large amount of water.In morphology,there was no significant difference between the wet rGO hydrogel and FeC2O4/rGO hydrogel.TEM experiments were conducted for further exploring the structure of as-prepared materials.Different from SEM,TEM could provide more information about the sample in nanoscale.TEM image of rGO hydrogel is shown in FIG.3(b),and the graphene nanosheet seemed to be neat along with remarkable decentralization. Meanwhile,the degree of stacking of graphene sheets was light that could be illustrated by high light transmittance.For the composite,actually,the aggregation of the FeC2O4nanoparticles can be found which constituted porous rod-like structure of FeC2O4with some nanospheres,which were in accordance with the result of SEM,as shown in FIG.3(c)and(d).Compared with SEM,the size of rod-like FeC2O4in TEM was not obviously changed in the presence of rGO(FIG.S2 in supplementary materials).TEM studies further illustrated that the aggregation of FeC2O4nanoparticles was restricted on account of the assembly efficiency between graphene sheets and FeC2O4nanoparticles in hydrothermal reaction.

FIG.4 N2adsorption and desorption isotherms of(a)the as-prepared dried rGO hydrogel,(b)FeC2O4,and(c)the asprepared dried FeC2O4/rGO hydrogel.(d)Pore size distributions.
The N2adsorption-desorption isotherm and pore size distribution of all the samples were studied(FIG.4).The Barret-Joyner-Halenda(BJH)method is suited to analyze the mesoporous structure of the material and the Horvath-Kawazoe(HK)method is fit for analyzing the microporous structure of the material,the DFT method can be applied to analyze the pore size distribution.As can be seen from the isothermal adsorption curve of the samples,due to the microporous filling process,there was a rapid increase in the amount of gas adsorption in the low relative pressure region(FIG.4(a−c)).Subsequently,the platform near the level indicates that the micropores have been filled,no further adsorption occurred.Isothermal curve type of sample can illustrate the presence of micropores.Moreover,the BJH adsorption curve was consistent with the BJH desorption curve at the peak position that proved the existence of mesopores. Similar to the analysis method above,the dried FeC2O4/rGO hydrogel can be proven to have macroporous-microporous-mesoporous structure,the pore size distribution ranges from 0.5 nm to 20 nm(FIG.4(d)),indicating a multilevel porous structure.
In spite of the fact that the dried FeC2O4/rGO hydrogel retained a 3D multilevel porous structure,the irreversible collapse and serious stacking have occurred during the dried process,which leads to a low surface area. Therefore,the specific surface areas of the graphene-based materials measured through N2adsorption-desorption were not accurate.By calculating the degree of methylene blue solution absorbed by the graphene materials,we can obtain a relatively accurate real specific surface area of the as-prepared materials,as Liet al.reported[31].The specific surface area maximum value of wet rGO hydrogel is calculated to be 958 m2/g,while it is 1170 m2/g for wet FeC2O4/rGO hydrogel.When these as-prepared materials are used for supercapacitor electrodes,relatively large surface area and porous structure provide more active sites.On one hand the structure of wet rGO hydrogel can enhance the rate of charge migration,on the other hand it can shorten the paths of electrolyte ions by diffusion during the electrochemical experiments.Without the addition of conductive additives and polymer binders,the self-supported 3D multilevel porous structure is conducive to acquire a larger voltage window in the aqueous electrolyte to achieve greater energy density and power density.
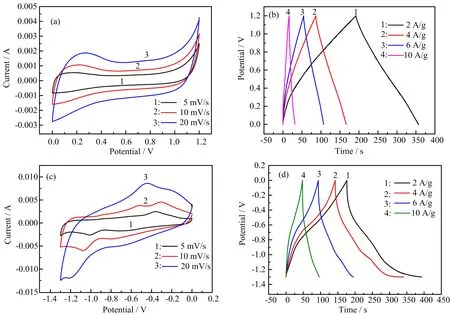
FIG.5 Cyclic voltammograms of(a)rGO hydrogel and(c)FeC2O4/rGO hydrogel in a three-electrode system.Galvanostatic charge-discharge curves of(b)rGO hydrogel and(d)FeC2O4/rGO hydrogel.All of samples were measured in 1 mol/L Na2SO4solution.
The effects of different electrolytes on the electrochemical properties were studied.FIG.5 shows the electrochemical performance of rGO hydrogel and FeC2O4/rGO hydrogel in a three-electrode system measured in 1 mol/L Na2SO4solution were different from these measured in 1 mol/L KOH(FIG.S3 in supplementary materials).Jacheet al.found that solvated sodium ions can be co-embedded in the graphene layers[34]. Of course,they can be co-embedded in the rGO hydrogel that was formed by stacking graphene sheets.The reaction mechanism is as follows:(alkali metal),y(solvent molecule)][2]. The solvation of sodium ions in graphene was accompanied by a reversible phase transition during its insertion and extraction. Similar to the lithium ions in lithium batteries,sodium ions can be used to store energy and sodium-ion batteries have received more and more attention[35].There were many defects in graphene sheets prepared in this work,which could provide a large number of special sites for the storage of sodium ions and increase the adsorption of sodium ions.In the sodium sulfate solution,the rGO hydrogel gained larger voltage window and specific capacitance through solvent co-implantation.Potassium ions did not find similar effects.Sodium ions can form similar NaxFeyOzlayered materialswith ferrousoxalate,activate Fe2+/Fe3+redox pair,and make them have higher electrochemical activity[36].TheCVcurves of rGO hydrogels were rectangle-like while redox peaks apparently appear for the FeC2O4/rGO hydrogel(FIG.5(a)and(c)),indicating the pseudocapacitive of iron oxalate. Meanwhile,GCD curves of rGO hydrogel also become irregular.At the current density of 2,4,6,and 10 A/g,the rGO hydrogel exhibited a specific capacitance that reached up to 192,153,160,and 135 F/g.The expansion of the voltage window effectively enhances the specific capacitance of the electrode material,as well as the possibility of assembling the asymmetrical supercapacitor to obtain a larger voltage window. ForCVcurves of FeC2O4/rGO hydrogel,the position of redox peak changes. The oxidation peak appears at about−0.5 V and the reduction peak appears at about−1.1 V.The redox peak position will be slightly changed along with different scan rates ofCV.GCD curves of FeC2O4/rGO hydrogel also obtained high specific capacitance values,591,576,527,and 343 F/g,when the GCD curves at current density of 2,4,6,and 10 A/g,respectively. The reason why the shapes of the GCD curves were more irregular than before was presumed to be related to the electrolyte solution,on the other hand during the charge and discharge process the structure of electrode materials that were soaked in the electrolyte for a long time would change,such as the break of loading of ferrous oxalate particles.
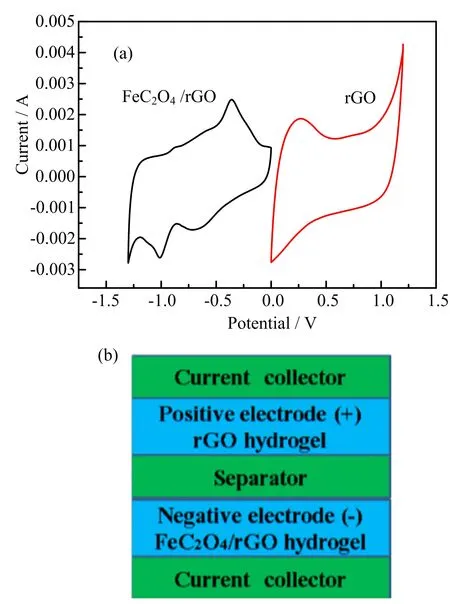
FIG.6(a)Cyclic voltammograms of rGO and FeC2O4/rGO electrodes performed in a three-electrode system in 1 mol/L Na2SO4aqueous solution.(b)The structure of an assembled asymmetric supercapacitor.The rGO hydrogel was used as the positive electrode and the FeC2O4/rGO hydrogel was used as the negative electrode.
FIG.6(a)shows the stable potential window is between−1.3 and 0 V for the FeC2O4/rGO hydrogel electrode while it is between 0 and 1.2 V for the rGO hydrogel in a three-electrode system.After the voltage window was determined,the asymmetric supercapacitor can be assembled(FIG.6(b)).
The voltage window in the aqueous electrolyte is limited by hydrogen evolution reaction and oxygen evolution reaction,which is the main factor to limit the application of supercapacitors.An asymmetrical supercapacitor can be assembled to enlarge the voltage window effectively.In order to assemble into an asymmetric supercapacitor,the FeC2O4/rGO hydrogel was used as the negative electrode and the rGO hydrogel wasused as the positive electrode(FIG.6(b)).As shown in FIG.7(a),the asymmetric supercapacitor voltage window reached 2.5 V in 1 mol/L Na2SO4electrolyte,far more than the decomposition of water voltage.CVcurves of the FeC2O4/rGO∥rGO tended to a rectangle.Similarly,the asymmetric charge-discharge curves revealed the charging time seems a bit long.

FIG.7Electrochemical property of the asymmetric supercapacitor of FeC2O4/rGO∥rGO.(a)CV curves of the FeC2O4/rGOrGO at different scan rates of 5,10,and 20 mV/s.(b)GCD curves of the FeC2O4/rGO∥rGO at different current densities of 1,2,4,3,and 5 A/g.All of samples were measured in 1 mol/L Na2SO4solution.
In general,the relationship between the energy density and the power density in the asymmetric supercapacitor is negatively correlated,with the increase of energy density,the power density decreases[33].The energy densities were calculated to be 59.7,53.2,50.0,43.2,and 37.7 Wh/kg,the corresponding current densities are 1,2,3,4 and 5 A/g,respectively.Meanwhile the power densities are calculated to be 0.14,0.24,0.36,0.47 and 0.58 kW/kg(FIG.8(a)).Compared with the results of Penget al.,when Fe2O3/rGO was used as the electrode material,the energy density is only 23.2 Wh/kg with a power density of 1.0 kW/kg,which is lower than that of our results.The stability of the asymmetric supercapacitor was evaluated by the charging-discharging cycling test.At a current density of 3 A/g,as FIG.8(b)shows,it retained nearly 68%of the initial specific capacitance after 1500 cycles.The poor cycle stability was mainly due to the destruction of the microstructure of the material.When metal oxides are charged and discharged,the low-valence state and the high-valence state are mutually converted,the structure of metal oxides will change and metal oxides may fall o fffrom carbon materials[26].The ferrous oxalate prepared in this work had a large particle size,and its adhesion stability was relatively poor.The ferrous oxalate particles also agglomerated during charge and discharge,and were more easily detached from the rGO hydrogel,which made the cycle stability poor.It is the main reason why the capacitance retention decreased quickly at the early stage of cycling.In subsequent work,we will strive to optimize the size of ferrous oxalate particles and composite structure and enhance the cycling stability of electrode materials.In addition,performance of the asymmetric supercapacitor of the same system in KOH electrolyte was also studied(FIG.S3−S5 in supplementary materials),and the voltage window of the asymmetric supercapacitor is 1.7 V.

FIG.8(a)Ragone plot and(b)cycling performance of the FeC2O4/rGO∥rGO asymmetric supercapacitor of in 1 mol/L Na2SO4solution.
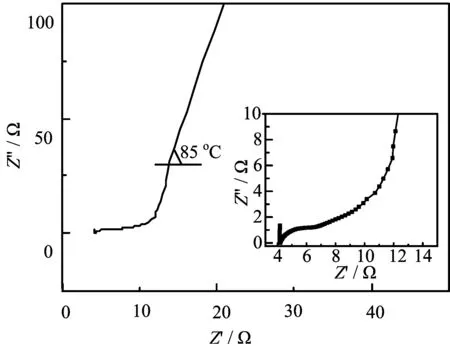
FIG.9 Nyquist plots of the FeC2O4/rGO||rGO in 1 mol/L Na2SO4electrolyte.
A series of experiments show that,whether in a threeelectrode system or assembled into an asymmetric super capacitor,1 mol/L Na2SO4electrolyte is more suitable compared with 1 mol/L KOH electrolyte in terms of the as-prepared materials.FIG.9 shows the Nyquist plots of the FeC2O4/rGO||rGO in 1 mol/L Na2SO4electrolyte.The intercept of the semicircle and the X-axis is the equivalent series resistance in the high-frequency region,about 4.2 Ω.The diameter of the semicircle represents the contact resistance,which affects the charge transfer process,about 2 Ω.The diffusion impedance can be re flected by the angle of the straight line with the real axis in the low-frequency region,which affects the diffusion of electrode reactants or products.The angle with the real axis is 85◦,which is consistent with the description of the typical region of the container impedance characteristics.The internal resistance of the device is about 8 Ω.
IV.CONCLUSION
Through a series of reactions and self-assembly process,a 3D macro-micro-mesoporous FeC2O4/graphene hydrogel has been successfully prepared.In consideration of the unique structure,FeC2O4/graphene hydrogel was used as the negative electrode and rGO hydrogel was used as the positive electrode to assemble into an asymmetric supercapacitor.The asymmetrical supercapacitor voltage window is up to 2.5 V that is conducive to acquire a prominent energy density of 59.7 Wh/kg.The method provides a general route to prepare various kinds of multilevel porous 3D graphene based hydrogels,which can be used as binder-free electrodes for high-performance supercapacitors.
Supplementary materials:SEM and TEM images of rGO or FeC2O4nano fiber,and CV and electrochemical property of the asymmetric supercapacitor of FeC2O4/rGO||rGO in 1 mol/L KOH solution are given.
V.ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China(No.51673180 and No.51673180).
[1]M.Winter and R.J.Brodd,Chem.Rev.104,4245(2004).
[2]M.Gong,Y.Li,H.Zhang,B.Zhang,W.Zhou,J.Feng,H.Wang,Y.Liang,Z.Fan,J.Liu,and H.Dai,Energy Environ.Sci.7,2025(2014).
[3]Y.Zhai,Y.Dou,D.Zhao,P.F.Fulvio,R.T.Mayes,and S.Dai,Adv.Mater.23,4828(2011).
[4]Y.Zhu,S.Murali,M.D.Stoller,K.J.Ganesh,W.Cai,P.J.Ferreira,A.Pirkle,R.M.Wallace,K.A.Cychosz,M.Thommes,D.Su,E.A.Stach,and R.S.Ruo ff,Science 332,1537(2011).
[5]G.Wang,L.Zhang,and J.Zhang,Chem.Soc.Rev.41,797(2012).
[6]L.L.Zhang and X.S.Zhao,Chem.Soc.Rev.38,2520(2009).
[7]P.H.Mutin and A.Vioux,J.Mater.Chem.A 1,11504(2013).
[8]Y.Cheng,H.Zhang,S.Lu,C.V.Varanasi,and J.Liu,Nanoscale 5,1067(2013).
[9]W.F.Chen,L.F.Yan,and P.R.Bangal,Carbon 48,1146(2010).
[10]C.Wu,Q.Shen,R.Mi,S.Deng,Y.Shu,H.Wang,J.Liu,and H.Yan,J.Mater.Chem.A 2,15987(2014).
[11]L.Deng,J.Wang,G.Zhu,L.Kang,Z.Hao,Z.Lei,Z.Yang,and Z.H.Liu,J.Power Sources 248,407(2014).
[12]S.S.Wu,W.F.Chen,and L.F.Yan,J.Mater.Chem.A 2,2765(2014).
[13]X.Peng,L.Peng,C.Wu,and Y.Xie,Chem.Soc.Rev.43,3303(2014).
[14]C.Long,D.Qi,T.Wei,J.Yan,L.Jiang,and Z.Fan,Adv.Funct.Mater.24,3953(2014).
[15]J.Yan,Q.Wang,C.Lin,T.Wei,and Z.Fan,Adv.Energy Mater.4 1400500(2014).
[16]N.Lu,W.Zhang,and X.Wu,Chin.J.Chem.Phys.30,553(2017).
[17]L.Sheng,L.Jiang,T.Wei,Z.Liu,and Z.Fan,Adv.Energy Mater.7,1700668(2017).
[18]X.Wu,L.Jiang,C.Long,and Z.Fan,Nano Energy 13,527(2015).
[19]J.Yan,Z.Fan,W.Sun,G.Ning,T.Wei,Q.Zhang,R.Zhang,L.Zhi,and F.Wei,Adv.Funct.Mater.22,2632(2012).
[20]K.J.Datta,M.B.Gawande,K.K.R.Datta,V.Ranc,J.Pechousek,M.Krizek,J.Tucek,R.Kale,P.Pospisil,R.S.Varma,T.Asefa,G.Zoppellaro,and R.Zboril,J.Mater.Chem.A 4,596(2016).
[21]W.F.Chen and L.F.Yan,Nanoscale 3,3132(2011).
[22]W.Chen,S.Li,C.Chen,and L.Yan,Adv.Mater.23,5679(2011).
[23]W.F.Chen,S.R.Li,C.H.Chen,and L.F.Yan,Adv.Mater.23 5679(2011).
[24]P.Yang,Y.Ding,Z.Lin,Z.Chen,Y.Li,P.Qiang,M.Ebrahimi,W.Mai,C.P.Wong,and Z.L.Wang,Nano Lett.14,731(2014).
[25]Z.W.Qu,H.Zhu,V.May,and R.Schinke,J.Phys.Chem.B 113,4817(2009).
[26]M.Yu,D.Lin,H.Feng,Y.Zeng,Y.Tong,and X.Lu,Angew Chem.Int.Ed.Engl.56,5454(2017).
[27]H.Gao,F.Xiao,C.B.Ching,and H.Duan,ACS Appl.Mater.Interf.4,2801(2012)
[28]Y.H.Hwang,E.G.Bae,K.S.Sohn,S.Shim,X.Song,M.S.Lah,and M.Pyo,J.Power Sources 240,683(2013).
[29]Z.Fan,J.Yan,T.Wei,L.Zhi,G.Ning,T.Li,and F.Wei,Adv.Funct.Mater.21,2366(2011).
[30]T.Brousse,P.L.Taberna,O.Crosnier,R.Dugas,P.Guillemet,Y.Scudeller,Y.Zhou,F.Favier,D.B´elanger,and P.Simon,J.Power Sources 173,633(2007).
[31]Q.T.Qu,S.B.Yang,and X.L.Feng,Adv.Mater.23,5574(2011).
[32]N.T.Koga,Solids Thermochim.Acta 388,41(2002).
[33]T.Cottineau,M.Toupin,T.Delahaye,T.Brousse,and D.B´elanger,Appl.Phys.A 82,599(2006).
[34]B.Jache and P.Adelhelm,Angew Chem.Int.Ed.Engl.53,10169(2014).
[35]K.Park,D.Han,J.Shon,S.G.Doo,and S.Lee,RSC Advances 5,6340(2015).
[36]P.Singh,K.Shiva,H.Celio,and J.B.Goodenough,Energy Environ.Sci.8,3000(2015).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- A Simple,Compact and Rigid Scanning Tunneling Microscope
- Extraction of Lignin from Tobacco Stem using Ionic Liquid
- Gd Doped Hollow Nanoscale Coordination Polymers as Multimodal Imaging Agents and a Potential Drug Delivery Carriers
- Gamma Ray Radiation Effect on Bi2WO6Photocatalyst
- Ag-Cu Nanoparticles Supported on N-Doped TiO2Nanowire Arrays for Efficient Photocatalytic CO2Reduction
- UV Laser Regulation of Surface Oxygen Vacancy of CoFe2O4for Enhanced Oxygen Evolution Reaction
