Gd Doped Hollow Nanoscale Coordination Polymers as Multimodal Imaging Agents and a Potential Drug Delivery Carriers
Go-zheng ZhoZhen GuoQin-wng Chen
a.Hefei National Laboratory for Physical Sciences at the Microscale,Department of Materials Science&Engineering,University of Science and Technology of China,Hefei 230026,China
b.Anhui Key Laboratory for Cellular Dynamics and Chemical Biology,School of Life Sciences,University of Science and Technology of China,Hefei 230027,China
Key words:Coordination polymers,Magnetic resonance imaging,Fluorescence optical imaging,Drug delivery potentials
I.INTRODUCTION
The early detection of cancers,as the very first and most crucial step before treatment,determines the chances of survival of patients[1,2],and a variety of imaging methods provide good auxiliary assistance to the diagnosis.The requirements for the detection are not only tracking the lesion location[3,4],but also well con firming the margins of the tumor for the next surgical resection[5,6].Inevitably,one single way of imaging could encounter with their respective limitations[7,8],thus resulting in the possible inaccuracy or even misdiagnosis.So a step forward aims at conceiving nanomaterials that could serve as multimodal imaging probes,of which different modalities could have complementary effects.
Among various imaging modalities,magnetic resonance imaging(MRI)and fluorescence optical imaging tools are broadly applied to medical and biological fields.For the MRI technique,the need for higher imaging effect puts forward the request of contrast agents(CAs)for contrast enhancement.Generally,there are two types of MRI CAs:one type is the positive contrast agent[9,10]that shortens the longitudinal relaxation time(T1)of water protons,and the other type is the negative contrast agent[11,12]that reduces the transverse relaxation time(T2)of water protons.The advantages and disadvantages of both modes have been discussed in previous reports[13,14],and thus dual modal contrast agents combining T1 and T2 CAs are necessary in order to adopt the advantages and avoid the shortcomings.As for the fluorescence optical imaging,the fluorophores labeled with targeting ligands have been applied to delineating tumor margins with the help of intraoperative optical devices during the surgery[15].However,the intraoperative pathology cannot be correlated with preoperative diagnostic images.Hence,in many researchs, fluorescent organic dyes(such as FITC[16]or RITC[17]),inorganic quantum dots[18,19]or nanoparticles containing rare earth ions[20,21]are often decorated onto MRI CAs.Integrated with the MRI CAs,the multimodal imaging probes will offer aid to the preoperative diagnosis and the accurate intraoperative resection.
Typically,the common idea is to combine different materials in the form of composites to attain multifunctionality[22,23].This way to some extent has proven to be effective,but still faced with a series of drawbacks:except for the complicated synthesis steps,usually nanocomposites may result in the undesirable interactions between different materials[24,25].So an increasing number of researches[26–28]are focused on the one single material that integrates different imaging tools to facilitate the detection of the lesion locations.Recently,our group has developed a novel contrast agent Mn3[Co(CN)6]2which combines both the MRI(T1 and T2)and fluorescence optical imaging in one material[29].As we expected,the bimetallic coordination polymers present the properties of each element:the T1 contrast effects originate from the Mn element,while the Co element contributes to the T2-relaxation property.In the meantime,the energy level transition of Mn2+brings about the nanomaterials fluorescent optical imaging effect.Further experiments demonstrate the viability of bio-application.Unfortunately,ther1value of the Mn-based contrast agents still remains at a lower level in contrast with the Gd-based ones[30],and in theory replacing Mn element by Gd could effectively improve ther1value,but simply replacing all the manganese by the Gd element would abandon the fluorescence optical imaging effect derived from the intrinsic nature of the Mn ions[29].A compromise way to overcome the situation might be partial substitution of the manganese ions to realize the co-residency of the three metal elements coexisting in the structure.On the other hand,ther2value of Mn3[Co(CN)6]2is also relatively lower than the commonly used T2-weighted CAs Fe3O4,which has room for promotion.Herein,we prepare a highly integrated system through a solvothermal method.Gd doped hollow nanoscale coordination polymers(Gd doped prussian blue analogue,denoted as GPBA)are formed as a multimodal imaging contrast agents,and the hollow structure formed without acid etching also exhibits prospect as a drug carrier system.Although the metal ions and CN−may be toxic alone,the coordination polymers of these units show good biocompatibility due to the intrinsic stability under room temperature.After the silica coating process,both the MRI contrast effects and biocompatibility have been improved.
II.EXPERIMENTS
A.Materials
Materials used in the experiment are as follows:potassium cobalticyanide(K3[Co(CN)6])was purchased from J&K chemical Ltd.(Shanghai,China);tetraethylorthosilicate(TEOS),gadolinium oxide(Gd2O3),manganese acetate(Mn(COOH)2·4H2O),nitric acid(HNO3),aqueous ammonia,and polyvinyl pyrrolidone(PVP)were purchased from Shanghai Chemical Reagent Company(Shanghai,China).Gadolinium oxide was dissolved in the nitric acid and Gd(NO3)3was crystallized through evaporation of the acid solution.
B.Synthesis of the hollow nanoparticles and silica coating
A slight change was carried out to synthesize the precursor according to the previous report.18.375 mg manganese acetate and 0.3 g PVP(K-30)were dissolved in a mixed solution containing 15 mL ethanol and 5 mL deionized water,and the solution was denoted as solution A.0.04 mmol potassium cobalticyanide was dissolved in 5 mL deionized water and then added to solution A using a syringe under stirring.The white precipitates of the precursor were formed.Then adequate amounts of Gd(NO3)3together with 1 g PVP(K-30)were added into the above solution,afterwards,adequate amounts of ethanol and water were added to form a 30 mL C2H5OH/15 mL H2O system.The solution was then added into a Te flon autoclave(50 mL),then the sealed autoclave was heated for 40 h at 170◦C.After it was cooled to room temperature,the precipitate was centrifuged and washed a few times with mixed solution containing ethanol,purified water,and DMF.
The silica coating procedure was performed through suspending 18 mg nanocubes in 18 mL of ethanol and then dissolving 1.08 mL aqueous ammonia in 18 mL ethanol(3%V/V).One solution was poured into the other one under stirring and kept stirring for 10 min.68µL tetraethylorthosilicate was injected into the suspension above and the solution was stirred for 2 h.In order to get a thicker silica layer,the process can be repeated once again.The coated nanoparticles were centrifuged and washed several times using ethanol and purified water repeatedly.
C.DOX-loading and releasing studies
The prepared nanoparticles(1 mg)were added into 1.4 mL DOX solution(1 mg/mL)for drug loading experiments.The mixture was shaking for 24 h at 37◦C and then centrifuged and washed several times.All the supernatants after centrifugation were retained for the next measurement of loading capacity and encapsulation efficiency,which was calculated according to the previous report[31].
For the DOX release behavior investigation,the drug loaded nanoparticles were placed in a dialysis bag and then immersed in 20 mL PBS solution(pH=7.4).Every 3 h we would collect 1.0 mL solution outside the dialysis bag to measure the concentration of DOX by UV-Vis spectrophotometer and further calculate the release amount.We conducted three sets of experiments to reduce the deviations.
D.Cell viability test
Cytotoxicity of the NPs and the silica-coated ones were determined by the tetrazolium dye(MTT)method using H520 and A549 cell lines. Cells were incu-bated in a 96-well plate at 37◦C in a moist atmosphere with 100%CO2,and then the medium above was modified with fetal bovine serum(10%),penicillin(100 units/mL)and streptomycin(100 units).After cultured for 24 h,the solution containing uncoated and coated GPBA nanoparticles was added to replace the original medium.Then,MTT solution was added into each well for another 4 h incubation.Finally,an ELISA reader was applied to test the absorbance of each well.
E.Fluorescence imaging measurement in vitro
The GPBA nanoparticles were cultured with A549 cells for 24 h,followed byin vitrolaser confocal scanning fluorescence measurements.The detailed procedure can be found in the previous literature[47]and after incubation images were taken with a laser scanning microscope(Zeiss L SM 710)equipped with a 63−1.3 numerical aperture PlanApo objective.
F.Magnetic resonance imaging measurement
Based on the metal(Gd+Mn)content of GPBA measured by ICP-AES,different concentrations of GPBA were dispersed in deionized water and clinical magnetic resonance scanner(GE Signa HDxt 3.0 Tesla MRI system)was applied to measure the relaxation characteristics.T1-weighted magnetic resonance images were acquired by utilizing a saturated recovery spin echo sequence(TE=10 ms;TR=4000,2000,1000,500,200,100 ms). T2∗weighted images are obtained by the Carr-Purcell-Meiboom-Gill method with RARE sequences(parameters:TR=120 ms;TE=2.328,6.112,9.896,13.68,17.46,21.24 ms;the filp angle=30o;bandwidth=31.25 Hz;FOV=180×180 mm2).
BALB/c mice bearing tumors were employed to perform MRI exprerimentin vivo.The mouse was anesthetized and then intravenously injected at tail by GPBA nanoparticles(5 mg/mL in PBS,100µL).MR imaging was obtained at preinjection,20 min and 24 h post-injection.
G.Characterization
The morphology of the nanostructured material was observed using a field emission scanning electron microscopy(FE-SEM,JEOL JSM-6700M)and a transmission electron microscope(TEM,Hitachi H7650).The crystal structure of the material was measured by an X-ray diffractometer(XRD,Rigaku D/MAX-cA,Japan),the 2θscanning range was 10o−70o.Metal ion concentrations were measured with an Optima 7300DV Inductively Coupled Plasma Atomic Emission Spectrometer(ICP-AES).The surface electronic structure was characterized by X-ray photoelectron spectroscopy

FIG.1(a,b)SEM images and(c,d)TEM images of GPBA.
(XPS,VGESCALAB MKII).High-resolution transmission electron microscopy(STEM,JEM2100F)was used to characterize the distribution of Fe,O,C,and N elements in the sample.Ultraviolet-visible(UV-Vis)absorption spectra were measured with a UV-visible spectrophotometer(TU-1810 DSPC)over a measuring wavelength at 480 nm.
III.RESULTS AND DISCUSSION
The morphology of the products was revealed by the SEM images as shown in FIG.1(a)and(b).It can be seen that the cube-shaped nanoparticles ranging from 120 nm to 160 nm in size have been prepared,which have little changes in the size and shape with the precursor.Some cracks on the surface(shown in the inset of the FIG.1(a))reveal that it may be hollow in the interior,which is further proven by TEM results.The high magnification image in FIG.1(b)indicates the surface of the nanoparticle may be composed of tiny nanograins.From TEM micrographs,we can see that after the solvothermal process,the uniform hollow structures are formed with an outer shell in thickness of about 10 nm,while in stark contrast,the diameter of the cavity is more than 100 nm.This kind of thin-shelled hollow structure provides a huge space for encapsulating therapeutic drugs.
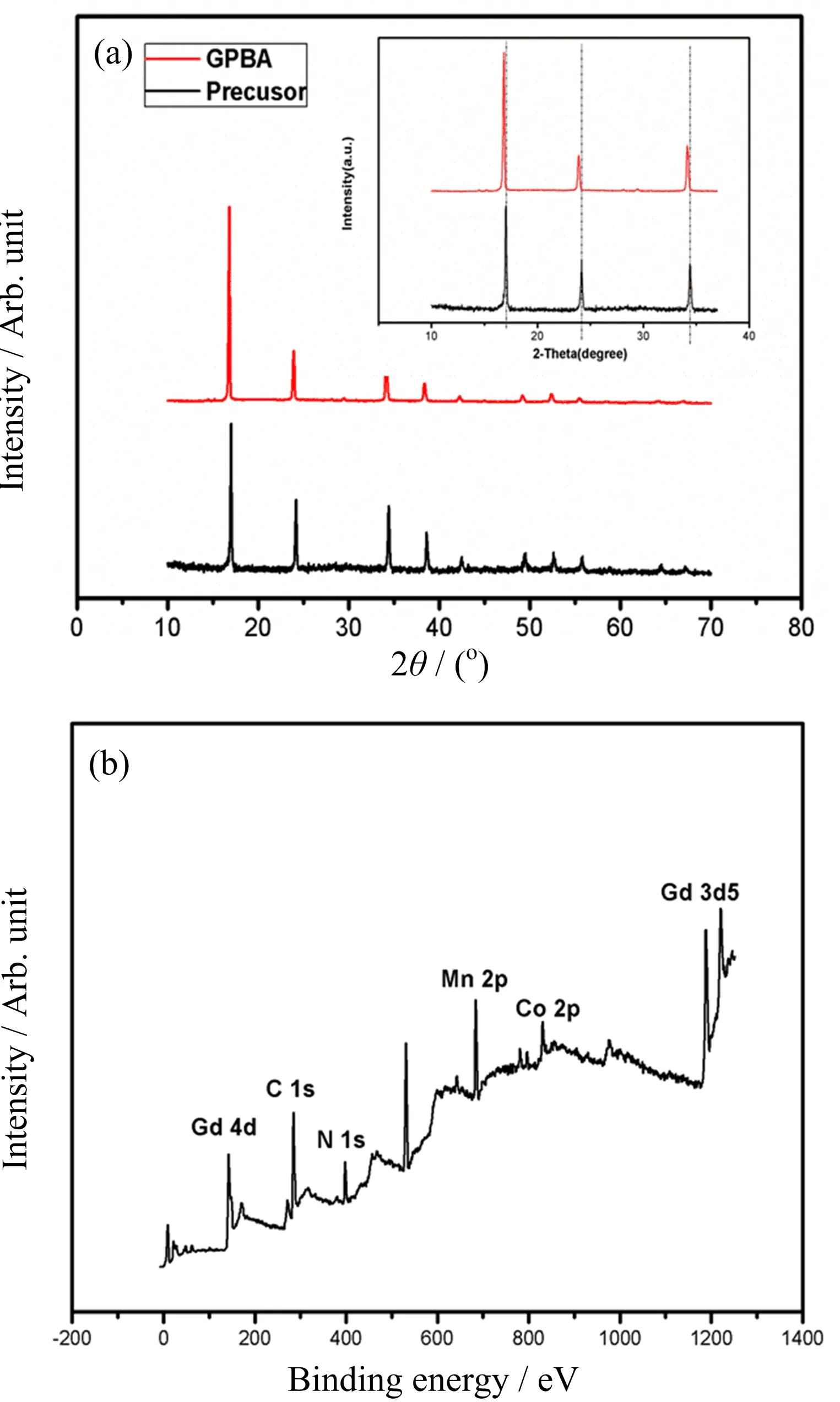
FIG.2(a)XRD pattern of GPBA and the inset is magnified XRD pattern from 10oto 37o,showing slightly left-shifted peaks compared with the precursor.(b)X-ray photoelectron spectroscopy(XPS)of GPBA.
X-ray diffraction(XRD)patterns of the precursor and GPBA are shown in FIG.2(a).The peak position has a good consistence with the standard XRD pattern of the precursor(Mn3[Co(CN)6]2),but has a slightly left shift as shown in the inset of FIG.2(a),indicating the lattice constant enlargement,which could be caused by Gd3+doping.The molar ratio of Gd to Mn was measured to be approximately 2.9:6.2 by ICP-AES.The X-ray photoelectron spectroscopy(XPS)measurement was carried out with the peak at 1188.8,614.88,and 781.27 eV in XPS spectra(FIG.2(b)),demonstrating existence of Gd,Mn,and Co elements,respectively.Dark- field STEM image of the same nanoparticle clearly shows the spatial distribution of Gd,Mn,Co elements in the nanoprobes(see FIG.3).Obviously,the three elements are mainly distributed in the outer layer,while absent in the middle,further indicating the hollow interior structure.The position of the three elements matches roughly well with the shell.The three elements are cano-bridged to form the trimetallic coordination polymers in the shell of the NPs.Through the measurements above,it is con firmed that the hollow trimetallic coordination polymers have been successfully fabricated.
The nanoporous characteristic and hollow structure of the coordination polymers suggest it is a potential drug delivery vehicle.DOX,as one of the most widely used antitumor drug,was employed to perform the drug loading procedure[31].It is determined that the DOX loading content was as high as 1166 mg/g(53.8 wt%),and encapsulation efficiency reached 83.29%,the loading capacity showed quite excellent results in contrast with previous reports[32,33],in which the loading capacity was usually several hundred milligrams per gram.The high loading capacity demonstrated the great potentials of our platform as antitumor drug delivery vehicle.Drug release behavior was also studied as shown in FIG.4.Under buffers of pH=7.4,DOX gradually released over time,eventually entering plateau phase with around 40%of DOX being released.
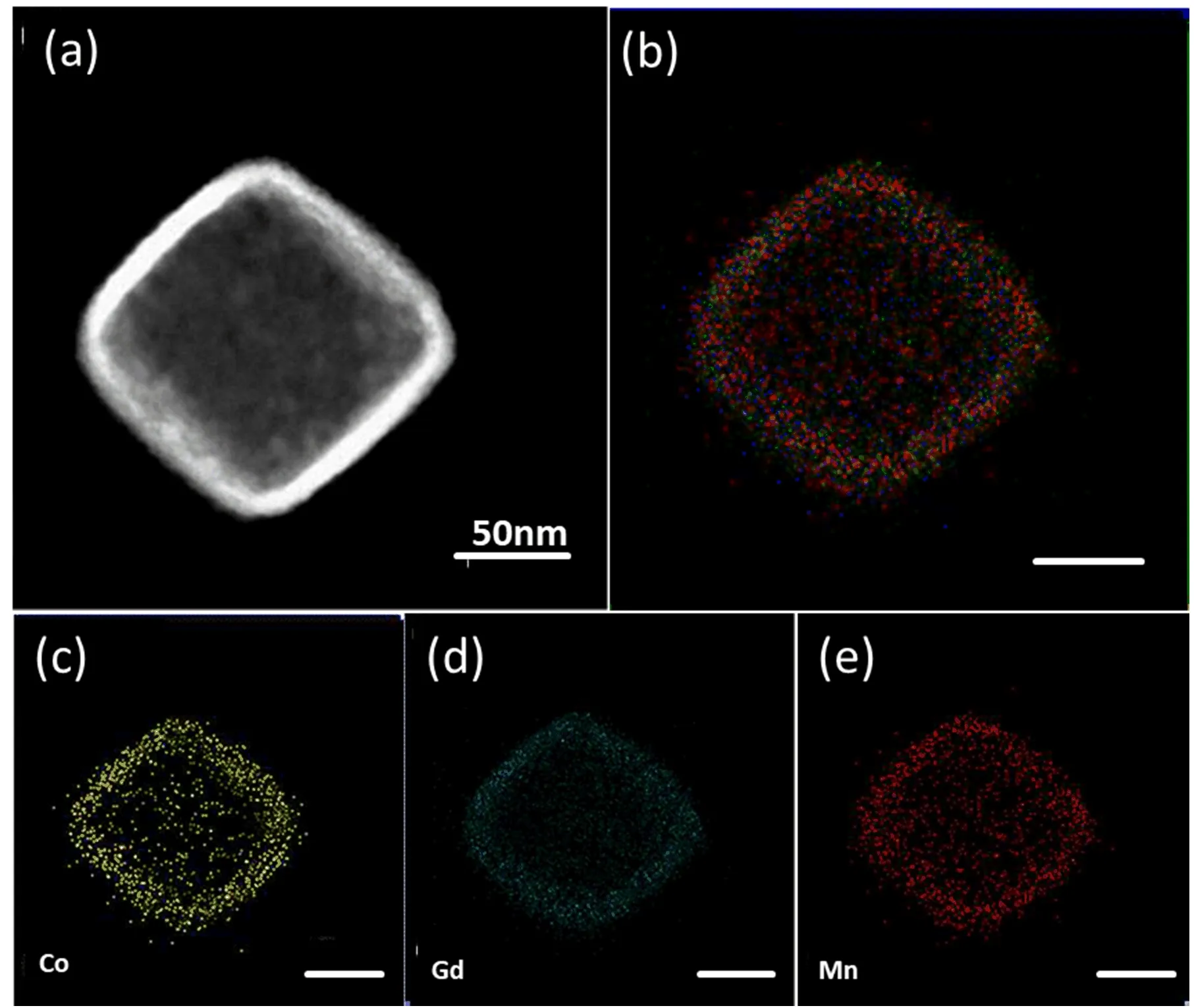
FIG.3(a)HAADF-STEM image of the Gd doped coordination polymer.(b)The merged image and(c)−(e)EDX element mapping of the same nanoparticles.
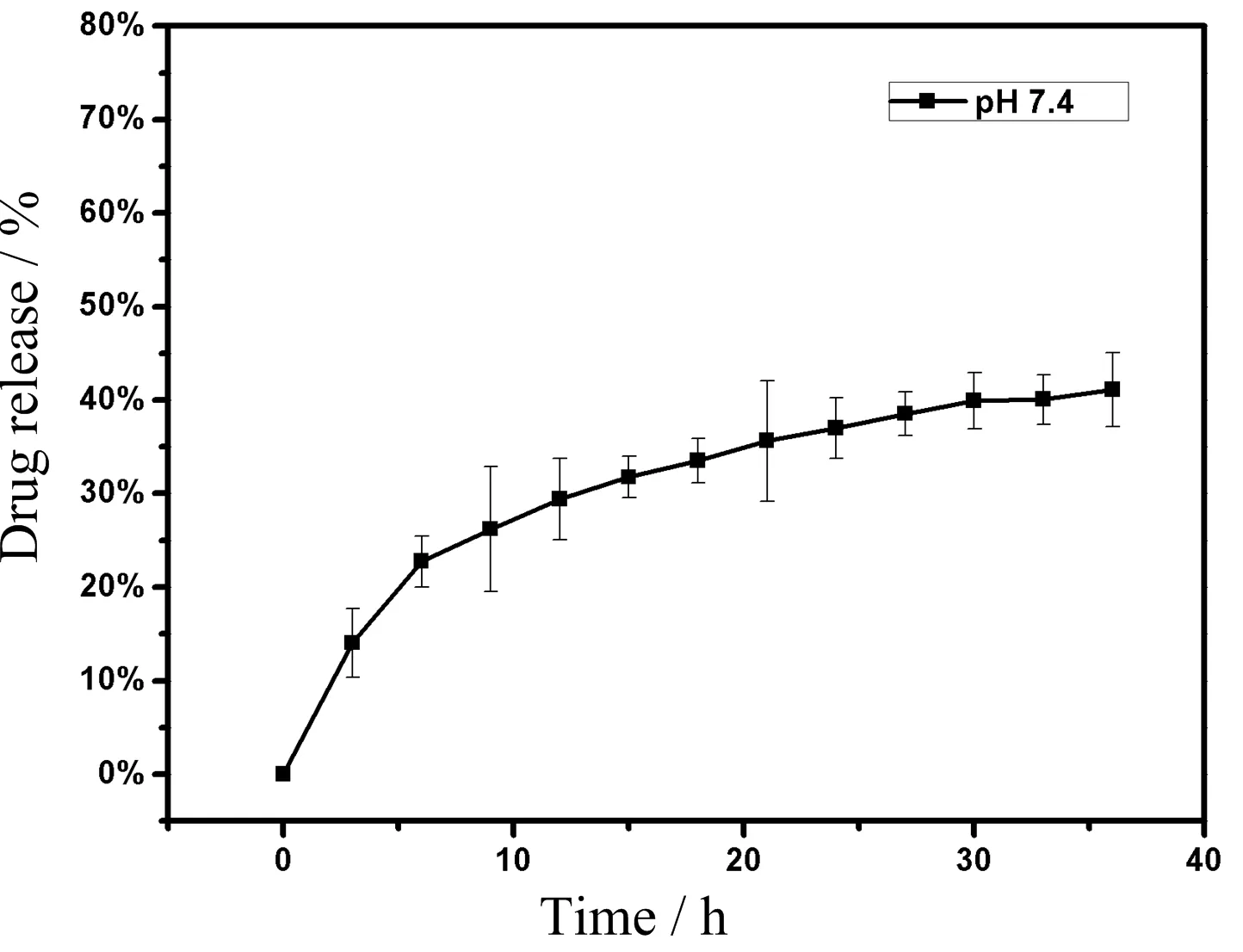
FIG.4 The pro file of DOX release in PBS solution(pH=7.4).
The excellent drug loading performance of the NPs obviously bene fits from the hollow structure.We found that the hollow structure gradually evolved as the reaction time proceeded until a completely hollow structure was formed as shown in FIG.5.Although our previous study[34]has provided reasonable explanations for the forming mechanism of Pd-doped hollow Prussian blue analogue nanoparticles,however,the mechanism of Gd doped ones should be different due to the distinct formation process.The formation progress of the hollow structure was an action of evacuating and recently a research[35]about the formation of the hollow MOFs provided some guidance for us,and the explanation may account for the possible mechanism:just like Rubik-cube,the big nanocubes of the precursor Mn3[Co(CN)6]2are composed of great amounts of small nanocubes;under the high temperature condition,Gd3+gradually substitutes Mn2+to form a thin layer of coordination polymers on the surface of the particles,then an inside-out formation process takes place;the surface-energy-driven mechanism makes the inner nanocubes to dissolve and migrate to the surface,recrystallizing with the little surface nanocubes to form Gd dopped hollow coordination polymers.Further studies should be carried out to make the mechanism clear.
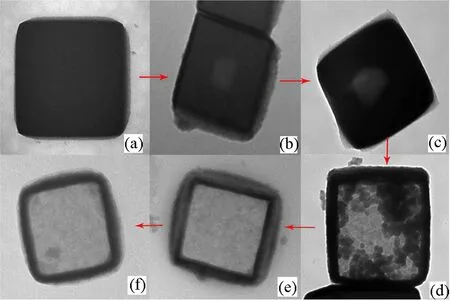
FIG.5 The formation progress of the hollow structure of the NPs.
As our previous report[29]revealed,the nanoparticles could enhance both biocompatibility and MRI contrast effect simultaneously after a silica layer coating on the surface,so our NPs were also coated by silica layer and then MRI measurements were carried out by a clinical magnetic resonance scanner.Uncoated and silica-coated nanoprobes were respectively dispersed in deionized water with different concentration to determine the longitudinal(r1)and transverse(r2)relaxivities.As illustrated in FIG.6,uncoated and silica-coated GPBA exhibitedr1of 7.38 and 13.57(mmol/L)−1·s−1,andr2of 180.6 and 304.8(mmol/L)−1·s−1,exhibiting fairly good contrast effect. Compared with the precursor,r1value of the uncoated NPs could reach 7.38(mmol/L)−1·s−1,53%enhancement,while ther2value is 2 times as high as the previous one.Initially we intended to enhance the T1 imaging effect through partial substitution,however,the final results showed that both T1 and T2 imaging effects were well strengthened.Through comparison with CAs in previous reports(as shown in Table I),we concluded that no matter as T1 CA or as T2 CA individually,the nanoprobes showed no weaker imaging effects than other single modal CAs.Thus,the hybrid system could well satisfy the imaging demands as dual T1&T2 CAs.Apparently T1 enhancement is derived from Gd doping as we have anticipated;seven unpaired electrons of Gd ions would help shorten longitudinal(T1)relaxation time,while the T2 contrast enhancement may result from changes of coordination environment.On the other hand,there are reports[36]revealing magnetic properties enhancement due to magnetic coupling effect via doping magnetic element.We can also make a reasonable inference:Gd ions partially substitutes Mn ions,which could enhance magnetic coupling,thus consequently getting a promotion at the T2 relaxation rates.
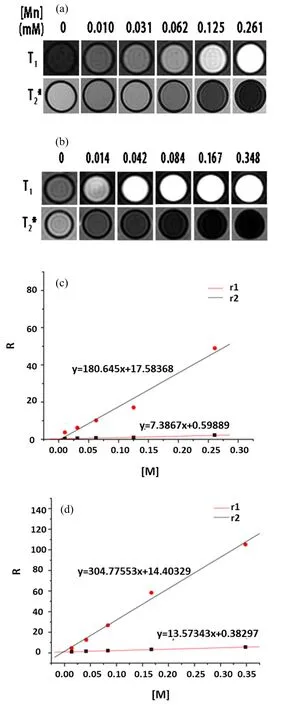
FIG.6 T1 and T2 weighted MR images of(a)uncoated and(b)silica-coated nanoparticles with different concentrations of metal ions,respectively.T1 and T2 relaxation rates as a function of metal concentration of(c)uncoated nanoparticles and(d)silica-coated nanoparticles,respectively.
Uncoated nanoparticles as CAs for MRI have alsobeen investigatedin vivo.0.5 mL saline solution containing 0.5 mg NPs was injected into a mouse bearing an A549-tumor via the tail vein and measured by clinically used 3T MR scanner mentioned above.Theoretically the CAs would enter the tumor readily as a result of EPR effect and be retained there for long periods.As shown in FIG.7 above,within 20 min after injection,T1 contrast effect had slightly enhancement,while after 24 h,MRI tests exhibited excellent T1 imaging effect,helping delineate the margins of the tumor clearly,which would be of great advantages for the next surgical resection.As for T2-weighted imaging,the MRI contrast effect at 20 min showed no obvious changes,but after 24 h injection,we could see negative contrast enhancement at partial regions of the tumor.Thein vivoexperiments showed the potential of our nanoparticles for use as preoperative diagnosis.
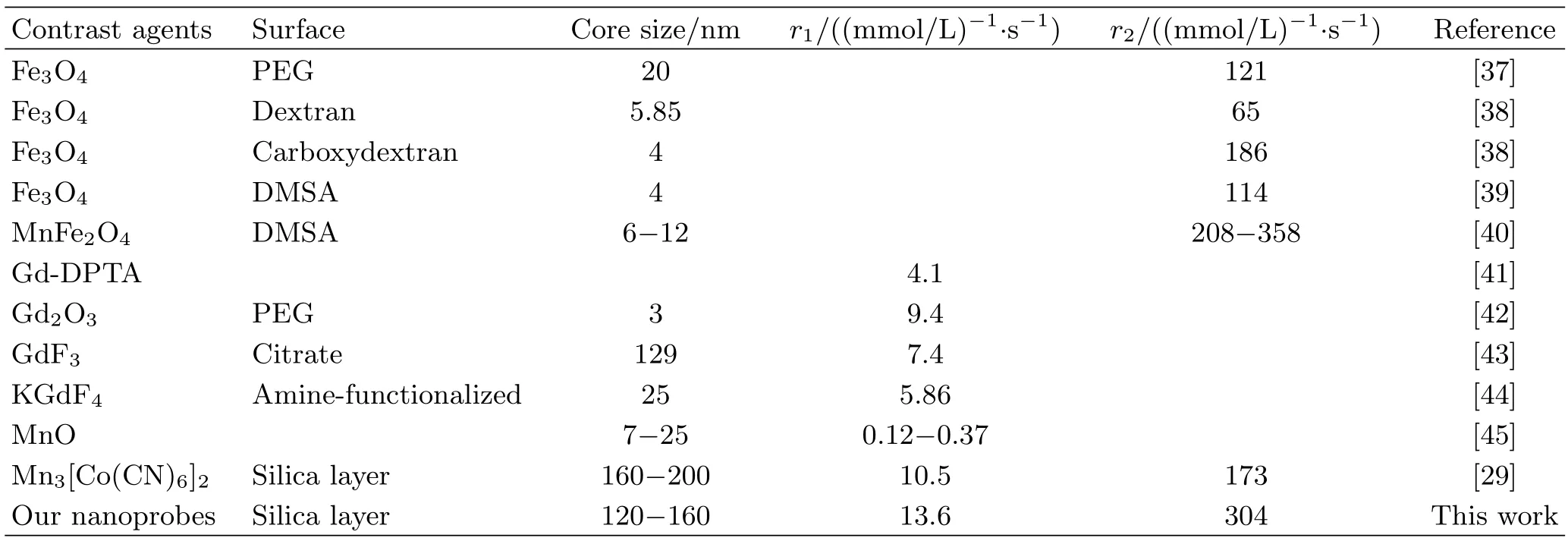
TABLE I The relaxivities rates of some typical contrast agents reported in previous articles.

FIG.7 in vivo T1 images and T2 images of a mouse bearing an A549-tumor at different time intervals before or after injection with uncoated nanocubes.
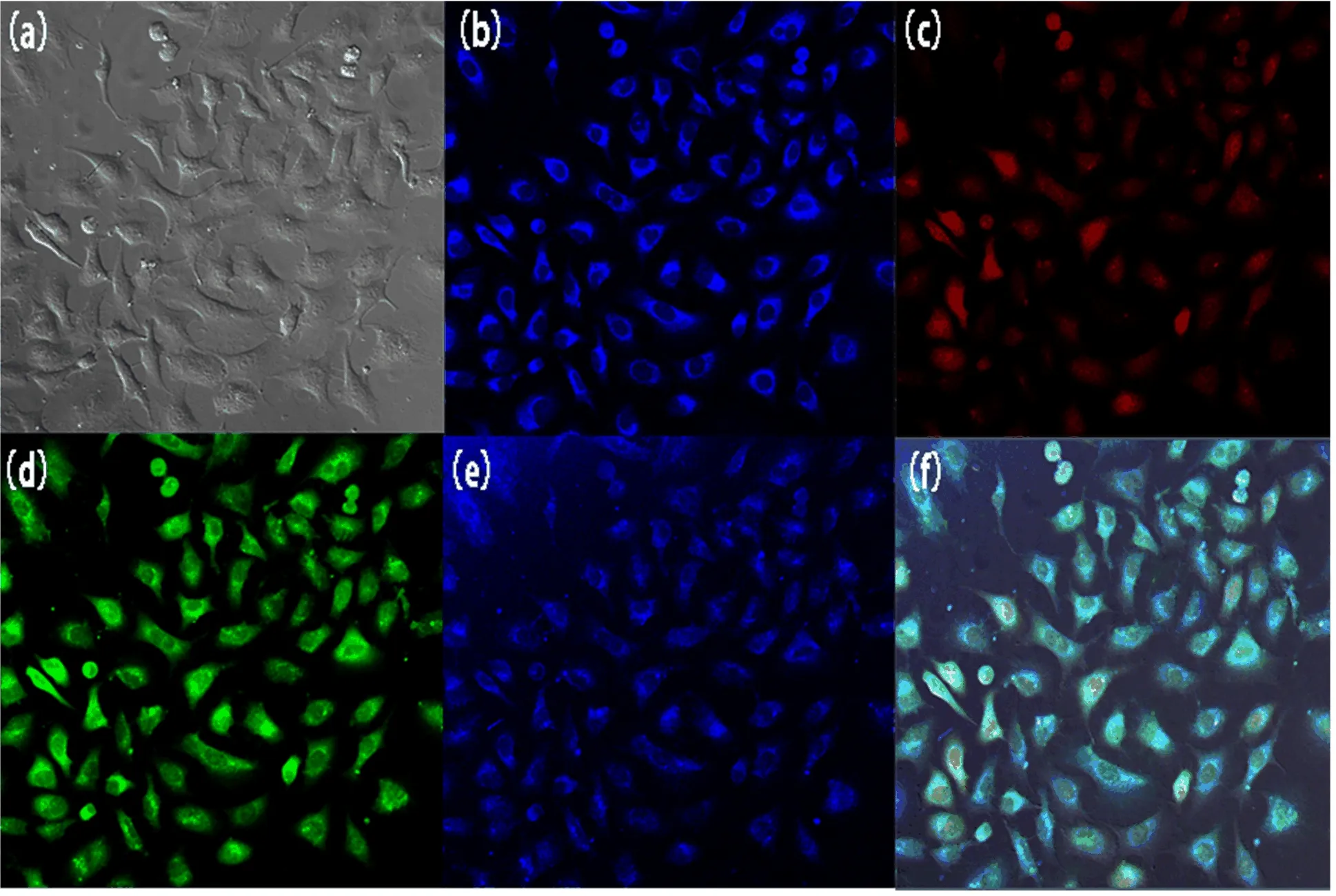
FIG.8CLSM images of GPBA incubated with A549 cells with different excitation wavelengths:(a)bright field,(b)720 nm two-photon excitation,(c)−(e)543,488,and 360 nm single-photon excitation,and(f)their merged image.
The uncoated GPBA nanoparticles were incubated with A549 cell lines for 24 h without any further dying in concentration of 50µg/mL for fluorescence optical imaging tests by confocal laser scanning microscopy(CLSM).Irradiated by laser beams of various wavelengths of single photon excitation(λex=360,488,543 nm),the cultured tumor cells emitted multicolor fluorescence as shown in FIG.8(c)−(e).It was observed that the uncoated NPs were localized in cytoplasm,and the fluorescence from unstained cells should be caused by the NPs themselves.However,due to the drawbacks of UV-excited imaging such as undesired tissue photodamage,two-photon fluorescence(TPF)microscopy was thus proposed.With the aids of powerful femtosecond pulse irradiation,the nanoprobes can simultaneously absorb two photons to the excited state and then emit fluorescence after a ground-state relaxation.Upon biphotonic excitation at 720 nm(equivalent to 360 nm single-photon excitation),the NPs displayed outstanding fluorescence in the blue spectrum region(FIG.8(b)). The excellent fluorescence effect above demonstrated the nanocubes offered a multiple-choice platform for biological labels.It is reported that the photoluminescence of4T1-6A1transition of Mn2+can offer two-photon fluorescence in doped quantum dots,such as Mn-doped ZnS QDs[37],and our previous work[34]had also demonstrated the materials containing Mn2+ions applied in fluorescence optical imaging.The abundant energy levels of Mn2+could account for the multicolor fluorescence.
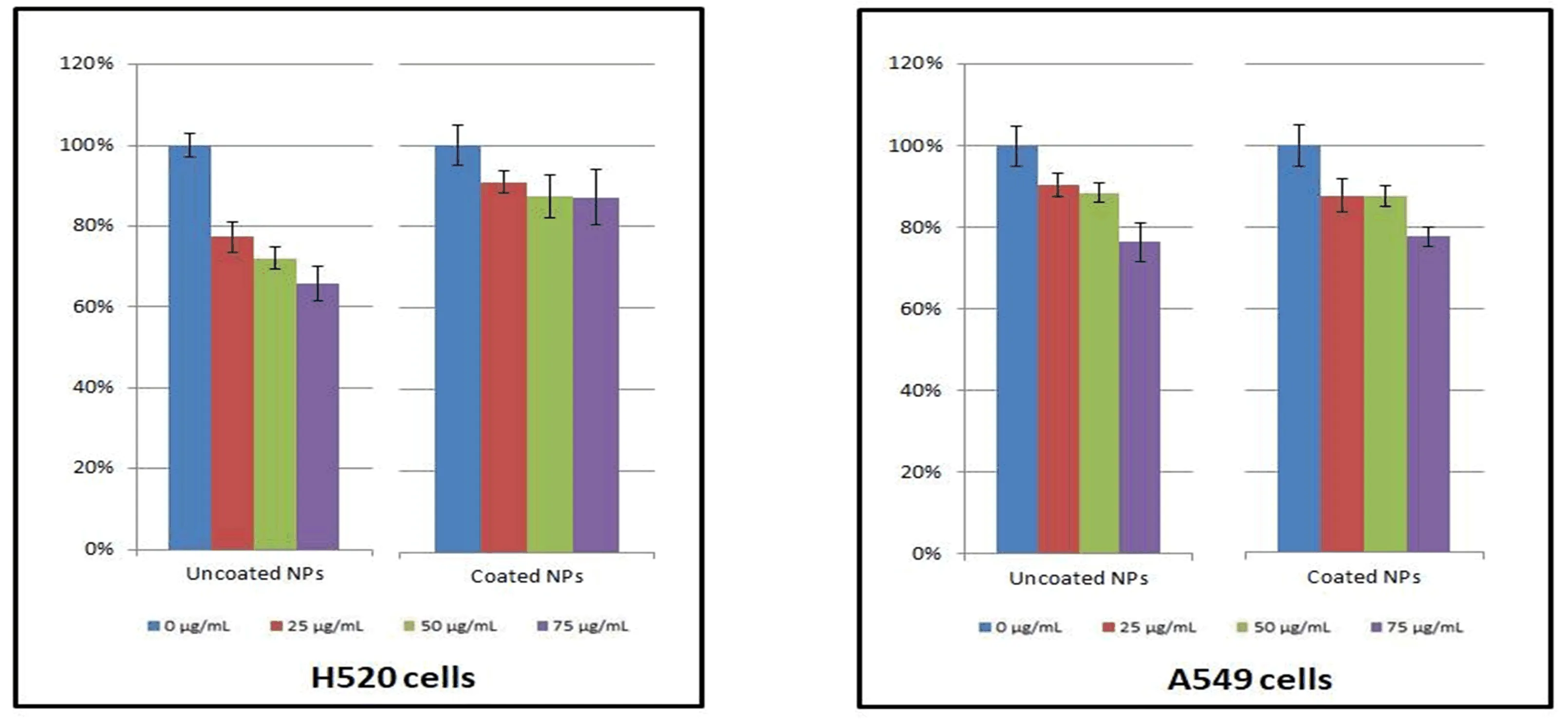
FIG.9 in vitro cytotoxicity of uncoated and silica-coated nanocubes on the viability of H520 and A549 cells after 24 h incubation.
As well known,CN−in the matrix is extremely toxic,but it does not mean our nanoparticles cannot be usedin vivo.In 2003,prussian blue(Fe4[Fe(CN)6]3)had already been approved by US Food and Drug Administration(FDA)as a pharmaceutical drug in clinic.Although CN−could be toxic alone,the coordination between metal ions and CN−is quite stable,which allows prussian blue to be applied clinically. As one of prussian blue analogues,Mn3[Co(CN)6]2exhibited excellent chemical stability even at strong acid environment[46].The inner coordination sphere between Co3+and CN−was especially stable,and the stability of the structure can effectively prevent the leakage of ions from reducing the toxicity.To evaluate the cytotoxicity of the as-prepared nanoprobes before and after SiO2coating,MTT assays were carried on H520 and A549 cell lines,and different concentration levels ranging from 25µg/mL to 75µg/mL were assessed.The results showed that no obvious toxicity was exhibited at tested concentrations,especially after SiO2coating(see FIG.9).The MTT assays above can demonstrate low toxicity of the NPs to be applied to bioimaging.
IV.CONCLUSION
In summary,Gd doped hollow prussian blue analogue was prepared by solvothermal method and demonstrated good T1 and T2 dual-mode magnetic resonance imaging capabilities.The longitudinal relaxation rate(r1)was 13.57(mmol/L)−1·s−1and transverse relaxation rate(r2)was 304.8(mmol/L)−1·s−1after silica coating,which was higher than many reported singlemode magnetic resonance probes.Under various wavelengths of laser irradiation,the nanoparticles emitted multiple colors of fluorescence.This multi-modal imaging could complement each other to make up for the defects of one single imaging mode.On the other hand,the NPs with hollow structure have a high loading capacity(1166 mg/g)for the chemotherapeutic drug doxorubicin,showing potentials as a drug delivery system,andin vitrocytotoxicity tests revealed that the obtained silica-coated nanoparticles have good biocompatiblity.The highly integrated nanoplatfrom showed great prospect for cancer theranostics.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.21571168),the Ministry of Science and Technology Grant(No.2016YFA0101202,No.U1232211,and No.31501130),CAS/SAFEA international partnership program for creative research teams and CAS Hefei Science Center(No.2016HSCIU011).
[1]I.R.Whittle,Curr.Opin.Neurol.15,663(2002).
[2]R.C.Bast Jr.,B.Hennessy,and G.B.Mills,Nat.Rev.Cancer 9,415(2009).
[3]L.An,H.Hu,J.Du,J.Wei,L.Wang,H.Yang,D.M.Wu,H.L.Shi,F.H.Li,and S.P.Yang,Biomaterials 35,5381(2014).
[4]J.V.Frangioni,Curr.Opin.Chem.Biol.7,626(2003).
[5]S.Arii,S.Tanaka,Y.Mitsunori,N.Nakamura,A.Kudo,N.Noguchi,and T.Irie,Oncology 78,125(2010).
[6]H.Hirschberg,G.N.Wu,and S.J.Madsen,Minim.Invasive Neurosurg.50,318(2007).
[7]J.Schnorr,S.Wagner,C.Abramjuk,R.Drees,T.Schink,E.A.Schellenberger,H.Pilgrimm,B.Hamm,and M.Taupitz,Radiology 240,90(2006).
[8]C.Bremer,V.Ntziachristos,and R.Weissleder,Eur.Radiol.13,231(2003).
[9]P.Caravan,Chem.Soc.Rev.35,512(2006).
[10]K.M.L.Taylor,W.J.Rieter,and W.B.Lin,J.Am.Chem.Soc.130,14358(2008).
[11]S.Laurent,D.Forge,M.Port,A.Roch,C.Robic,L.V.Elst,and R.N.Muller,Chem.Rev.108,2064(2008).
[12]A.K.Gupta and M.Gupta,Biomaterials 26,3995(2005).
[13]H.B.Na,I.C.Song,and T.Hyeon,Adv.Mater.21,2133(2009).
[14]M.Liong,J.Lu,M.Kovochich,T.Xia,S.G.Ruehm,A.E.Nel,F.Tamanoi,and J.I.Zink,ACS Nano 2,889(2008).
[15]S.Walter,S.Susanne,W.Simon,S.Herbert,F.Clemens,G.Claudia,E.G.Alwin,K.Rainer,and J.R.Hans,Neurosurgery 42,518(1998).
[16]Y.L.Pei,J.Li,J.H.Sui,Z.G.Li,and W.Cai,J.Nanosci.Nanotechnol.13,3928(2013).
[17]H.Lee,D.Sung,J.Kim,B.T.Kim,T.Wang,S.S.A.An,S.W.Seo,and D.K.Yi,Int.J.Nanomedicine 10,215(2015).
[18]P.Zrazhevskiy,M.Sena,and X.H.Gao,Chem.Soc.Rev.39,4326(2010).
[19]S.Z.Wang,B.R.Jarrett,S.M.Kauzlarich,and A.Y.Louie,J.Am.Chem.Soc.129,3848(2007).
[20]X.M.Li,D.Y.Zhao,and F.Zhang,Theranostics 3,292(2013).
[21]H.Y.Chen,B.Qi,T.Moore,D.C.Colvin,T.Crawford,J.C.Gore,F.Alexis,O.T.Mefford,and J.N.Anker,Small 10,160(2014).
[22]H.X.Peng,B.Cui,L.L.Li,and Y.S.Wang,J.Alloys Compd.531,30(2012).
[23]L.J.Zhou,X.P.Zheng,Z.J.Gu,W.Y.Yin,X.Zhang,L.F.Ruan,Y.B.Yang,Z.B.Hu,and Y.L.Zhao,Biomaterials 35,7666(2014).
[24]J.S.Choi,J.H.Lee,T.H.Shin,H.T.Song,E.Y.Kim,and J.Cheon,J.Am.Chem.Soc.132,11015(2010).
[25]Z.J.Zhou,D.T.Huang,J.F.Bao,Q.L.Chen,G.Liu,Z.Chen,X.Y.Chen,and J.H.Gao,Adv.Mater.24,6223(2012).
[26]Y.Zhang,J.D.Lin,V.Vijayaragavan,K.K.Bhakoo,and T.T.Y.Tan,Chem.Commun.48,10322(2012).
[27]C.Y.Liu,Z.Y.Gao,J.F.Zeng,Y.Hou,F.Fang,Y.L.Li,R.R.Qiao,L.Shen,H.Lei,W.S.Yang,and M.Y.Gao,ACS Nano 7,7227(2013).
[28]E.Chelebaeva,J.Larionova,Y.Guari,R.A.S.Ferreira,L.D.Carlos,A.A.Trifonov,T.Kalaivani,A.Lascialfari,C.Gurin,K.Molvinger,L.Datas,M.Maynadier,M.Gary-Bobo,and M.Garcia,Nanoscale 3,1200(2011).
[29]Y.M.Huang,L.Hu,T.T.Zhang,H.Zhong,J.J.Zhou,Z.B.Liu,H.B.Wang,Z.Guo,and Q.W.Chen,Sci.Rep.3,2647(2013).
[30]F.Q.Hu and Y.S.Zhao,Nanoscale 4,6235(2012).
[31]J.Chen,Z.Guo,H.B.Wang,M.Gong,X.K.Kong,P.Xia,and Q.W.Chen,Biomaterials 34,571(2013).
[32]X.Y.Yang,Y.S.Wang,X.Huang,Y.F.Ma,Y.Huang,R.C.Yang,H.Q.Duan,and Y.S.Chen,J.Mater.Chem.21,3448(2011).
[33]J.N.Shen,Q.J.He,Y.Gao,J.L.Shi,and Y.P.Li,Nanoscale 3,4314(2011).
[34]Y.Wang,S.X.Bao,R.Li,G.Z.Zhao,Z.H.Wang,Z.A.Zhao,and Q.W.Chen,ACS Appl.Mater.Interf.7,2088(2015).
[35]Z.C.Zhang,Y.F.Chen,X.B.Xu,J.C.Zhang,G.L.Xiang,W.He,and X.Wang,Angew.Chem.Int.Ed.53,429(2014).
[36]L.A.Li,H.X.Jin,D.F.Jin,Q.Lu,L.N.Sun,Q.Tang,M.Chen,H.L.Ge,and X.Q.Wang,Rare.Metal.Mat.Eng.39,479(2010).
[37]C.J.Xu,J.Xie,D.Ho,C.Wang,N.Kohler,E.G.Walsh,J.R.Morgan,Y.E.Chin,and S.H.Sun,Angew.Chem.Int.Ed.47,173(2007).
[38]Y.X.J.Wang,S.M.Hussain,and G.P.Krestin,Eur.Radiol.11,2319(2001).
[39]Y.W.Jun,Y.M.Huh,J.S.Choi,J.H.Lee,H.T.Song,S.Yoon,K.S.Kim,J.S.Shin,J.S.Suh,and J.Cheon,J.Am.Chem.Soc.127,5732(2005).
[40]J.H.Lee,Y.M.Huh,Y.W.Jun,J.W.Seo,J.T.Jang,H.T.Song,S.Kim,E.J.Cho,H.G.Yoon,J.S.Suh,and J.Cheon,Nat.Med.13,95(2007).
[41]J.L.Bridot,A.C.Faure,S.Laurent,C.Riviere,C.Billotey,B.Hiba,M.Janier,V.Josserand,J.L.Coll,L.V.Elst,R.Muller,S.Roux,P.Perriat,and O.Tillement,J.Am.Chem.Soc.129,5076(2007).
[42]M.A.Fortin,R.M.Petoral Jr.,F.S˜oderlind,A.Klasson,M.Engstr˜om,T.Veres,P.O.K¨all,and K.Uvdal,Nanotechnology 18,395501(2007).
[43]F.Evanics,P.R.Diamente,F.C.J.M.van Veggel,G.J.Stanisz,and R.S.Prosser,Chem.Mater.18,2499(2006).
[44]Q.Ju,D.T.Tu,Y.S.Liu,R.F.Li,H.M.Zhu,J.C.Chen,Z.Chen,M.D.Huang,and X.Y.Chen,J.Am.Chem.Soc.134,1323(2012).
[45]H.B.Na,J.H.Lee,K.An,Y.I.Park,M.Park,I.S.Lee,D.H.Nam,S.T.Kim,S.H.Kim,S.W.Kim,K.H.Lim,K.S.Kim,S.O.Kim,and T.Hyeon,Angew.Chem.Int.Ed.46,5397(2007).
[46]L.Hu,J.Y.Mei,Q.W.Chen,P.Zhang,and N.Yan,Nanoscale 3,4270(2011).
[47]D.D.Wang,Z.Guo,J.J.Zhou,J.Chen,G.Z.Zhao,R.H.Chen,M.N.He,Z.B.Liu,H.B.Wang,and Q.W.Chen,Small 11,5956(2015).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- A Simple,Compact and Rigid Scanning Tunneling Microscope
- Extraction of Lignin from Tobacco Stem using Ionic Liquid
- 3D Macro-Micro-Mesoporous FeC2O4/Graphene Hydrogel Electrode for High-Performance 2.5 V Aqueous Asymmetric Supercapacitors
- Gamma Ray Radiation Effect on Bi2WO6Photocatalyst
- Ag-Cu Nanoparticles Supported on N-Doped TiO2Nanowire Arrays for Efficient Photocatalytic CO2Reduction
- UV Laser Regulation of Surface Oxygen Vacancy of CoFe2O4for Enhanced Oxygen Evolution Reaction
