UV Laser Regulation of Surface Oxygen Vacancy of CoFe2O4for Enhanced Oxygen Evolution Reaction
Zhen-hong XiaoDao-chuan JiangHan XuJing-tian ZhouQi-zhong ZhangPing-wu DuZhen-lin LuoChen Gao
a.National Synchrotron Radiation Laboratory,University of Science and Technology of China,Hefei 230026,China
b.CAS Key Laboratory of Materials for Energy Conversion,Department of Materials Science and Engineering,University of Science and Technology of China,Hefei 230026,China
Key words:Oxygen evolution reaction,Spinel oxide,Transition metal oxide,Laser irradiation,Oxygen vacancy
I.INTRODUCTION
Electrochemically splitting water can store the electrical energy effectively into chemical bonds,and be regarded as one potential renewable and clean energy technique for replacing the burning of fossil fuel[1−3].The water splitting process consists of two half reactions:hydrogen evolution reaction(HER)and oxygen evolution reaction(OER).The slow kinetics of the OER requires high overpotential,resulting in considerable energy loss[4−6].In the past decades,many progresses have been made in developing active catalysts for the OER,and precious IrO2and RuO2are regarded as the most efficient candidates[7,8].Recently,considering the cost aspect for practical use,efforts have been devoted to exploring non-precious transition-metal-based catalysts for the OER[9,10].
The most valuable merit of transition metal oxide is that the cations therein possess variable valence states,which leads to tunable and rich functionalities.Theoretically,Manet al.found that the computed thermodynamic overpotentials show a volcano relation to the adsorption free energies of different reaction intermediates,which indicates the free energy as a descriptor for the OER electrocatalysis[11].Trasattiet al.investigated the OER potentials of different binary transition metal oxides,revealing a similar volcano trend between the potential and enthalpy,i.e.,a better catalyst should have neither strong nor weak affinity for oxygen[12].Theoretical and experimental investigations both show that these volcano-like behaviors are relevant to the electronic band structure of the catalysts.The band structure of outer shell electrons,say the egoccupancy,closely depends on the coordination between cations and oxygen anions[13].Shao-Hornet al.reported that the overpotential of the OER in perovskite oxides exhibits a volcano relationship with the egoccupancy[14,15].Subsequently,Xuet al.proved that this egtheory is also applicable to the spinel family of materials.As exemplified in MnCo2O4,the ORR/OER activity show a volcano shape with the Mn valence state in octahedral sites[16].On the other hand,various approaches had been utilized to regulate the egfilling state and thus the OER catalytic activity,such as thermo-treatment[17],plasma sculpturing[18],hydrogenated treatment,etc.[6,19].Very recently,pulse laser irradiation has exhibited its powerful ability in controlling the oxygen vacancy in VO2films[20].Considering the bene fits of quantitatively controllable pulse number,frequency,irradiation density of laser,this approach is adopted to treat the typical spinel CoFe2O4in this work,to exhibit its regulation ability of oxygen vacancy and the OER catalytic activity.

FIG.1 Schematic of laser irradiation process on spinel CoFe2O4.
As schematically presented in FIG.1,CoFe2O4is a typical transition-metal spinel oxide,in which oxygen atoms are in tetrahedral-coordinated and octahedralcoordinated form.Studies have shown that OER/ORR catalytic activities therein are governed by the egfilling of the active cations in octahedral sites[6].Therefore,it is reasonable to hypothesize that the escaped oxygen anions under irradiation will leave vacancies in the octahedral sites,thus to change the egfilling of the cations and to tune the OER activity of the catalyst.In this work,CoFe2O4films were fabricated and then irradiated by pulsed laser of UV light.Subsequently,XRD,SEM,XAS,XPS and OER measurements were performed to evaluate the effect of irradiation on the crystal structure,surface electronic structure,and catalytic activity.
II.EXPERIMENTS
The CoFe2O4films were deposited on FTO glass by pulsed laser deposition(PLD)at 600◦C under an oxygen pressure of 20 Pa.During deposition,half surface of the substrate was covered by metal mask in order to set aside the conductive area for electrochemistry test.After deposition the films were cooled down to room temperature in an O2atmosphere.The films were put in a vacuum chamber and subsequently irradiated by pulsed laser with wavelength of 248 nm.For the laser usage during irradiation,the repetition rate is 1 Hz,the laser energy density fallen on the film is 0.35 J/cm2per pulse,and the exposure time is used to control the irradiation dosage.Surface morphology is evaluated using scanning electron microscopy(SEM).The crystal structure and growth quality of the films were examined by X-ray diffraction(XRD)on a four-circle diffractometer(Rigaku SmartLab Film Version,Cu Kαradiation).The valence state of the elements in the CoFe2O4films was characterized by X-ray absorption spectrum(XAS)on the magnetic circular dichroism station at Hefei light source and X-ray photoelectron spectrum(XPS)using monochromatic Al Kαemission as the excitation source.To guarantee the comparability of the XAS/XPS results,we divided one film into five regions and then exposed these regions to the laser with different exposure time using metal mask,i.e.,0,15,35,70,and 100 min.The catalysis activity was measured using electrochemical station(CHI760E,Shanghai Chen Hua Instrument Co.,Ltd.)in a standard three-electrode system.
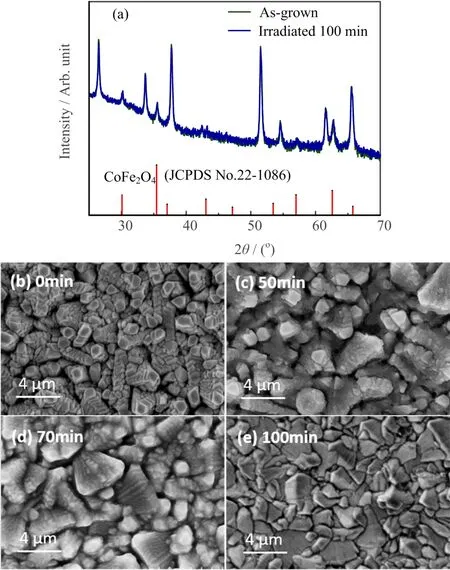
FIG. 2 (a) Typical X-ray diffraction patterns of CoFe2O4/FTO films as-grown or after-irradiation. The JCPDS No.22-1086 is shown as reference.(b−e)SEM images of the samples irradiated for different time.
III.RESULTS AND DISCUSSION
Typical XRD patterns of the as-grown CoFe2O4films under general conditions and after-irradiation are exhibited in FIG.2(a).And the inset photograph shows the optical image of the sample.Besides the strong reflections of the FTO substrate,the marked diffraction peaks at 30.10◦,35.54◦,43.20◦,57.02◦,and 62.70◦are well assigned to the(220),(311),(400),(511),and(440)re flections of CoFe2O4(JCPDS No.22-1086),indicating the successful synthesis of polycrystalline CoFe2O4film on FTO.Note that no obvious difference was observed in the XRD patterns of the as-prepared and irradiated films,indicating no significant difference in the bulk structure before and after UV irradiation.Similar results were found in the bulk composition information revealed by XAS spectra(FIG.S1 in supplementary materials).These results indicate that the crystal structure of the thin films and the element electronic structure(depth∼10 nm)did not change.In contrast,obvious change of the surface morphology can be seen in the SEM images(FIG.2(b−e)).Clear stacking growth mode can be observed in the as-grown film.However,after irradiation,the sample surface becomes more and more flat and dense,with less terraces and cracks.

FIG.3(a)O 1s XPS spectra of the CoFe2O4 film with varied exposure time under the UV irradiation.(b)The fitting peaks of O 1s spectra.
Surface-sensitive technique XPS was utilized to reveal the element valence state on the film surface,and the resulting O 1s spectra with varied exposure time are shown in FIG.3(Co 2p and Fe 2p XPS spectra are shown in FIG.S2 in supplementary materials).The O 1s curves were fitted using three peaks:O1 peak at 529.7 eV corresponds to the metal-oxygen bonds,O2 at a higher value of 531.4 eV stands for the surfaceadsorbed hydroxyl groups,and O3 peak at 532 eV is attributed to the absorbed molecular water.For comparison,the spectra shown in FIG.3(a)are normalized using the O1 peak.The sum of O2 and O3 could quantitatively re flect the “surface effective vacancy” which absorbs the reactants and thus affects the chemical reaction.In the XPS results,it is obvious that the relative ratio and position of the peak associated with such “surface effective vacancy” change a lot with the increasing irradiation time.The relative intensity of the merged O2-O3 peaks increases with the increasing exposure time until 70 min,and then decreases after that time.Meanwhile,the position of the merged O2-O3 peak initially shifts to a higher energy,and then to a lower energy.The quantity of“surface effective vacancy”presents a volcano shape,which could be ascribed to the competitive effects of vacancy-fabrication and surface-densification induced by UV irradiation.
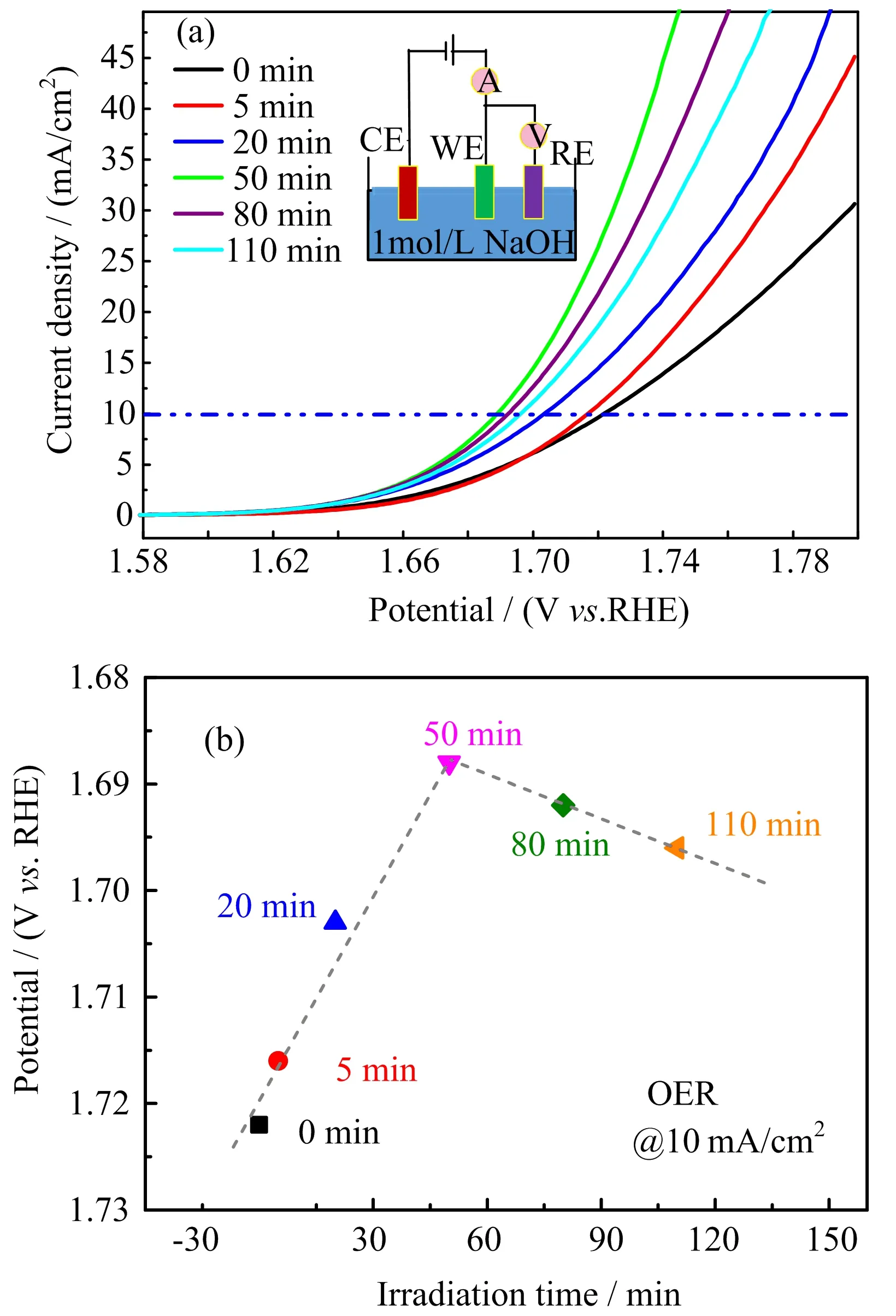
FIG.4 For the CoFe2O4 films with varied exposure time to the UV laser,(a)the current-potential curves for the OER measurements(inset is the schematic of the OER electrochemical test,in which CE is counter electrode,WE is working electrode and RE is reference electrode)and(b)the OER potential values at 10 mA/cm2as a function of irradiation time.
To evaluate the effect of UV-light irradiation on the catalytic activity of CoFe2O4towards oxygen evolution,the OER measurements were implemented in 1 mol/L NaOH.As shown in FIG.4(a),the OER performance of the CoFe2O4catalyst showed a decreasing overpotential and increasing current density under UV irradiation.The best catalytic activity was achieved after UV exposure for 50 min.At a current density of 10 mA/cm2,the required potential values versus the exposure-time are plotted in FIG.4(b).An obvious volcano shape is observed in this result.The applied potential for the OER reaches the lowest value of 1.69 V after 50 min UV irradiation,suggesting the best OER efficiency under this condition.The above electrochemical results indicate that UV-light irradiation can enhance the OER catalytic activity of CoFe2O4,which is consistent with the above XPS result.With the increasing exposure time under UV-irradiation,the surface effective vacancies increase,which in-turn changes the coordination environment and the filling of the egelectron of metal ions.The change in microstructure and electron energy state finally affects the macro-property of the OER.
It could be noticed that there is minor shift between the extremum values found in the XPS and OER measurements.It is partly because the specimens used for OER measurement is not the same one and therefore could not guarantee the same original state of the oxide vacancy.The sample with high comparability used for the above XPS characterization could not be utilized for the OER measurement due to the very limited area of the regions with different dosage of irradiation.So,there is a reasonable offset in the optimum irradiation time.
Supplementarymaterials:XAS spectra of the CoFe2O4film with varied exposure time under the UV irradiation are given.Co 2p and Fe 2p XPS spectra are also shown.
IV.CONCLUSION
In summary,we proved that irradiation with 248 nm UV-light could enhanced the OER activity of CoFe2O4films.The OER activity exhibits a volcano shape as a function of the irradiation time,consistent with the relative ratio of surface effective vacancy,which is a trade-o ffbetween the laser-induced increase of oxygen vacancy and the laser-induced decrease of surface area ratio.Since the irradiation source used in this work was a pulsed laser with merits of quantitatively controllable frequency and pulse number,this strategy provides possibility for quantitatively investigation of the relationship between surface cation valence,anion vacancy and physical/chemical properties of various transition metal based compounds.
V.ACKNOWLEDGMENTS
This work was supported by the National Key Basic Research Program of China(2016YFA0300102),the National Natural Science Foundation of China(No.11675179,No.U1532142,and No.11434009)and the Fundamental Research Funds for the Central Universities.This work was partially carried out at the USTC center for Micro and Nanoscale Research and Fabrication.We thank the support from the magnetic circular dichroism endstation at Hefei light source.
[1]N.S.Lewis and D.G.Nocera,Proc.Natl.Acad.Sci.USA 103,15729(2006).
[2]P.W.Du and R.Eisenberg,Energy Environ.Sci.5,6012(2012).
[3]D.C.Jiang,L.Zhu,R.M.Irfan,L.Zhang,and P.W.Du,Chin.J.Cataly.38,2102(2017).
[4]M.Qian,S.S.Cui,D.C.Jiang,L.Zhang,and P.W.Du,Adv.Mater.29,1704075(2017).
[5]W.T.Hong,M.Risch,K.A.Stoerzinger,A.Grimaud,J.Suntivichb,and Y.Shao-Horn,Energy Environ.Sci.8,1404(2015).
[6]J.Bao,X.D.Zhang,B.Fan,J.J.Zhang,M.Zhou,W.L.Yang,X.Hu,H.Wang,B.C.Pan,and Y.Xie,Angew.Chem.127,7507(2015).
[7]K.A.Stoerzinger,L.Qiao,M.D.Biegalski,and Y.Shao-Horn,J.Phys.Chem.Lett.5,1636(2014).
[8]E.A.Paoli,F.Masini,R.Frydendal,D.Deiana,C.Schlaup,M.Malizia,T.W.Hansen,S.Horch,I.E.L.Stephens,and I.Chorkendor ff,Chem.Sci.6,190(2015).
[9]W.F.Chen,J.T.Muckerman,and E.Fujita,Chem.Commun.49,8896(2013).
[10]X Sun,L.F.Gao,C.Y.Guo,Y.Zhang,X.Kuang,T.Yan,L.Ji,and Q.Wei,Electrochim.Acta 247,843(2017).
[11]I.C.Man,H.Y.Su,F.Calle-Vallejo,H.A.Hansen,J.I.Martinez,N.G.Inoglu,J.Kitchin,T.F.Jaramillo,J.K.Norskov,and J.Rossmeisl,ChemCatChem 3,1159(2011).
[12]S.Trasatti,J.Electroanal.Chem.111,125(1980).
[13]J.E.Huheey,E.A.Keitzer,and R.L.Keiter,Inorganic Chemistry:Principles of Structure and Reactivity,New York:Harper&Row(1993).
[14]J.Suntivich,K.J.May,J.B.Goodenough,H.A.Gasteiger,and Y.Shao-Horn,Science 334,1383(2011).
[15]J.Suntivich,H.A.Gasteiger,N.Yabuuchi,H.Nakanishi,J.B.Goodenough,and Y.Shao-Horn,Nat.Chem.3,546(2011).
[16]C.Wei,Z.X.Feng,G.G.A.Scherer,J.Barber,Y.Shao-Horn,and Z.C.J.Xu,Adv.Mater.29,1606800(2017).
[17]N.Zhang,X.Y.Li,H.C.Ye,S.M.Chen,H.X.Ju,D.B.Liu,Y.Lin,W.Ye,C.M.Wang,Q.Xu,J.F.Zhu,L.Song,J.Jiang,and Y.J.Xiong,J.Am.Chem.Soc.138,8928(2016).
[18]L.Xu,Q.Q.Jiang,Z.H.Xiao,X.Y.Li,J.Huo,S.Y.Wang,and L.D.Dai,Angew.Chem.128,5363(2016).
[19]G.M.Wang,Y.C.Ling,X.H.Lu,F.Qian,Y.X.Tong,J.Z.Zhang,V.Lordi,C.R.Leao,and Y.Li,J.Phys.Chem.C 117,10957(2013).
[20]H.T.Zhang,L.Guo,G.Stone,L.Zhang,Y.X.Zheng,E.Freeman,D.W.Keefer,S.Chaudhuri,H.Paik,J.A.Moyer,M.Barth,D.G.Schlom,J.V.Badding,S.Datta,V.Gopalan,and R.Engel-Herbert,Adv.Funct.Mater.26,6612(2016).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- A Simple,Compact and Rigid Scanning Tunneling Microscope
- Extraction of Lignin from Tobacco Stem using Ionic Liquid
- Gd Doped Hollow Nanoscale Coordination Polymers as Multimodal Imaging Agents and a Potential Drug Delivery Carriers
- 3D Macro-Micro-Mesoporous FeC2O4/Graphene Hydrogel Electrode for High-Performance 2.5 V Aqueous Asymmetric Supercapacitors
- Gamma Ray Radiation Effect on Bi2WO6Photocatalyst
- Ag-Cu Nanoparticles Supported on N-Doped TiO2Nanowire Arrays for Efficient Photocatalytic CO2Reduction
