Stabilization of Aqueous Graphene Oxide with Acetone under γ-Ray/UV Irradiation
Hong QinQin-yi ZhuHe-wen Liu
Key Laboratory of Soft Matter Chemistry,Chinese Academy of Sciences,Department of Polymer Science and Engineering,University of Science and Technology of China,Hefei 230026,China
Key words:Graphene oxide,Acetone,γ-Ray/UV,Reduction
I.INTRODUCTION
Graphene oxide(GO)is an oxygen-containing derivative of graphene with a large amount of hydroxyl,epoxy,and carboxyl groups on the surface,and can be easily reduced into graphene-like materials[1–3].These oxygencontaining groups make GO different from other kinds of graphene materials.For example,GO has excellent aqueous dispersibility,thus ideal processability[4],and perfect reactivity and functionality.GO has found some important applications in sensor[5],drug carriers[6,7],nanotechnology[8–10],environmental treatment[11,12],etc.For example,Zhanget al.enhanced chemical efficacy by sequential delivery of siRNA and anticancer drugs using PEI grafted graphene oxide[13].Tanet al.reported a dual platform for selective analyte enrichment and ionization in mass spectrometry using aptamer-conjugated graphene oxide[14].Stabilization of GO in aqueous environments is very important for the practical application of GO.
Radiation chemistry supplies a special chemical synthesis method dependent on activation of organic compounds using high-energy rays,such asγ-ray.The most widely usedγ-ray source is60Co sources.Each decay of the60Co nucleus emits twoγ-rays with energies of 1.17 and 1.33 MeV[15,16].The radiation chemistry of water is often the predominant process when aqueous solutions or dispersions are subjected toγirradiation,which mainly generates oxidizing hydroxide radical(·OH),reducing hydrogen radical(·H)and hydrated electron()at a very early stage[17–19].The generatedhas the strongest reducibility and a highGvalue(the number of transformed moieties per 100 eV absorbed energy).TheGvalue foris 2.63[18,19].Hydrogen radical(·H)can be neglected because theGvalue is too low(Gis ca.0.55).And·OH does not participate in the reduction of GO[20].Hence,the reduction of GO in water byγ-irradiation can be regarded to be induced predominantly by.Reduction of GO in gaseous phase has been investigated in Refs.[15,16].
GO consists ofπ-conjugated sp2domains which can exhibit a photoresponse or photoreactivity under UV irradiation[21].In other words,the sp2domains act as a photocatalyst under UV,and an electron is excited into theπ∗conduction band,thus a hole is created in theπvalence band by photoexcitation.The electron and hole contribute to reduction and oxidation of the GO nanosheets and water molecules,respectively[22].Therefore,UV irradiation can also cause the reduction of GO in aqueous environments[23,24].Because GO is very sensitive to either high energy ionic irradiation or UV exposure,stabilization of GO becomes an important issue for the important application of GO.Here,we investigated the stabilization of GO under high energy environments,and found that acetone can be selected to inhibit the reduction reaction of GO underγ-ray or UV radiation.
II.EXPERIMENTS
A.GO for γ-ray irradiation
Graphene oxide(GO),commercial brand No.XF002,was purchased from Nanjing Xianfeng Nanomaterials Technology Co.,Ltd. GO was dispersed in ultrapure water by ultrasonication at a concentration of 0.15 mg/mL.A Shepherd 109-68 Cobalt 60 source was used for steady-stateγ-irradiation at a rate of 9 kGy/h.In a typicalγ-ray irradiation process,the GO aqueous dispersion was placed in60Coγ-ray source for irradiation while magnetic agitation at ambient conditions.Then GO dispersions were sampled at a certain irradiation time(0.5,1,1.5,2,3,6,and 9 h).Each sample of GO was filtered,and washed three times with deionized water and ethanol,and then dried in vacuum at room temperature for 12 h.
Some effects on the irradiation of GO were tested in control experiment,including effects of O2compared by bubbling N2or air in GO,and effects of some organic chemicals:acetone(12wt%),and isopropanol(12wt%)in pure N2saturated GO aqueous dispersions.
B.GO for UV irradiation
GO was dispersed in ultrapure water by ultrasonication at a concentration of 0.15 mg/mL.In a general UV irradiation process,the GO aqueous dispersion in a quartz tube was exposed under a Hamamatsu LC8 UV spot light source(Optical energy density,95.3 mW/cm2)for irradiation while magnetic agitation at ambient conditions.Then GO dispersions were sampled at a certain irradiation time(0.5,1,2,and 3 h).Each sample of GO was filtered,and washed three times with deionized water and ethanol,and then dried in vacuum for 12 h.
Some effects on the UV irradiation of GO were tested in control experiment,including the effect of some organic chemicals,for example,acetone(12wt%)and isopropanol(12wt%)in GO aqueous dispersions.All UV irradiation was carried out in a sealed air atmosphere.
C.Reduction of GO by hydrazine hydrate[25]
GO was dispersed in ultrapure water by ultrasonication at a concentration of 0.15 mg/mL.In a typical procedure,85%hydrazine hydrate(12wt%)was added dropwise into the GO dispersion at 50◦C and the solution was heated in an oil bath at 60◦C.The GO dispersions were sampled at a certain reaction time(0,5,10,15,20,30,60,90,120,180,and 240 min).Then the product was filtered,washed copiously with deionized water and ethanol,and then dried in vacuum for 12 h.
D.Characterization
X-ray photoelectron spectroscopy(XPS)was performed with a Thermo-VG Scientific ESCALAB 250 XPS spectrometer with a MgKαX-ray source.Fourier transform infrared spectrum(FTIR)was recorded on a Vector-22 FTIR instrument.UV-Vis absorption spectra were acquired on a Mapada UV-1800PC spectrometer.Time dependent amperometricI-tcharacterization was performed on an electrochemical workstation CHI660D under a bias voltage at 0.8 V.Electrochemical tests were performed in 0.5 mol/L Na2SO4electrolyte using a typical three-electrode electrochemical cell configuration.0.1 mL GO aqueous solution(0.5 mg/mL)was dropped on the glassy carbon electrode and dried in vacuum for 2 h to form a GO film.The glassy carbon electrode was working electrode,Pt electrode was counter electrode,and Ag/AgCl electrode(saturated KCl aqueous)was reference electrode.
III.RESULTS AND DISCUSSION
A.γ-Irradiation of GO
UV-Vis absorption is very sensitive to the reduction of GO,because the difference of conjugation between GO and reduced GO(rGO)is large.The UV-Vis spectra of theγ-irradiated GO in different chemistry environments are shown in FIG.1(b)−(d).The chemical reduction of GO by hydrazine hydrate is often regarded as a complete reduction method,thus is used as reference in this work,and UV-Vis spectra at different reduction degrees are illustrated in FIG.1(a).The sharp absorption peak at around 230 nm is attributed toπ-π∗transition of aromatic C−C bonds,and is a characteristic peak of pristine GO[26].According to FIG.1(a),the UV absorption peak at 230 nm red-shifts to 270 nm with the increase in reduction degree,which is because of an increase in the conjugation degree in the rGO[27–29].As GO is reduced,the color changes from brownish yellow to deep black after reaction.Though all the UV-Vis spectra in FIG.1(b)−(d)were acquired in parallel,the peak shifts in these spectra dependent on the irradiation time are clearly different,indicative of a difference in the reduction rate in different chemical environments.The absorption peak ofπ-π∗transition of aromatic C−C bond of GO irradiated in air and isopropanol red-shifts to 270 nm after 9 h,as shown in FIG.1(c)−(d).However,the absorption peak ofπ-π∗transition of GO in acetone red-shifts to just 249 nm even after 9 hγ-ray irradiation,indicating a very slow reduction rate in acetone as shown in FIG.1(b).It shows that acetone can significantly stabilize GO inγ-irradiation.Furthermore,the absorbance ratioA270/A230of rGO(270 nm)and GO(230 nm)can directly reveal the degree of reduction of GO afterγ-irradiation.As shown in FIG.2,theA270/A230of GO in acetone is lower than that of GO in air and isopropanol.It further demonstrates that the reduction rate of GO in acetone is very slow compared with irradiation samples in air and isopropanol,because theγ-irradiation reduction of GO can be inhibited by acetone.
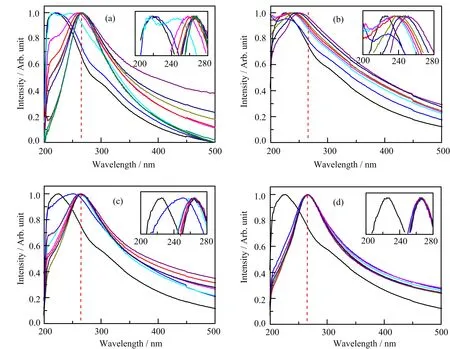
FIG.1 UV-Vis spectra of GO under reduction by(a)hydrozine,and(b)−(d) γ-irradiation under different conditions:(b)acetone,(c)air,(d)isopropanol.Different color lines represent different reaction time:(a)the black,blue,violet,magenta,red,dark yellow,navy,purple,wine,olive,and dark cyan lines represent 0,5,10,15,20,30,60,90,120,180,and 240 min;(b)−(d)the black,blue,violet,magenta,red,dark yellow,navy,and purple lines represent 0,0.5,1,1.5,2,3,6,and 9 h.
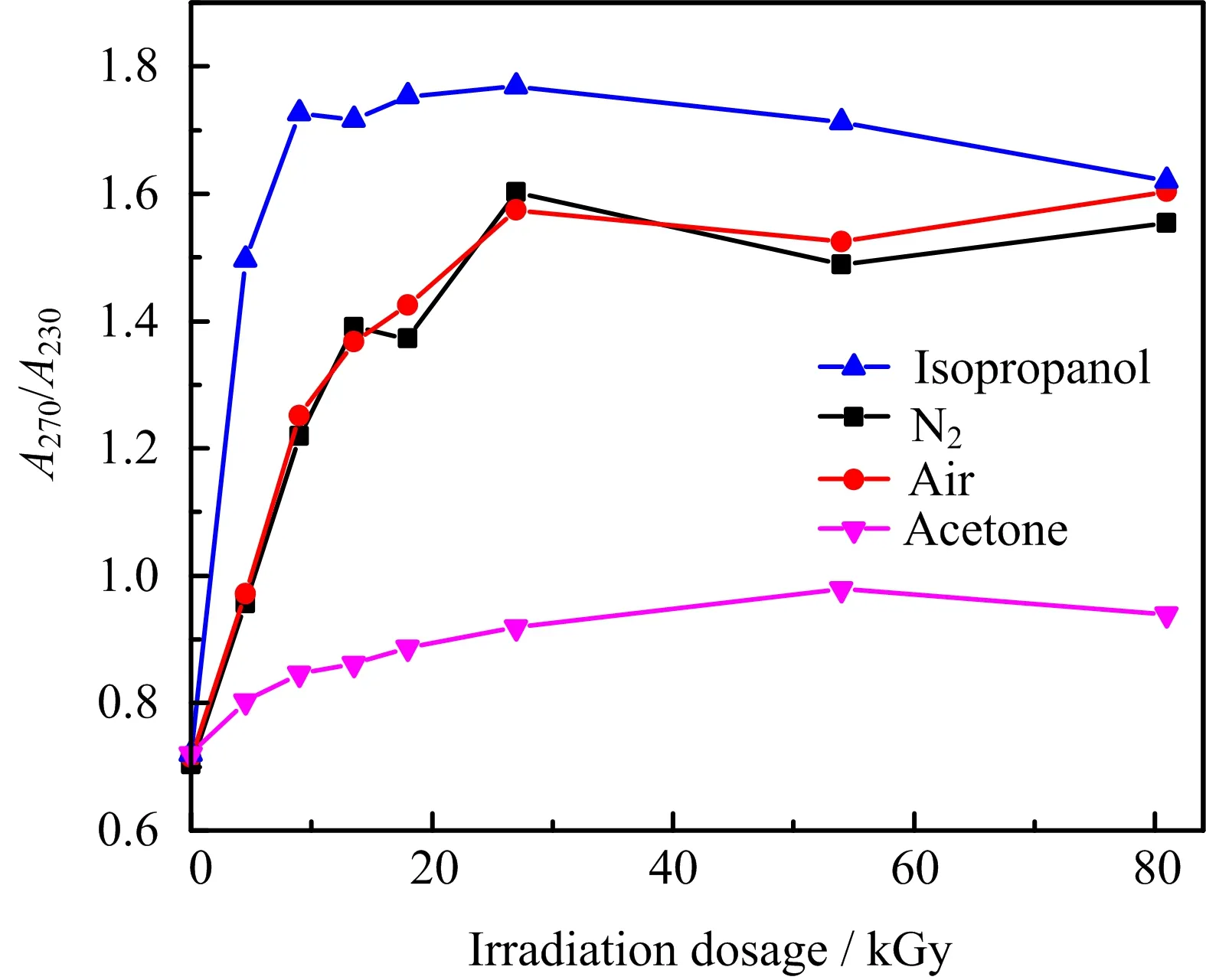
FIG.2 The ratio of the absorbance at 270 to 230 nm(A270/A230)of GO under reduction by hydrozine and γ-irradiation under different conditions.
The chemicalstructure changesofGO afterγ-irradiation were investigated by using FTIR.As shown in FIG.3,the peaks of 1727 and 1640 cm−1correspond to stretching vibration peak of C=O and C=C,respectively.The peak of 3500 cm−1is due to symmetrical stretching vibration of hydroxyl groups.According to FIG.3,the relative intensity of the C=O and hydroxyl group becomes weaker afterγ-ray irradiation.These phenomena can be explained by the removal of oxygen-containing functional groups from GO,suggesting thatγ-irradiation results in the reduction of GO.
XPS is an ideal characterization method to analyze the chemical state and atomic ratio of each element in GO.In this work,XPS is used to provide more evidence of the irradiation reduction mechanism of GO afterγ-irradiation.According to the survey scan of XPS shown in FIG.4,the peaks at 284−287 and 555−560 eV are the corresponding peak positions of C 1s and O 1s,respectively.It can be seen from FIG.4 that the overall intensity of the peak associated with O 1s decreases afterγ-irradiation or hydrated hydrazine reduction,whereas that of the C 1s increases.The peak intensity ratio of C 1s to O 1s(C/O)can re flect the degree of reduction of GO afterγ-irradiation.According to XPS analysis,the C/O of the pristine GO is 2.15,and the C/O of rGO completely reduced by hydrazine hydrate reduction is 5.90.The C/O of GO after 3 hγ-irradiation in air,isopropanol,and acetone are 5.63,5.85,and 3.20,respectively.It shows that the degree of reduction of GO afterγ-irradiation in acetone is the lowest among those in air and isopropanol,and also indicates that the reduction of GO underγ-ray is hindered by acetone.
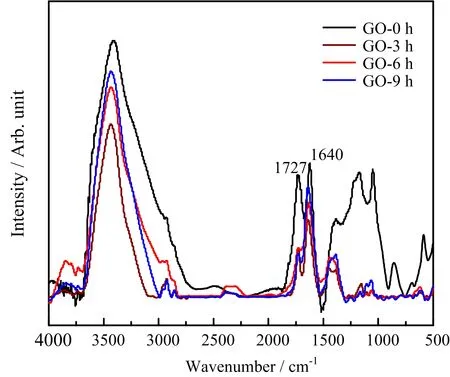
FIG.3 FTIR of GO after γ-irradiation in different time.
FIG.5 is the detailed C 1s XPS of GO,and C 1s spectrum can reveal the changes in the oxygen-containing groups of GO afterγ-irradiation.As shown in FIG.5,it clearly indicates five components attributable to carbon atoms involved in different functional groups or chemical bonds:the aromatic C=C(sp2hybridized carbon),C−C(sp3hybridized carbon),C−O/C−OH(hydroxyl and epoxy),C=O groups(carbonyl),and O−C=O(carboxyl),centered at 284.5,285.5,286.5,287.6,and 289.7 eV,respectively.The peak intensity of C−O/C−OH,C=O,and O−C=O decreases significantly in the C 1s spectrum of rGO prepared in hydrazine hydrate reduction,as shown in FIG.5(b).FIG.5 clearly demonstrates that the shoulder peaks at higher binding energies become significantly weak for these GO samples after irradiation in either air or N2,or irradiation in isopropanol,same as in the reduced sample in hydrazine,whereas the GO sample irradiated in acetone still shows a strong shoulder peak because of lower reduction degree.Irradiation atmosphere(in air or N2)did not result in a significant difference in the reduction degree.The quantitative results of the peak intensity of C−O/C−OH,C=O,and O−C=O of GO treated under different conditions are listed in Table I.Table I shows the chemical group percentage of GO treated under different conditions.It can be seen from Table I that the percentage of C=O of GO in acetone decreases from 40.37%to 17.52%afterγ-irradiation,but almost no changes in C−O/C−OH.Mainly the C=O groups were affected during GO irradiation in acetone.However,all oxygen-containing groups percentages of GO in isopropanol decrease afterγ-irradiation.It is demonstrated that acetone hasinhibitory effect on theγ-radiation reduction of GO.
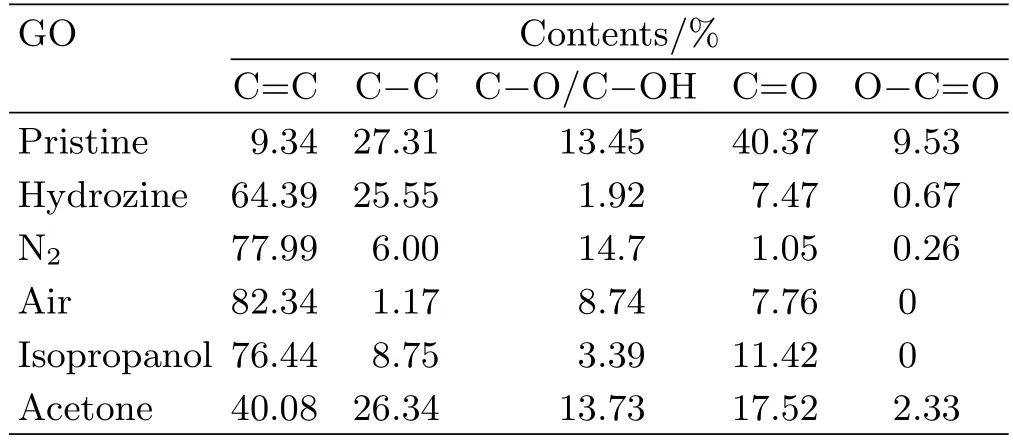
TABLE I Chemical group contents of pristine GO or reduced GO by hydrozine or γ-irradiation under different conditions in 3 h.
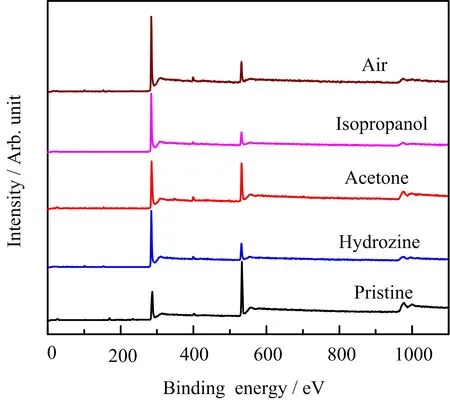
FIG.4 XPS survey scan of pristine GO and reduced GO by hydrozine and γ-irradiation under different conditions in 3 h.
B.UV irradiation of GO
It is established that UV irradiation can also reduce aqueous GO.But different fromγ-irradiation,UV irradiation can generally activate valence electrons,especially those in aromatic bonds,but cannot break the covalent bonds of water directly.Thus the reduction of GO by UV is totally different from that byγ-irradiation.The latter is carried out by the way of the radiation chemistry of water.We investigated the effects of acetone on UV reduction of GO with UV-Vis spectra.According to FIG.6(c)−(d),the absorption peak ofπ-π∗transition of aromatic C−C bond of GO in air and isopropanol red-shifts from 230 nm to 244 nm after 3 h UV-irradiation.However,the absorption peak ofπ-π∗transition of GO in acetone only red-shifts to 234 nm after 3 h UV irradiation,as shown in FIG.6(b).It shows that the reduction degree of GO by UV irradiation is lower than that ofγ-irradiated GO.This may be due to that the energy of UV is lower than the energy ofγ-rays.FIG.7 describes the peak position of GO versus UV irradiation time.According to FIG.7,the peak shift of GO in acetone is significantly slower than that of GO in air and isopropanol.Therefore,it proves that acetone also has inhibitory effect on the reduction of GO under UV,just like that underγ-rays.

FIG.5 C 1s XPS spectra of(a)pristine GO,reduced GO by(b)hydrozine,and γ-irradiation under different conditions(c)−(f)in 3 h:(c)air,(d)N2,(e)isopropanol,(f)acetone.
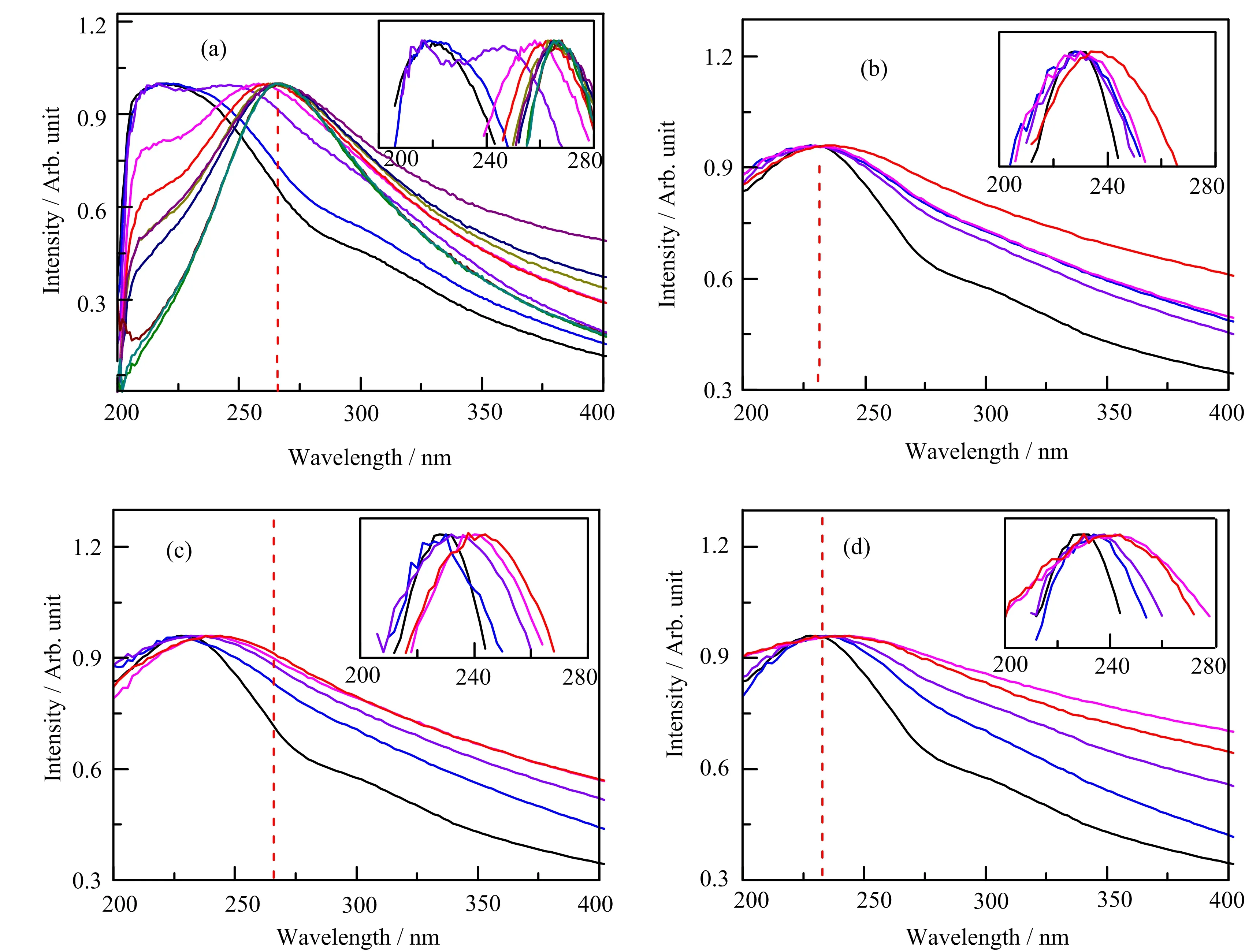
FIG.6 UV-Vis spectra of GO under reduction by(a)hydrozine,and(b)−(d)UV irradiation under different conditions:(b)acetone,(c)air,(d)isopropanol.Different color lines represent different reaction time:(a)the black,blue,violet,magenta,red,dark yellow,navy,purple,wine,olive,and dark cyan lines represent 0,5,10,15,20,30,60,90,120,180,and 240 min;(b)−(d)the black,blue,violet,magenta,and red lines represent 0,0.5,1,2,and 3 h.

FIG.7 Peak position change of GO in UV-Vis spectra after reduction by hydrozine and UV irradiation under different conditions.
The reduction of aqueous GO by UV involves an electron transfer process[18,19,22,30],though the electrons were predominantly generated in GO,not from water.We guess that acetone can interfere with the early electrons.To verify this hypothesis,we measured the photoelectric current generated during the UV irradiation of aqueous GO.The irradiation currents of GO in air,acetone,and isopropanol were monitored by using typical amperometricI-tcharacterization of electrochemical workstation CHI660D under a bias voltage at 0.8 V.Because the irradiation-generated electronhole pairs of GO can be separated under an electric field.So the changes of electrons of GO under irradiation can be directly indicated by the changes of irradiation current[31].Electrochemical tests were performed in a 0.5 mol/L Na2SO4electrolyte using a typical three-electrode electrochemical cell con figuration.The glassy carbon electrode covered with a GO film was working electrode,Pt electrode was counter electrode,and Ag/AgCl electrode(saturated KCl aqueous)was reference electrode.As shown in FIG.8,the average photocurrents of GO in air and isopropanol(12wt%in water)are 3.49 and 3.85 nA,respectively.But the average irradiation current of GO in acetone is only 2.76 nA,about 25%smaller than those in air and isopropanol.It indicates that acetone can capture the electrons generated by irradiation of GO aqueous.
IV.CONCLUSION
In this work,we have demonstrated that acetone can inhibit the reduction reaction of GO underγ-ray or UV irradiation.The inhibition of reduction of GO by acetone is because that acetone can capture electrons generated by GO aqueous underγ-ray or UV irradiation.Our findings would be interesting not only in radiation chemistry of GO,but also in understanding the redox properties of GO.
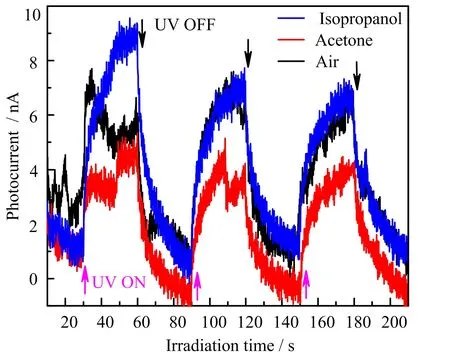
FIG.8 Photocurrent of GO during UV(365 nm)irradiation in different environments.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.51673181).
[1]K.Song,X.Y.Zhao,Y.M.Xu,and H.W.Liu,J.Mater.Sci.48,5750(2013).
[2]L.Peng,Z.Xu,Z.Liu,Y.Y Wei,and C.Gao,Nat.Commun.6,5716(2015).
[3]D.Chen,H.B.Feng,and J.H.Li,Chem.Rev.112,6027(2012).
[4]J.I.Paredes,S.V.Rodil,A.M.Alonso,and J.M.D.Tascón,Langmuir 24,10560(2008).
[5]S.Cinti and F.Arduini,Biosens.Bioelectron.89,107(2017).
[6]C.Chung,Y.K.Kim,D.Shin,and D.H.Min,Acc.Chem.Res.46,10,2211(2013).
[7]M.A.Lopez-Ramirez,D.F.Báez,and J.Wang,ACS Sens.1,3,217(2016).
[8]J.H.Hao,W.S.Yang,Z.Zhang,and J.L.Tang,Nanoscale 7,10498(2015).
[9]K.S.Novoselov,V.I.Falko,L.Colombo,and K.Kim,Nature 490,192(2012).
[10]F.Valentini,M.Carbone,and G.Palleschi,Anal.Bioanal.Chem.405,3449(2013).
[11]V.V.Singh,A.Martin,K.Kaufmann,and J.Wang,Chem.Mater.27,8162(2015).
[12]D.Vilela,J.Parmar,Y.F.Zeng,and S.Sánchez,Nano Lett.16,2860(2016).
[13]L.M.Zhang,Z.X.Lu,Q.H Zhao,J.Huang,H.Shen,and Z.J.Zhang,Small 7,460(2011).
[14]B.Gulbakan,E.Yasun,M.I.Shukoor,and W.H.Tan,J.Am.Chem.Soc.132,17408(2010).
[15]L.F.Dum´ee,C.F.Feng,L.He,and L.X.Kong,Carbon 70,313(2014).
[16]L.F.Dum´ee,C.F.Feng,L.He,and L.X.Kong,Appl.Surf.Sci.322,126(2014).
[17]J.Lee,W.H.Song,S.S.Jang,and J.H.Kim,Environ.Sci.Technol.44,3786(2010).
[18]J.W.T.Spinks and R.J.Woods,An Introduction to Radiation Chemistry,New York:John Wiley and Sons Inc.8(1990).
[19]R.Shen,K.Song,H.R.Liu,and H.W.Liu,J.Phys.Chem.C 116,15826(2012).
[20]T.H.Ji,Y.Y.Hua,M.Sun,and N.Ma,Carbon 54,412(2013).
[21]A.Bagri,C.Mattevi,M.Acik,and V.B.Shenoy,Nat.Chem.2,581(2010).
[22]Y.Matsumoto,M.Koinuma,S.Ida,and S.Amano,J.Phys.Chem.C 115,19280(2011).
[23]Y.H.Ding,P.Zhang,Q.Zhuo,and Y.Jiang,Nanotechnology 22,215601(2011).
[24]L.Guardia,S.V.Rodil,J.I.Paredes,and R.Rozada,Carbon 50,1014(2012).
[25]M.Venkanna and A.K.Chakraborty,AIP Conf.Proc.1591,574(2014).
[26]Q.Lai,S.F.Zhu,X.P.Luo,and S.H.Huang,AIP Adv.2,032146(2012).
[27]K.Bramhaiah and N.S.John,Adv.Nat.Sci.:Nanosci.Nanotechnol.3,045002(2012).
[28]X.Zhao,Q.H.Zhang,D.J.Chen,and P.Lu,Macromolecules 43,2357(2010).
[29]Y.W.Zhang,H.L.Ma,M.L.Zhai,and Z.Z.Yu,J.Mater.Chem.22,13064(2012).
[30]D.Stewart and C.T.Imrie,Chem.Comm.1383(1996).
[31]X.Qi,X.H.Zou,Z.Y.Huang,and L.Ren,Appl.Surf.Sci.266,332(2013).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- A Simple,Compact and Rigid Scanning Tunneling Microscope
- Extraction of Lignin from Tobacco Stem using Ionic Liquid
- Gd Doped Hollow Nanoscale Coordination Polymers as Multimodal Imaging Agents and a Potential Drug Delivery Carriers
- 3D Macro-Micro-Mesoporous FeC2O4/Graphene Hydrogel Electrode for High-Performance 2.5 V Aqueous Asymmetric Supercapacitors
- Gamma Ray Radiation Effect on Bi2WO6Photocatalyst
- Ag-Cu Nanoparticles Supported on N-Doped TiO2Nanowire Arrays for Efficient Photocatalytic CO2Reduction
