Comparative tree-ring anatomy of Fraxinus excelsior with Chalara dieback
Mirela Tulik •Barbaros Yaman •Nesibe Köse
Abstract We tested the hypothesis that the biometrical characters of wood elements in ash trees(Fraxinus excelsior L.)become modified in response to the progression of disease caused by Chalara fraxinea.Anatomical analyses were performed on wood samples collected at breast height from the trunks of groups of ash trees which contained healthy,weakened and dead trees.We measured(1)treering width,(2)early wood vessel diameter,(3)earlywood vessel element length,(4) fibre length,(5) fibre diameter,(6) fibre lumen diameter,and(7) fibre wall thickness.We showed that tree-ring width diminished in all analysed groups during disease progression.However,the greatest suppression of growth was observed in dead trees.In both weakened and dead ash trees,the reduction in tree-ring width was accompanied by diminished vessel diameter in the earlywood of the outermost annual rings.The annual rings of dead trees had shorter fibres having greater lumen diameter and thinner cell walls.Consequently,water conduction in the sapwood of dead ash trees was less efficient owing to reduced vessel diameter,and this seems to be one of the greatest disease-induced morphological modifications.All the anatomical modifications might be due to leaf loss and crown dieback triggered by Chalara fraxinea.
Keywords Ash·Dieback·Fibres·Vessels·Wood
Introduction
European ash(Fraxinus excelsior L.,Oleaceae),like many other broad-leaved trees,is economically valuable and ecologically important.It provides high quality wood for the timber industry.The natural range of F.excelsior
includes most of Europe.Ash is considered to be the most demanding tree species in terms of its requirement for soil richness and humidity(Bugala 1995),and it is sensitive to drought.Stands with inadequate water availability suffer damage most often seen as reduced crown size.However,European ash possesses drought tolerance mechanisms resulting from the reduction of osmotic potential(osmotic adjustment)and an increase in the elastic modulus(elastic adjustment;Marigo et al.2000).The first mechanism maintains turgor pressure and the second leads to the continued uptake of water from drying soils(Marigo and Peltier 1996).Water supply has a major effect on the growth of ash(Kerr and Cahalan 2004).Severe winters and late spring frosts,among other factors(Wardle 1961),cause cracking of stems.This is due mainly to the low resistance of ash to internal tension in the xylem and to the large vessels characteristic of the earlywood(Savill 1991).
The secondary xylem(wood)of ash,a useful source of data concerning the external environment,is composed of highly specialized cell types.Ash wood is a ring-porous wood and contains vessels,tracheids, fibres as well as axial and radial parenchyma cells.The mean diameter of ash vessels reaches 250–350 μm in earlywood(Jacquiot et al.1973),decreasing to 50 μm in latewood(Jacquiot et al.1973).The large earlywood vessels facilitate water and solute transport between organs.However,due to the large diameter of their lumina,they are vulnerable to embolization induced by frost,drought or other factors,including biotic factors(Carlquist 1988;Cochard et al.1997).Moreover,earlywood vessels are functional only during the year that they are formed(Zimmermann and Brown 1977),a common feature of trees having ring-porous wood.The length of vessel elements of ash is ca.400 μm(Jacquiot et al.1973).
The fibres range in length from 150 to 1600 μm and have cell walls that range from 0.75 to 2.6 μm in thickness(Jacquiot et al.1973).These provide mechanical support for the plant.Vessels and fibres have thick,lignified cell walls,the deposition of the secondary wall being preceded by the radial and tangential expansion of vessel elements and the axial growth of fibres.The process of wood element differentiation is terminated by programmed cell death(PCD)(Tulik and Myskow 2015).
Since the 1990s,large-scale ash dieback has been reported from several Baltic countries and recently has spread to other regions of Europe(Kowalski 2006;Thomsen et al.2007).In Poland,this was recorded for an area covering about 10,000 ha in 86%of forest districts,including stands containing ash(Zachara et al.2007).Since 1996,in Lithuania,especially in the north,large-scale decline in ash has been observed.There,stands covering over 30,000 ha,i.e.,approximately 60%of the total area occupied by this species,have been affected by the disease(Lygis et al.2005;Bakys et al.2009a).In Sweden,Denmark,and Germany,the first symptoms of ash dieback have been recorded since 2002(Barklund 2005;Heydeck et al.2005;Schumacher et al.2007;Bakys et al.2009b).In subsequent years(2007–2008),it has been reported in the Czech Republic,Estonia,Switzerland,Slovenia,France,Norway,Slovakia and Hungary(Zúbrik and Kunca 2007;Jankovskýetal.2008;Kowalskiand Holdenrieder 2008,2009;Szabo 2008).Ash dieback also affects other tree species, ‘e.g.,F.angustifolia,F.ornus’in the UK(Mitchell et al.2014).It is noteworthy that ash dieback occurs not only in Europe,but has also been reported from North America and Asia(Bricker and Stutz 2004;Ward et al.2009;Palik et al.2011),where F.nigra and F.velutina are mainly subject to the disease.Many countries have studied the factors involved in ash dieback and it has been reported that trees can become sensitive to biotic factors.It is assumed that there is a significant relationship between extreme climate events and the decline of forest trees.However,fungi that occur mainly in sick trees and their dead tissues are also believed to promote the progression of the disease.A fungus described by Kowalski(2006,2007)and referred to as Chalara fraxinea T.Kowalski(whose teleomorph stage is currently named Hymenoscyphus fraxineus Baral et al.2014)is believed to be the primary cause of ash dieback.Skovsgaard et al.(2010)pointed out that dieback of ash mainly affects trees of average or below-average size within a stand,and that the resistance of a particular tree to the disease diminishes with declining tree growth or vigour.Ash dieback is probably an example of a complex(spiral)disease where the interaction of both biotic and abiotic factors contributes to the progressive decline of ash stands.Symptoms of the disease can be observed on various parts of the plant body and include the wilting of leaves,cankers on young shoots and necrosis of stem bark(Barklund 2005,2006;Juodvalkis and Vasiliauskas 2002;Przybyl 2002;Thomsen et al.2007).From the outset,symptoms are mainly visible at the tops of trees and often lead to extensive top-dry or foliage dieback(Kowalski and Lukomska 2005).Subsequently,the disease can involve the lower parts of the stem,where the bark becomes discoloured and reddish(Hibben and Silverborg 1978).The decay of the root systems is also noticeable(Thomsen and Skovsgaard 2012).Owing to the fact that ash dieback appears to be the result of multiple factors,we set our null hypothesis as:The biometrical characters of wood elements of ash trees in decline are unaffected by the progression of ash dieback.
There is a gap in the knowledge of the tree-ring anatomy of F.excelsior in response to ‘Chalara dieback’.Our study aimed to determine the impacts of Chalara fraxinea on the tree-ring anatomy of the host ash trees by measurements of the annual ring width,earlywood vessel diameter,earlywood vessel element length, fibre length,fibre diameter, fibre lumen diameter and fibre wall thickness.
Materials and methods
Sampling and study sites
Disc samples of wood were collected in July 2010,at breast height(bh=1.3 m)from the main trunk of 13 European ash trees growing in the Srokowo Forest District(21°36′20′E, 54°20′48′N; Warminsko-Mazurskie Voivodeship,northeast Poland).Based on the degree of defoliation,we categorized the 13 sampled trees into three groups based on health/decline:dead trees(Group 1);weakened trees(Group 2);and healthy trees(control-Group 3,Table 1).The first group consisted of 5 trees,and the two latter groups consisted of 4 trees each.Dead trees occupied a moist,mixed broad-leaved forest.

Table 1 Mean values of biometric characters of European ash selected for studies during the course of sampling.The biometric parameters represent the mean value for each group
Wood preparation and statistical analysis
A single strip of wood containing all annual increments from pith to bark,without knots,and sufficiently wide for studying both dendrochronology and wood anatomy of tree-rings,was removed from a single radial axis of each of the 13 tree stem discs.Tree-ring widths were measured to an accuracy of 0.01 mm using a PAST4Lt measurement system.COFECHA software was used to evaluate the quality of cross-dating(Holmes 1983;Grissino-Mayer 2001).Tree-ring measurement series for each group were standardized by fitting a linear or negative exponential regression equation.Bi-weight robust mean values were used to build mean chronologies for each separate group.These analyses were performed using ARSTAN software(Cook 1985;Grissino-Mayer et al.1996).We observed sudden suppression of growth post-1999 when investigating the mean chronologies of dead and weakened trees.In order to investigate any growth changes that occurred pre-1999 and post-1999,‘Routine IMP’in Dendrochronology Program Library(DPL),which uses tree-ring measurement series or chronologies to compare mean growth before and after an impact,was employed for each group.In this research we used standardized mean chronologies to calculate growth changes.
Anatomical analysis of wood was performed on three strips for each group.This involved taking small,cubeshaped samples of wood including post-1999 tree-rings,from these strips.After boiling and softening in glycerol and water(1:1)for 1 week,20 μm—thick transverse sections of the wooden cubes were cut using a G.S.L.-1 microtome.Routine procedures were applied(Gärtner and Schweingruber 2013)for the preparation of all thin sections.Measurements of tangential and radial earlywood vessel diameters were made semi-automatically from calibrated micrographs based on the protocol ‘making wood anatomical measurements with ‘Image J’described by Jansen and Choat(2011).Measurements of vessel elements length, fibre length, fibre diameter and fibre lumen diameter were determined for isolated vessel elements and fibres following maceration of wood splinters sampled for both 2008–2009 and 1999–2000.For this purpose,wood splinters were macerated in a solution of sodium chlorite acidified with acetic acid(Spearin and Isenberg 1947).An Olympus light microscope(CX-21)with micrometre eyepiece was used to measure the characteristics of the macerated material.We measured vessel element length for earlywood vessels only.Fibre wall thickness was considered half the difference between fibre diameter and fibre lumen diameter.When calculating mean values,n=50 for vessel elements length,but n=60 for all fibre parameters.Statistical analysis was carried out using SPSS 20.0(IBM)software.For both TVD and RVD(tangential and radial vessel diameter)measurements throughout tree-rings from 1999 to the bark,differences among Groups 1,2 and 3 were tested using one-way ANOVA followed by Scheffe’s post hoc test.T Test was employed at the 99%con fidence level for vessel element length and all fibre parameters because only two periods of the sample strip for each group were measured.
Results
Anatomical examination of five dead trees showed that some died in the years preceding the study.Two trees formed annual rings dated to 2009,two trees dated to 2008,and the fifth tree had insect galleries dating to 2006.

Fig.1 Standardized mean chronologies of each group
Different standardized mean chronologies were built for the trees of each of the three condition categories(Fig.1).Compared with the chronology of healthy trees,abrupt reductions in growth were observed for dead and weakened trees post-1999,those of dead trees being more pronounced.These visually observed reductions in growth were also documented by the results of routine IMP(‘‘Impact before and after event’’)analysis for ± 11 years(Table 2).Each group showed post-1999 growth reduction,even the group of healthy trees(Group 3).However,dramatic variations in growth were calculated as 44%for dead trees(Group 1,Fig.1),61%for weakened trees(Group 2),and 77%for healthy trees(Group 3,Table 2).From 1999 to 2010(2009 for Group 1)there was a reduction in mean tangential vessel diameter(TVD)and radial vessel diameter(RVD)for Groups 1 and 2(Figs.2,3 and 4).Quadratic polynomial R2values for TVD trend-lines from 1999 to 2010 were calculated as 0.91 (p≤0.001), 0.89(p≤0.001),and 0.30(p>0.001)for Group 1,Group 2 and Group 3,respectively.The values for RVD were 0.95(p≤0.001),0.93 (p≤0.001)and 0.08 (p>0.001),respectively.TVD and RVD differed by health category.Mean TVD for Group 1(171.4 μm)was less than that of Group 2(193.6 μm).However,TVD means for Groups 1 and 2 were statistically similar to the mean for Group 3(184.53 μm).RVD for Group 1(207.50 μm)was significantly lower than RVD for Group 2(250.69 μm)and Group 3(241.12 μm).Groups 2 and 3 had similar mean RVD.Vessel element length(VEL)differed by condition category(F:17.5,p≤0.001).VEL means for Group 1(276.80 μm)and Group 2(288.35 μm)were significantly greater than for Group 3(257.40 μm).VEL means were similar for Groups 1 and 2.Fibre characters varied significantly by condition category(p≤0.001).Mean fibre lengths(FL)for Group 1(762.10 μm)and Group 2(819.92 μm)were significantly less than for Group 3(1032.51 μm).Fibre wall thickness(FWT)for Group 1(4.12 μm)was significantly less than FWT for Groups 2 and 3(5.76 μm and 5.31 μm respectively).Fibre lumen diameter(FLD)for Group 1(16.13 μm)was greater than for Groups 2 or 3(12.48 and 12.58 μm,respectively).Fibre diameters(24.35,24.02 and 23.21 μm for Groups 1,2 and 3,respectively)did not vary by condition category(F:4.1,p>0.001).
When comparing two parts of a strip(LTR:last formed tree-rings (2008–2009)and CTR:control tree-rings(1999–2000))for each group,mean vessel element length(VEL)formed in LTR was less than VEL for CTR in Group 1.However,for all groups,these mean differences were not significant(p>0.001)(Fig.5).For Groups 1 and 2,mean fibre length(FL)formed in LTR was significantly less than that for CTR(p<0.001).Mean FL for Group 3 was similar to means for LTR and CTR(Fig.6).Mean fibre diameters(FD)for all groups were similar for LTR and CTR(p>0.001)(Fig.7).However,mean fibre lumen diameter(FLD)in LTR is significantly greater than that in CTR for Groups 1 and 2(p<0.001;Fig.8),and mean fibre wall thickness(FWT)in LTR is significantly less than that in CTR for Groups 1 and 2(p<0.001;Fig.9).But,mean FLD and FWT for Group 3 were similar to means for LTR and CTR.
Discussion
Compared with healthy trees,the outermost tree-rings of dead and weakened ash trees were significantly narrower and the narrowest rings occurred in dead trees(Group 1,Fig.1).Similar results were reported by Danek and Chuchro(2015).Dead,weakened and healthy trees of ash showed a reduction in growth post-1999,but a dramatic reduction in growth was also observed for dead trees(44%compared with 77%in healthy trees-Table 2).Both tangential and radial vessel diameters in the outermost treerings of dead trees were significantly smaller than those of weakened and healthy trees.Moreover,between 1999 and the year that the outermost annual ring was formed in each case,the vessel diameters demonstrated a diminishing trend for both dead and weakened trees(Figs.2,3 and 4).
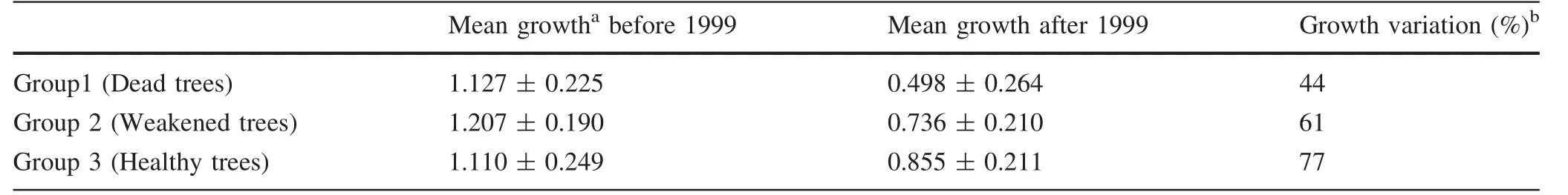
Table 2 Mean growth 11 years pre-/post-1999

Fig.2 Transverse section of wood of tree 3 in Group 1

Fig.3 Variation in mean tangential vessel diameter from 1999 to 2009 for Group 1 and to 2010 for Groups 2 and 3
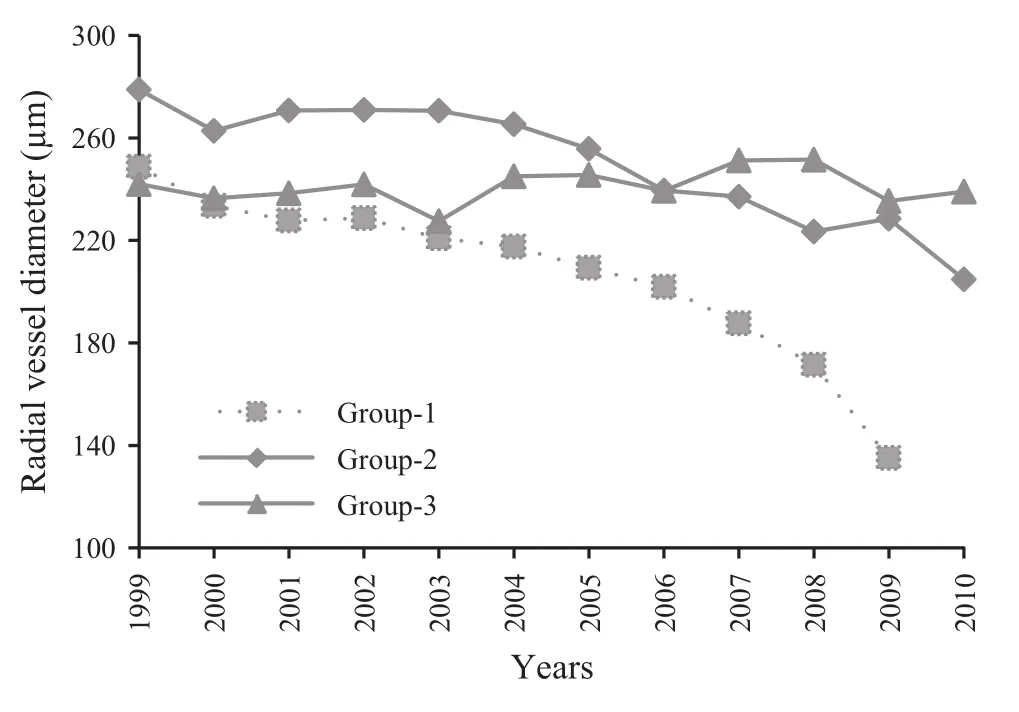
Fig.4 Variation in mean radial vessel diameter from 1999 to 2009 for Group 1 and to 2010 for Groups 2 and 3

Fig.5 Mean vessel element length in LTR(last formed tree-rings)and CTR(control tree-rings)for each group.Vertical bars represent standard deviation.LTR and CTR compared in each group separately using the Independent-Samples T Test.The same letters on each bar of each group indicate that there is no statistical significant difference between LTR and CTR of related group(p≤0.001)
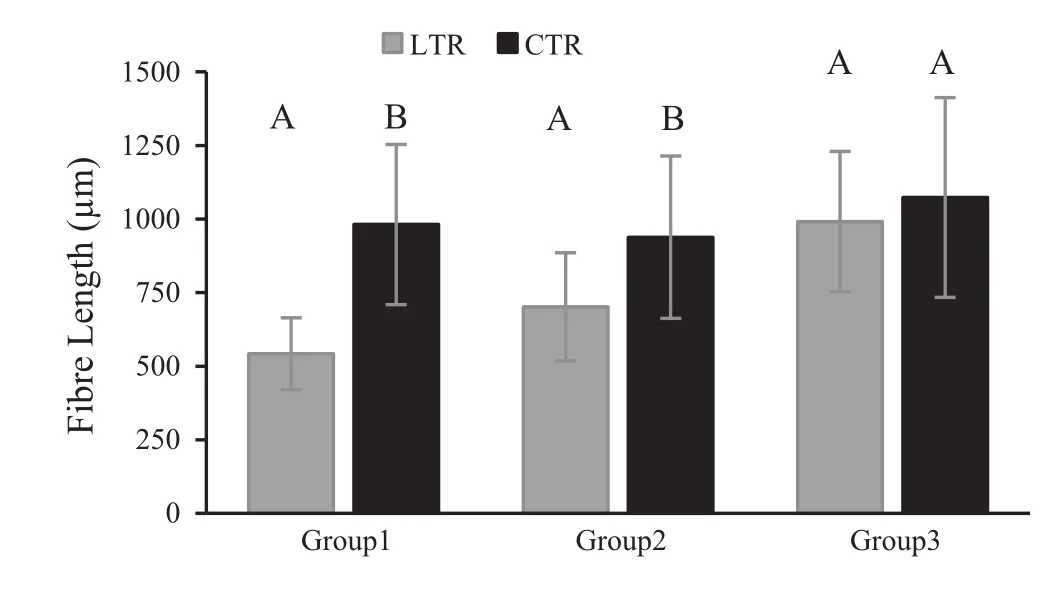
Fig.6 Mean fibre length in LTR(last formed tree-rings)and CTR(control tree-rings)for each group.Vertical bars represent standard deviation.LTR and CTR compared in each group separately using the Independent-Samples T Test.The same letters on each bar of each group indicate that there is no statistical significant difference between LTR and CTR of related group(p≤0.001)

Fig.7 Mean fibre diameter in LTR(last formed tree-rings)and CTR(control tree-rings)for each group.Vertical bars represent standard deviation.LTR and CTR compared in each group separately using the Independent-Samples T Test.The same letters on each bar of each group indicate that there is no statistical significant difference between LTR and CTR of related group(p≤0.001)
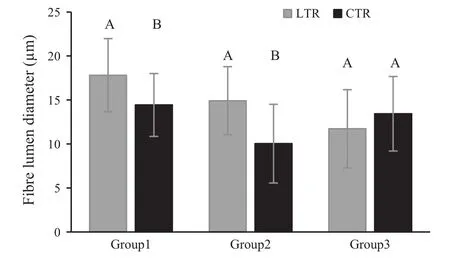
Fig.8 Mean fibre lumen diameter in LTR(last formed tree-rings)and CTR(control tree-rings)for each group.Vertical bars represent standard deviation.LTR and CTR compared in each group separately using the Independent-Samples T Test.The same letters on each bar of each group indicate that there is no statistical significant difference between LTR and CTR of related group(p≤0.001)
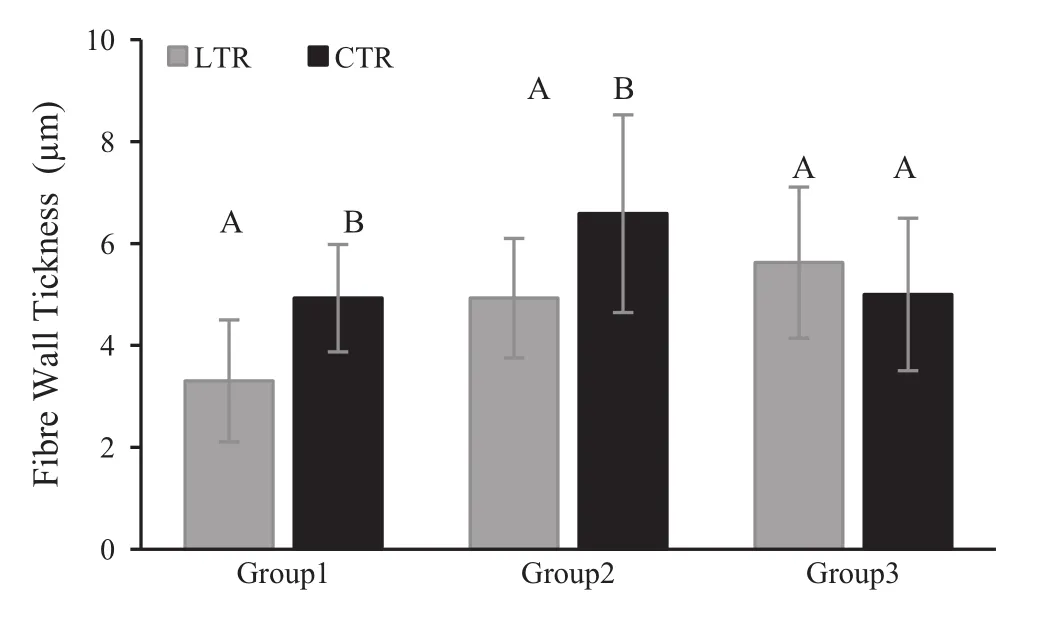
Fig.9 Mean fibre wall thickness in LTR(last formed tree-rings)and CTR(control tree-rings)for each group.Vertical bars represent standard deviation.LTR and CTR compared in each group separately using the Independent-Samples T Test.The same letters on each bar of each group indicate that there is no statistical significant difference between LTR and CTR of related group(p≤0.001)
We discuss together the first and second points above because the formation of annual rings depends mainly on environmental factors(Schweingruber1993).Severe drought,scarcity of soil water,or a reduced C sink(Eilmann et al.2010;Brzostek et al.2014)are all possible causes of reduction in annual ring formation.In terms of abiotic stresses,drought,frost and changing winter conditions are considered to be associated with ash dieback(Schumacher et al.2007,2010).However,there is a gap in the knowledge whether and how environmental factors interact with Ch.fraxinea to cause this disease.Due to the fact that this fungus causes leaf loss and crown dieback in affected trees,in terms of carbon gain by plants,it is clear that the loss of photosynthetic organs negatively affects cambial activity in woody plants,and therefore tree-rings form narrower(Schweingruber 2007).The least reduction in the growth of annual rings occurred in the control group,which demonstrated ca.20%defoliation.
At the anatomical level,the smallest cellular structures(narrower vessels,shorter fibres,thinner cell wall etc.)were observed for weakened and dead groups of ash trees,and it is probable that this is caused by low cambial activity or changes to the process of wood differentiation(Zweifel et al.2006;Steppe and Lemeur 2007).As previously mentioned,it is a fact that reduced photosynthetic activity during leaf loss and crown dieback may lead to low carbon accessibility and allocation for cell division.It is known that crown dieback is a significant growth stressor,reducing photosynthesis(Manion 1991).In addition,tree species like Fraxinus excelsior that have ring-porous wood are strongly affected by growth rate changes(Panshin and deZeeuw 1970).In this study,owing to the drastic reduction in growth for the period from 1999 to the formation of the outermost rings(especially the narrowest tree-rings formed prior to the year of death)in dead trees(Group 1),we might suppose that this chronological section of wood is mechanically weak.This is due to the narrowness or absence of latewood(Fig.2)and the biometrical parameters of fibres(thin fibre wall and large fibre lumen in narrow tree-rings,Figs.8 and 9).
It is well known that while narrow vessels ensure safe conduction of water,large vessels are an indicator of conductive efficiency(Baas 1982;Carlquist and Hoekman 1985;Carlquist 1988;De Micco and Aronne 2012).A diminishing trend in the vessel diameters of dead and weakened ash trees may be due to ash dieback,triggered by Ch.fraxinea under associated with abiotic stresses.Pouzoulet et al.(2014)have proposed a hypothesis for explaining the relationship between vessel dimensions and fungal vascular wilt diseases in woody plants.Although ash dieback triggered by Ch.fraxinea is not a vascular wilt disease such as occurs in Ulmus sp.and Vitis sp.(Yadeta and Thomma 2013;Pouzoulet et al.2014),the question still arises whether ash phenotypes with larger vessel diameters are more vulnerable to this fungus when subjected to different environmental stress factors.Unfortunately,this question remains to be answered.However,in contrast to dead and weakened ash trees,the earlywood of healthy trees containing smaller vessel diameters during the period 1999–2000,yet not exhibiting a diminishing trend in vessel diameters as one passes from 1999 to the outermost annual ring might possibly indicate a degree of resistance to this disease.Alternatively,it is worth noting that cambial cell derivatives that differentiate into vessels increase in size(both radially and tangentially)and that this process is both guided by turgor(Gonzalez and Eckstein 2003)and utilizes the previous year’s reserve of carbohydrate(Atkinson and Denne 1988).
It would appear that dead and weakened trees attempt to adjust their water transport system by making it more resistant to cavitation.This however comes at a price,namely,reduced conductive efficiency(Sperry et al.2008).Ch.fraxinea take place in outer and inner sapwood at a high rate,and in axial direction,many hyphae occur mainly in the vessels as well as in the paratracheal parenchyma(Schumacher et al.2010).Both hyphae barriers in the sapwood and reduction in the vessel diameter makes host ash trees less efficient in transporting water.Thus host ash trees might be more vulnerable to the stress effects of abiotic factors compared to those of control trees,where a diminishing trend in vessel diameter was not observed.
The biometrical characters of ash fibres derived from the dead group were seemingly affected to a greater extent by adverse environmental factors than those of the control group,as their diameters remained rather more constant during the years prior to and during the progress of ash dieback.The formation of shorter fibres with thinner cell walls by the dead group of ash trees may result in discernibly poorer CO2uptake than that realized when there is sufficient soil moisture to sustain photosynthesis(Anderegg et al.2012;Choat et al.2012)and continued growth.
Based on our results,on the one hand,the dead group of ash trees modified their water-conducting system when subjected to stress by building a less effective but safer water vascular system comprising vessels of small diameter.On the other hand,reduced carbon investment resulted in reduced thickness of the fibre cell wall.It is a fact that derivatives of cambial cells that embark on the process of differentiating into fibres grow intrusively(Larson 1994).Shortened xylem fibres in dead ash trees may indicate a problem with the water content of the differentiating cell.It thus appears that this growth strategy may be insufficient for survival following such stress.It is worth noting that,based on studies of the colonization of wood and cortical tissues following artificial wound inoculation with Ch.fraxinea,that this pathogen develops intracellularly,moving from cell to cell,thus easily colonizing the phloem,axial paratracheal parenchyma and radial parenchyma cells(Dal Maso et al.2012,2014).Schumacher(2011)proposed an invasion model for woody tissue,where the pathogen spreads to the pith from infected shoots via rays,and moves axially through pith,vessels and fibres and subsequently spreads outwards causing necrotic lesions in the bark.Considering the pathway of Ch.fraxinea,it appears likely that secondary metabolites produced by the pathogen affect the cambial cells and its derivatives and lead to the formation of wood cells of modified character that precipitate the complex phenomenon of ash dieback.
Our results reveal that more focused research into the morphology of vessels and fibres derived from different ash phenotypes occurring within the natural range for the species should now be planned and undertaken so as to challenge the complex and significant threat to biodiversity and forest ecosystems that ash dieback poses,especially since its potential economic and aesthetic impacts are believed to be immense.
AcknowledgementsThe authors acknowledged the assistance of Katarzyna Marciszewska,PhD,Department of Forest Botany at Warsaw University of Life Sciences—SGGW during the collecting of wood samples.We thank to Jeffrey Wall for his English editing.
 Journal of Forestry Research2018年6期
Journal of Forestry Research2018年6期
- Journal of Forestry Research的其它文章
- Black locust(Robinia pseudoacacia L.)as a multi-purpose tree species in Hungary and Romania:a review
- The impact of the environmental factors on the photosynthetic activity of common pine(Pinus sylvestris)in spring and in autumn in the region of Eastern Siberia
- Osmoregulators in Hymenaea courbaril and Hymenaea stigonocarpa under water stress and rehydration
- Effect of nitrogen levels on photosynthetic parameters,morphological and chemical characters of saplings and trees in a temperate forest
- Free amino acid content in trunk,branches and branchlets of Araucaria angustifolia(Araucariaceae)
- Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress
