Free amino acid content in trunk,branches and branchlets of Araucaria angustifolia(Araucariaceae)
Crizane Hackbarth•Patrícia Soffiatti•Flávio Zanette•Eny Iochevet Segal Floh•Amanda Ferreira Macedo•Henrique Aparecido Laureano
Abstract Araucaria angustifolia (Bertol.)O.Kuntze exhibits dimorphism in its stem structure,where the trunk is orthotropic and branches and branchlets(primary and secondary branches)are plagiotropic.These stems exhibit different behavior when used for vegetative propagation,and only segments of trunk can form a complete plant.The physiological and biochemical mechanisms that characterize these stems are still little known.The aim of this study was to describe the free amino acid pro files in trunks,branches,and branchlets of A.angustifolia.Segments of 5 cm in length were excised from young individuals below the stem apex.The needles were removed and samples were frozen and lyophilized.The determinations were made by high-performance liquid chromatography,and the results were expressed as μg/g fresh weight(FW).The trunks and branches had the highest content of total amino acids, which were 112.23 ± 20.57 μg/g FW and 111.97 ± 27.78 μg/g FW,respectively.The amino acids—glutamine,aspartate and γ-aminobutyric acid and tyrosine—were noticeably higher in the three types of stems.In the trunk,a higher amount of asparagine and tryptophan,was also detected.Glutamic acid and glutamine were found in higher quantities in the branches.The branchlets had very low total amino acid content(30.79 ± 4.19 μg/g FW),wherein asparagine is the only amino acid not detected.Thus,it was observed that the pro file of the free amino acid differs among trunks,branches,and branchlets in A.angustifolia,indicating that they perform different functions.
Keywords Brazilian pine·Physiological mechanisms·Stem’s dimorphism ·Free amino acids
Introduction
Araucaria angustifolia(Bertol.)O.Kuntze(Araucariaceae),commonly known as araucaria,Brazilian pine,or Paraná pine,is the only conifer native to Brazil with economic importance(Elbl et al.2014).The wood is of excellent quality,for both construction and manufacturing paper;also,the seed of araucaria is used as food by both humans and wildlife,and has excellent nutritional value(Steiner et al.2008;Zanette et al.2017).The commercial exploitation of araucaria seeds is currently performed by extraction from natural stands,but some studies have aimed at developing vegetative propagation of the species for the formation of clonal orchards,mainly through grafting(Wendling 2011;Zanette et al.2011).However,there are limitations to the application of vegetative propagation,especially regarding the use of plagiotropic branches,which predominate in the architecture of the species(Kageyama and Ferreira 1975).
The branches of A.angustifolia exhibit dimorphism,and according to their growth pattern,they can be classified as orthotropic or plagiotropic(Zanette et al.2011,2017).The orthotropic branches grow in bifurcations or apexes of the trunk,and have a vertical orientation(Kageyama and Ferreira 1975).Only orthotropic segments coming from the trunk are of interest for forming vegetative propagation of commercial clonal orchards of A.angustifolia,because only they are capable of producing complete individuals(Zanette et al.2011).
Plagiotropic branches,on the other hand,exhibit lateral or horizontal growth,bilateral symmetry,and limited growth and life span(Iritani et al.1992;Zanette et al.2017).When used in vegetative propagation,plagiotropic branches do not form normal individuals because they maintain their lateral growth form and are incapable of forming a complete canopy(Iritani et al.1992;Wendling 2011).This characteristic remains even if they are grafted to an orthotropic stem(Zanette et al.2011).Branch orthotropism and plagiotropism characterize plants of the family Araucariaceae,and these branches remain as such,regardless of the orientation or treatment imposed on them(Haines and Fossard 1977).Moreover,the issue of branch dimorphism goes beyond orthotropism or plagiotropism,since graft branches grown in vertical orientation(orthotropic)continued with typical branch morphology(Constantino 2017).
Another interesting feature of A.angustifolia is that the trunk does not produce reproductive structures.Male and female strobili normally develop at the extremities of branches,rarely in branchlets(Zanette et al.2017).These peculiar characteristics indicate that these stems have different functions in the plant.
The mechanisms that regulate the behavior of these three types of stems are poorly understood.Some studies have limited their investigation to hormonal regulation,especially auxin,in orthotropic branches of other species(Muday 2001;Veierskov et al.2006).However,branches intended to be permanently plagiotropic might have a different regulatory system than the one which controls the vertical main trunk,which is likely regulated from within the branch itself(McSteen and Leyser 2005;Veierskov et al.2006).There have been no biochemical or physiological studies of the behavior of these stems in A.angustifolia,even though several physiological aspects beyond simply tropism,such as hormonal regulation and transcription factors,among other molecules,such as amino acids,should be involved in the differences between the three types of stems.
Amino acids play several important roles in plants.In addition to their role in protein biosynthesis,they also represent building blocks for several other bio-synthetic pathways(Hildebrandt et al.2015)and are essential for nitrogen transport in plants(Cánovas et al.2007;Lea et al.2006).In addition to their structural roles,they are important for a multitude of cellular reactions,influencing various physiological processes such as plant growth and development,intracellular pH control,metabolic energy generation or redox power,and resistance to both abiotic and biotic stresses(Fagard et al.2014;Galili et al.2014;Zeier 2013).More recently,a signaling role has been attributed to several amino acids in plants(Häusler et al.2014).
The function of specific amino acids and their degradation have been extensively investigated in different plant organs(Fait et al.2008;Gu et al.2010;Krübel et al.2014).In general,amino acid content varies widely depending on the developmental and physiological state of a plant.In A.angustifolia,free amino acid content has been evaluated in studies involving zygotic and somatic embryogenesis and seed development(Astarita et al.2003a,b).However,there have been no studies of the free amino acid content of stems.
The objective of this research was to describe the free amino acid pro file of the stems of A.angustifolia,from segments removed from the main trunks,branches and branchlets,in young plants with the aim of determining if there are differences among them.
Materials and methods
The work was conducted at the Plant Cell Biology Laboratory,at the Biosciences Institute of the University of São Paulo(USP),in São Paulo—SP.The plants used in this experiment came from seedlings located at Department of Plant Science,Federal University of Parana(UFPR)in Curitiba—PR(25°25′S latitude and 49°16′W longitude,934 meters high).The plants were grown in 20 L pots containing soil and substrate,with one plant per pot,under homogeneous conditions.Samples were collected during July 2016.
The stem segments of 5 cm of the seven plants,located below the apex of branches,branchlets,and main trunk of A.angustifolia were collected of the last whorl formed from 2-year-old plants.The stem needles were removed and the samples were immediately frozen in liquid nitrogen and lyophilized(Edwards®freeze drier,with a Savant II high-vacuum pump and a 30 L volume).Each sample was weighed before and after lyophilization and a calculation was made for the relationship between fresh weight and lyophilized weight of the samples.
The free amino acids were extracted using 0.2 g fresh weight(FW).The samples were macerated in 6 mL of 80%ethanol,and then concentrated in speed vacuum at 45°C until the ethanol was eliminated.The samples had their volume adjusted to 2 mL with water and were centrifuged at 20.000 g force for 10 min at 4°C.The supernatant was purified,prior to the chromatographic analysis,in Waters Sep-Pak C18 minicolumn with 12 mL of methanol,and then the samples were again concentrated in a speed vacuum at 45°C,until methanol was eliminated.The volume of the free amino acid extracts was adjusted to 1.5 mL with water.The samples underwent pre-column derivatization with OPA-Borate solution(140 mg of o-phthaldialdehyde,2.8 mL of methanol 100%,18.2 mL of borate buffer 400 mM pH 9.5 and 166.2 μL of β-mercaptoethanol).Aliquots of 20 μL of derivatized samples were injected.
The identification and quantification of the free amino acids was done according to Astarita et al.(2003b)and Wu and Meininger(2008).The amino acids were analyzed using high-performance liquid chromatography with fluorescence detector(RF-20A)(HPLC/F)(Shimadzu®LC-10AD pump,SIL-10AF autoinjector,and RF-20A detector)using a column Supelcosil LC-18 of reverse phase with 25 cm×4.6 mm,silica-gel coating,and particle size of 5 μm(Sigma/Supelco®).The content of the free amino acids was expressed in μg/g of FW.The reagents used for the mobile phase were obtained from Merck®Lichrosolv.
The eluent A was made up of sodium acetate trihydrate(100 mM),5 mL/L tetrahydrofuran,60 mL/L methanol,and deionized water to the final volume of 1 L.The pH 7.2 was adjusted with 48 μL/L HCL(6 N).The eluent B was 100%methanol.The elution program of 100%methanol relative to the other eluent defined the run gradient,at a flow rate of 1.0 mL/min.In the first 15 min,the concentration of B automatically remained at 14%;from there,the gradient was modified as follows:14–30%between 15 and 20 min,30–35%between 20 and 24 min,35–47%between 24 and 26 min,47–50%between 26 and 34 min,50–70%between 34 and 38 min,70–100%between 38 and 40 min,and maintaining 100%up to 42 min for column cleaning,and returning to 14%for up to 49 min for stabilization and start of new a run.The temperature of the column oven was maintained at 40°C.
The fluorescence detector was set for excitation at 250 nm and emission at 480 nm.The areas and retention times of each amino acid(Fig.S2–S4)were evaluated by comparison with standard amino acids at known concentrations(Fig.S1):aspartate,glutamate,asparagine,serine,glutamine,histidine,glycine,arginine,threonine,alanine,acid γ-aminobutyric+tyrosine,methionine,tryptophan,valine,phenylalanine,isoleucine,leucine,ornithine,lysine,and citrulline.
The experimental design was completely randomized,with seven replicates(plants)for each stem.Analysis of Variance(ANOVA)and Turkey tests were carried out to verify the existence of significant differences in the content of amino acids between the stems and to compare the averages observed,with 95% significance level,using R software.
Results
The composition of free amino acids in segments of the trunk,branches,and branchlets are shown in Table 1.The trunk and branches had the highest total amino acid content.The mean total content of free amino acids present in the trunk was 112.23 ± 20.57 μg/g FW,while the branches had 111.97 ± 27.78 μg/g FW and the branchlets 30.79 ± 4.19 μg/g FW,the value of the latter was approximately 73%lower than that observed in trunks and branches.A significant difference was observed between at least one pair of stems for the sixteen amino acids evaluated.The amino acids—ornithine,threonine,and lysine—were not detected in the analyzed stems.
The proportion of amino acids varied according to the type of stem evaluated.Aspartate and glutamine were prominent,predominating in the three stem types(Table 1),especially in the trunk and branches.Despite the extensive amount of γ-aminobutyric acid+tyrosine found in the present study,these data only provide qualitative,rather than quantitative information due to the low resolution of the obtained peak.It was not possible to quantify tyrosine alone because it was quantified together with γaminobutyric acid.Branchlets possessed very low levels of total amino acids(Table 1),and asparagine,although present in trunk and in branches,was not detected in branchlets.The amino acids,histidine and phenylalanine,were found in consistent amounts among the three stem types,but at slightly higher levels in the trunk(Table 1).Asparagine was also detected in greater quantity in the trunk,while glutamine and the glutamic acid were detected in greater quantity in the branches(Table 1).
Asparagine content(21.51 ± 6.39 μg/g FW)was higher in the main trunk,compared to the branches and branchlets(Table 1).Tryptophan content was higher in the trunk(4.25 ± 1.12 μg/g FW)and branches(2.12 ± 0.5 μg/g FW)(Table 1).
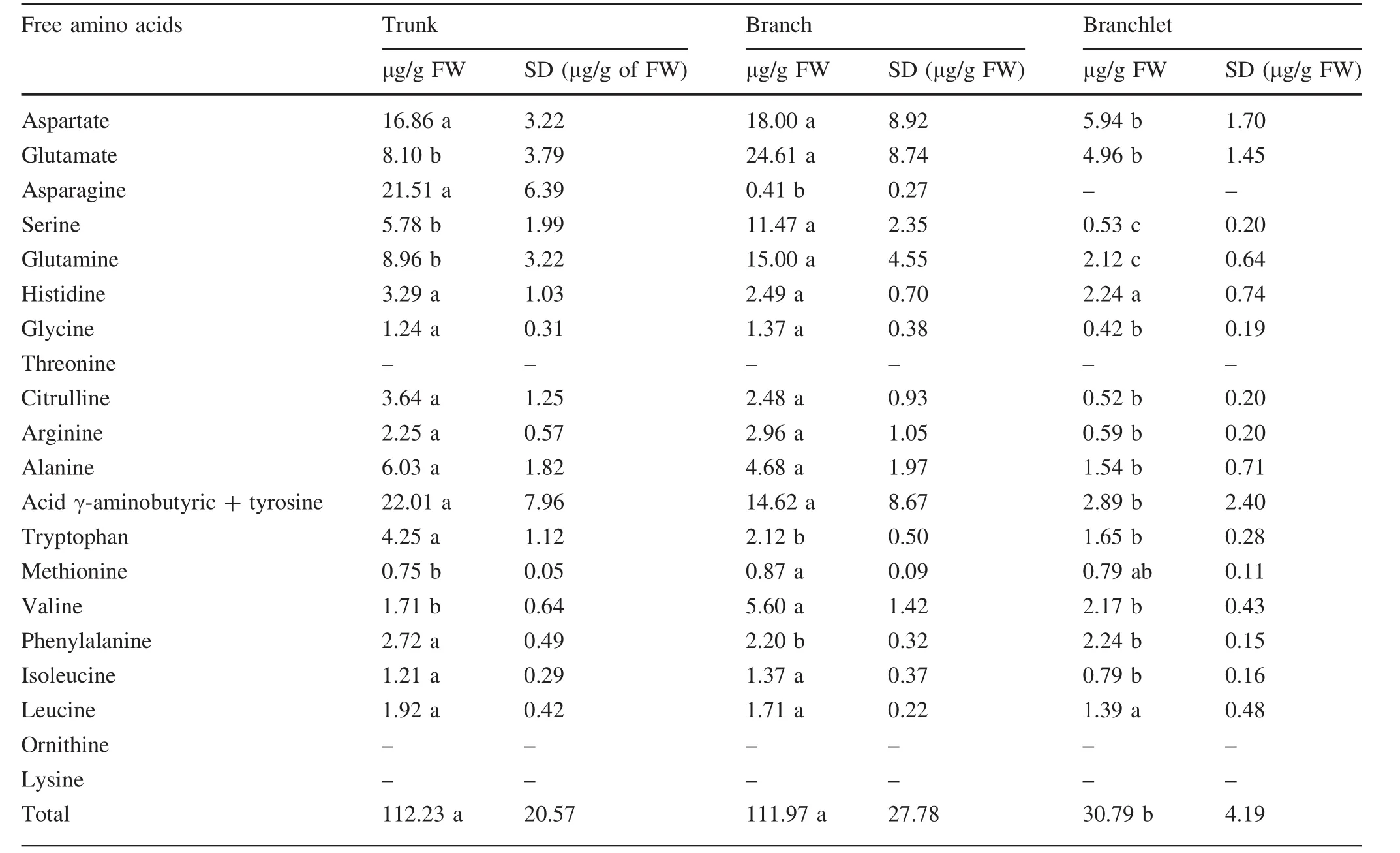
Table 1 Total content of free amino acids(μg/g FW)in trunks,branches,and branchlets of A.angustifolia with 2-year old and individual content of aspartate,glutamate,asparagine,serine,glutamine,histidine,glycine,threonine,citrulline,arginine,alanine,acid γ-aminobutyric,tyrosine,tryptophan,methionine,valine,phenylalanine,isoleucine,leucine,ornithine,lysine in trunk,branch and branchlet(μg/g of FW)
Discussion
Dimorphism between stems and branches is not a unique characteristic of Araucariaceae;it has been observed in species belonging to a wide variety of genera belonging to other gymnosperm and angiosperm families,such as Gossypium,Theobroma,Castilla,Musa,Hedera,Coffea and Pinus(Carvalho et al.1950;Oliveira et al.2010;Flores-Rentería et al.2013).In Gossypium and Castilla,this dimorphism is manifested by the occurrence of vegetative and floriferous branches.On the other hand,in Araucaria,Hedera,Theobroma and Coffea,the dimorphism is related to the direction of growth,that is,plagiotropic or orthotropic growth taken by the branches during development(Carvalho et al.1950;Oliveira et al.2010;Zanette et al.2017).However,in A.angustifolia we also observed dimorphism in relation to vegetative and floriferous branches.This is due to the induction of a vegetative meristem within the floriferous meristem occurring predominantly in branches,and very rarely in branchlets and never in the trunk(Zanette et al.2017).More research is needed on the physiology and biochemistry of these stems.
The structural function of amino acids,as constituents of protein,is already well understood.Proteins have important roles in plants,being involved in a multitude of cellular processes(Hasan et al.2014;Hildebrandt et al.2015).Enzymes are proteins that catalyze many biological reactions and are vital for plant metabolism(Hasan et al.2014).In addition to protein synthesis,amino acids are involved in a variety of processes of primary and secondary metabolism in plants,and act directly or indirectly on various aspects of plant growth and development(Oliveira et al.2001).Due to the multiple functions that amino acids perform in plants,it is assumed that the regulation of amino acid catabolism involves a broad set of general and specific regulators,and that there would be significant differences among plant species,culture sites,tissues and stages of development(Galili et al.2014;Hasan et al.2014;Häusler et al.2014).
In our study,although the amino acid content was analyzed only in stems,with samples collected from the different stems of the plant,there was a great variation in the pro file and amount of amino acids detected in each,reinforcing the idea that each stem type presents distinct and very specific functions in A.angustifolia.Amino acids involved in the biosynthesis of other amino acids and nitrogen stock,as well as in secondary metabolism,were found to be predominant in the trunks,branches and branchlets.A large amount asparagine and acid γaminobutyric+tyrosine were found in the trunk,while branches presented high amounts of serine and glutamine.The total content of free amino acids was very similar between trunk and branch,whereas branchlets were found to possess a lower total content of free amino acids.
In another conifer,Picea glauca,the proportion and content of amino acids differed among parts of the plant and the time of year(Durzan 1967).This author demonstrated that buds,shoot apexes,and terminal leaves of the branches accumulated high levels of soluble nitrogen in spring,late summer,and early winter,with the major components being arginine,glutamine and proline(Durzan 1967).These changes represent the storage and mobilization of nitrogen compounds during the onset of plant dormancy or the growth of the branches.Amino acid content was also found to vary among plant parts of Rubus amabilis,with a greater amount of free amino acids being found in the stem in relation to the leaves(Caidan et al.2014).Most of the amino acid composition of this species comprised methionine or cysteine,and GABA,with high levels of arginine in the stem(Caidan et al.2014).
When assimilated by plants in the form of glutamate and glutamine,nitrogen is readily assimilated by plant metabolism(Lea et al.2006).These amino acids function as the source of nitrogen for the biosynthesis of other amino acids,nucleic acids,and other nitrogenous compounds.Alternatively,they may be incorporated into the amino acids aspartate and asparagine(Lea et al.2006),the latter of which stores nitrogen in plants(Azevedo et al.2006).
Glutamic acid and glutamine are considered the main precursors of the other amino acids(Coruzzi and Last 2000),and so their accumulation in the stem of A.angustifolia may be related to their use as a source of nitrogen for the synthesis of other amino acids during plant growth,such as in the plants of the present work,which were undergoing full vegetative growth.In addition to being involved in nitrogen translocation,aspartate is a precursor to the synthesis of asparagine,threonine,methionine,lysine,and isoleucine(Lam et al.1995).In Picea glauca,dramatic seasonal changes in soluble nitrogen composition of trunk-buds and leaves were found related to plant morphogenetic changes.When the stem of this species ceases elongation,the amino acid arginine acts as a source of nitrogen reserve(Durzan 1967).
The cyclic inter-conversion between arginine,glutamine and asparagine plays a central role in nitrogen metabolism of conifers and its regulation is critical for maintaining the nitrogen economy throughout the life cycle of the species(Cánovas et al.2007).In shoots of Piceae glauca,the main components of the soluble nitrogen were arginine,glutamine,and proline.Other amino acids were readily detected,but many contributed only a small fraction to the total.Alanine and γ-Aminobutyric acid,among other unidentified compounds,were present in small quantities(Durzan 1967).
Asparagine accumulates in most plant organs during periods of low protein synthesis and reduced nitrogen supply(Cánovas et al.2007).In A.angustifolia,there is a reduction in metabolic activity during autumn and winter,possibly due to lower temperatures and radiation levels,which consequently results in a decrease in stem growth rate(Assumpc¸ão Neto 2008;Pereira et al.2016).The results obtained in the present study verify that when a plant is experiencing low metabolic activity,asparagine accumulates preferentially in the trunk,to the detriment of branches and the branchlets,and that this asparagine plays a crucial role in nitrogen metabolism during vegetative growth of A.angustifolia.
The higher asparagine content in the trunk is due to the morphogenic behavior of the stem of this species,which exhibits finite longitudinal growth (60–70 years)and unlimited radial growth(Zanette et al.2017),while the branches present definitive radial growth and a de fined life span(50–60 years).On the other hand,branchlets present definitive longitudinal and radial growth,in addition to a short life span(5–7 years)(Zanette et al.2017).
The rapid accumulation of γ-aminobutyric acid in tissues in response to biotic and abiotic stresse is important in cell elongation.In addition,among other functions,GABA plays an important role in determining the identity of cells in leaves and the apical meristem(Häusler et al.2014).This amino acid has a central role in pH regulation and nitrogen storage,being an intermediate for the synthesis of protein amino acids(Rodriguez et al.2008).The content of GABA is closely related to the concentrations of Ca/cytosolic and Ca/calmodulin,which act as cellular signals for several physiological processes,and calcium acts indirectly in the synthesis of GABA from glutamate(Häusler et al.2014).There are indications that there are GABA receptors on the cell membrane,which con firms the important role of GABA as a marker(Kinnersley and Lin 2000).Derivatives of GABA also appear to be involved in signaling processes.
Accumulation of a product derived from GABA transaminase led to a severe delay in the growth of Arabidopsis(Fait et al.2008).It is noteworthy that in this study,GABA content was evaluated in conjunction with tyrosine.Since the studied plants of A.angustifolia were in homogeneous and well-supplied conditions,it is unlikely that GABA had accumulated in higher concentrations due to stress,but there may have been a greater accumulation of tyrosine.In Picea glauca,GABA was also detected in the stem in small amounts(Durzan 1967).Tyrosine is closely related to lignin biosynthesis because it provides carbon skeletons for this metabolic pathway(Cánovas et al.2007).
Tryptophan,together with phenylalanine and tyrosine,comprise the aromatic amino acids,which,in addition to being constituents of proteins,are precursors of a variety of secondary compounds in plants,some with signaling functions(Häusler et al.2014).Tryptophan is the precursor to the synthesis of 3-indoleacetic acid,the most abundant auxin in plants(Mano and Nemoto 2012).Greater amounts of this amino acid accumulate during the winter,possibly for use during spring plant growth(Kim and Glerum 1995).This auxin is directly related to several types of tropisms present in plants,besides being directly involved in their exchange activity(Kim and Glerum 1995).When naturally vertical branches are placed horizontally,an asymmetric accumulation of auxin occurs as a characteristic response of gravitropism(Muday 2001).Asymmetric distribution of auxin results in the expression of certain genes and regulatory transcription factors(Moyle et al.2002).Exploratory studies point to auxin as one of the main regulators of orthotropism and plagiotropism(Muday 2001;Veierskov et al.2006).Gibberellins and abscisic acid might also be involved(Lamote and Pickard 2004).
Phenylalanine is the precursor amino acid to the phenylpropanoid pathway,which leads to,among other influences,a significant increase in lignin content in woody plants.Phenylalanine(tyrosine)ammonia-lyase(PAL)catalyzes the deamination of phenylalanine and tyrosine to cinnamic and p-coumaric acids,respectively.This is a crucial metabolic step connecting primary nitrogen metabolism through the shikimate pathway,with the allocation of carbon for the biosynthesis of phenylpropanoids.
Most of the metabolic flux through this pathway leads to the biosynthesis of lignin,an important constituent of wood(Gallardo et al.2003).The content of phenylalanine was constant in all three stem types studied,with a slight increase in content in the trunk.This result shows that lignification in A.angustifolia begins when the plants are still quite young.Conifers,in general,use large amounts of carbon in the biosynthesis of phenylpropanoides for lignin synthesis(Cánovas et al.2007).Although the lignin molecule does not contain nitrogen,the phenylpropane skeleton required for this metabolic pathway is provided by the deamination of the amino acids phenylalanine or tyrosine(Van Heerden et al.1996).In addition,lignified cells consume numerous methyl groups,which are generated through carbon metabolism associated with the catabolism of serine and glycine.
In the present study,branches—in addition to a high content of glutamic acid(24.61 ± 8.74 μg/g FW)and glutamine(14.99 ± 4.54 μg/g FW)—high values of serine(11.47 ± 2.35 μg/g FW)were also found(Table 1).This may indicate the involvement of this amino acid,together with phenylalanine and tyrosine,in the synthesis of lignin in A.angustifolia.However,it seems that A.angustifolia prioritizes tyrosine.In conifers,the existence of several mechanisms for re-assimilation of nitrogen by lignifying cells is crucial to maintaining the process of lignin synthesis without affecting nitrogen savings by the plant(Cánovas et al.2007).
The low accumulation of free amino acids in branchlets may indicate that this organ has a physiological function distinct from that of branches and the trunk,such as greater photosynthetic activity.In Picea glauca,the amount of amino acids found in leaves was much lower than the amount found in the stem and buds(Durzan 1967).This matches our results and reinforces the hypothesis that these stems have different functions and that the branchlets may function as leaves.
The content of some amino acids,such as glutamate,glutamine,glycine,serine,and aspartate,are closely related to photosynthetic rates and processes in leaves(Noctor et al.2002).Aspartate and glutamate,for example,account for about 25%of the total free amino acid content in branchlets.The determined growth of a branchlet,and its shorter life span on the plant while becoming senescent with the leaves(Oliveira 2011;Zanette et al.2017),are also indications that this stem has a function different from branches and trunk.However,this hypothesis needs further investigation.
In a study of vegetative propagation,both cutting and grafting,with segments of branches and branchlets of A.angustifolia,the plants were found to maintain horizontal growth(Oliveira 2011;Wendling 2011).In the specific case of grafting branchlets,even when performed in young plants,a limitation to growth and life span of the grafted plants was found,with senescence after the third year(Oliveira 2011).
Conclusions
Amino acid content of the stem of A.angustifolia differs significantly among trunk,branches,and branchlets,which shows that the stem does not form a unique system,and that there are biochemical differences among these stem types that vary accordingly to their functions.Greater amounts of amino acids related to the lignification process in the trunk indicate that its function is related to the presence of a large amount of wood and its main function in the plant support and hydraulic conduction.The low total amino acid content,as well as the prevalence of amino acids related to the photosynthetic mechanism,indicates that the main function of the branchlets is directly related to photosynthesis.However,further studies involving other physiological,biochemical,and genetic components are necessary to elucidate the role and regulatory mechanisms of these stems.
 Journal of Forestry Research2018年6期
Journal of Forestry Research2018年6期
- Journal of Forestry Research的其它文章
- Black locust(Robinia pseudoacacia L.)as a multi-purpose tree species in Hungary and Romania:a review
- The impact of the environmental factors on the photosynthetic activity of common pine(Pinus sylvestris)in spring and in autumn in the region of Eastern Siberia
- Osmoregulators in Hymenaea courbaril and Hymenaea stigonocarpa under water stress and rehydration
- Effect of nitrogen levels on photosynthetic parameters,morphological and chemical characters of saplings and trees in a temperate forest
- Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress
- Pilot study on the effects of elevated air temperature and CO2 on artificially defoliated silver birch saplings
