Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress
Jinfeng Song•Hongguang Zhang•Chengwei Duan•Xiaoyang Cui
Abstract Larix olgensis A.Henry(Changbai larch)is a productive commercial species and good candidate for afforestation in northeast China.It is widely planted in lead-stressed soils which can induce oxidative damage in this plant.Increasing tolerance to lead(Pb)stress is therefore of keen interest.A greenhouse experiment was conducted to identify the biomass,physiological responses and Pb accumulation of L.olgensis seedlings to Pb stress under succinic acid(SA)application and to explore the interaction of exogenous SA applications and stress resistance.L.olgensis seedlings were planted in Pb-stressed or unstressed haplic cambisols in pots.In Pb-contaminated soils the seedlings were treated daily with concentrations of SA solutions at a rate approximately equivalent to 0,0.04,0.2,1.0,or 2.0 mmol kg-1of soil for 10,20,and 30 days,respectively.Pb treatment induced damage in the seedlings and led to the inhibition of biomass accumulation in roots,stems and leaves,and a rise in Pb accumulation in fine roots and leaves.Malondialdehyde(MDA)content and electrolyte leakage in leaves significantly increased while peroxidase(POD)activities,soluble protein and photosynthetic pigment contents in leaves were all reduced.Physiological toxicity was promoted with increasing Pb treatment times.When Pb-stressed seedlings were exposed to SA (especially 10.0 mmol L-1over 20 days),the physiological responses for Pb-only were reversed and the biomass of roots,stems,and leaves dramatically increased.SA facilitated Pb uptake in fine roots and leaves but more Pb accumulated in fine roots.The results demonstrate that exogenous SA alleviates Pb-induced oxidative injuries and improves the tolerance of L.olgensis seedlings to Pb stress.
Keywords Soil Pb stress·Succinic acid·Larix olgensis·Physiological response·Pb uptake
Introduction
Soil contamination by heavy metals is a widespread and serious problem for ecosystem and human health as a result of their toxicity effects(Shakoor et al.2013).Lead(Pb)is a hazardous and persistent heavy metal and much research has focused on understanding the extent and mechanisms of its toxicity in the environment(Schreck et al.2012).Excessive Pb interferes with plant physiological processes such as the inhibition of protective enzyme activities and photosynthetic rates that can result in disturbed mineral nutrition and water balance.These disruptions have been shown to induce reductions in seed germination and plant growth rates,as well as overall plant quality(Sharma and Dubey 2005;Shahid et al.2014b).Pb also induces secondary oxidative stress in plantsby catalyzing the formation of harmful reactive oxygen species(ROS)(Sharma and Dietz 2009;Wahsha et al.2012)that interrupt the metabolic activities of plants and harm biological molecules such as proteins,lipids,and nucleic acids(Pourrut et al.2011).In extreme cases,ROS can lead to cell death.
Larix olgensis is a commercial forestry species and is considered a good candidate for afforestation in northeast China.It is widely planted in Pb-polluted soils and Pbtailing substrates in forested areas.However,Pb induces oxidative damage in L.olgensis.Therefore,alleviating Pb toxicity in L.olgensis is of significant interest.Studies of some agricultural species have indicated that organic acids can change heavy metal speciation,and hence toxicity(Kim et al.2010;Li et al.2014;Shahid et al.2014a).Furthermore,organic acids such as oxalic acid,citric acid,malic acid,salicylic acid,and ethylene diamine tetraacetic acid(EDTA)are involved in the regulation of plant growth and development, flowering,and response to abiotic stresses through the regulation of physiological and biochemical processes(Rivas-San and Plasencia 2011;Shakoor et al.2014;Song et al.2016).
Ten types of organic acids have been found in forest ecosystems of northeast China,especially in L.olgensis forests(Song and Cui 2003).Our previous study found that exogenous oxalic and citric acids affected physiological attributes and growth of cadmium-stressed L.olgensis seedlings,resulting in cadmium tolerance in the previously stressed plants(Song et al.2014).In addition to oxalic and citric acids,succinic acid(SA)is another dominant organic acid in stands of L.olgensis(Song and Cui 2003),and here the hypothesis that exogenous SA may protect larch against Pb toxicity by mediating physiological and biochemical responses was tested.It could help guide future afforestation of Pb-contaminated soils with localized amendments with SA and also improve local forestry economics.Thus,L.olgensis seedlings were cultivated in Pb-contaminated soils that were supplemented with different concentrations of SA.The response to Pb stress and those induced by exogenous SA after different Pb exposure times were assessed.Additionally,the relation between SA and Pb tolerance in L.olgensis seedlings was evaluated.
Materials and methods
Measurements were made of Pb accumulations,biomass and physiological parameters in seedlings cultivated in Pbcontaminated soils amended with different concentrations of SA,and the effect on these parameters of the time seedlings were exposed to treatment with SA.
Plant cultures and treatments
Seeds of L.olgensis were collected from the Maoershan Forest Research Station of the Northeast Forestry University,Harbin,Heilongjiang Province(127°30′–127°34′E,45°21′–45°25′N)where the climate is continental temperate monsoon with an annual average temperature of 2.4°C(range-40 to 34°C).Mean annual precipitation is 700 mm and the frost-free period is 125 days with a growing season between 120 and 140 days.The soil type is typic bori-udic cambosols(Cooperative Research Group on Chinese Soil Taxonomy 2001),corresponding to haplic cambisols(greyic,dystric)(IUSS working Group WRB 2006).At the end of April 2015,the seeds were disinfected,vernalized, and sown in pots (aperture:42.5 cm×19.0 cm;base:33.0 cm×10.0 cm,height:18.0 cm) filled with the A1horizon of haplic cambisols with uniform loam texture and high organic matter content.A total of 60 seeds were planted in each of the 180 pots and the pots placed in the glasshouse.At the end of May,only 30 seedlings were left per pot.
After one month,the soil was treated with Pb(NO3)2solution,to a Pb2+content of 100 mg kg-1.SA treatments(0,0.2,1.0,5.0,and 10.0 mmol L-1from pH 5.2 succinate solutions)were applied to three pots using root irrigation and an equal quantity of SA-free de-ionized water without Pb(NO3)2added to the control(Ck)pots.Root irrigation was performed by saturating soils with the 0,0.2,1.0,5.0,and 10.0 mmol L-1SA solutions at rates equivalent to 0,0.04,0.2,1.0,and 2.0 mmol kg-1of soil,respectively.As a result of incomplete root development of the seedlings,foliar applications of SA solutions were applied to enhance SA uptake which is a common approach with foliar fertilization(Pang et al.2010).A sprinkling can fully and uniformly wet the upper and lower surfaces with the appropriate concentration of SA solution.Irrigation and foliar SA treatments were applied once daily at 08:00 for 7 days.There were three replicates of the six treatments,with 10 pots per treatment(total 180 pots).At 10,20 and 30 days after SA treatments,the seedlings were assessed for biomass, Pb accumulation and physiological parameters.
Biomass determination
At 10,20,and 30 days after SA treatments,10 seedlings from each treatment were removed(n=10),washed thoroughly with tap water and cut into leaf,stem and root sections.After drying at 80°C until constant mass was achieved,the dry mass of the three plant parts was determined.
Physiological measurements
Leaf samples were taken at random from plants in each treatment from 10 pots and mixed to determine membrane permeability(expressed as relative electrical conductivity)with a conductivity meter DDS-6700(Shanghai Precision&Scientific Instrument Co.Ltd,Shanghai,China).Leaf samples of 3.0 g were collected,frozen,and ground in liquid nitrogen to determine other physiological parameters according to Li et al.(2000)and Song et al.(2014).Malondialdehyde(MDA)content that measures lipid peroxidation was determined based on 2-thiobarbituric acid reactive metabolites,peroxidase(POD)activity by a guaiacolassay,soluble protein content by coomassie brilliant blue G-250 staining,and chlorophyll and carotenoid contents by 80%acetone extraction spectrophotometry.Each determination was replicated three times.
Pb concentrations in fine roots and leaves
Fine roots(diameter≤2 mm)and leaves were randomly sampled from seedlings in each of the treatments(n=10).The leaf and root material were washed with de-ionized water,dried,de-enzymed for 15 min at 105°C,and dried at 70°C to a constant mass.The plant material was then powdered,sifted through 2-mm nylon screens and mixed.The Pb concentration(mg kg-1DW,DW=dry weight)wasmeasuredbyInductivelyCoupledPlasma-Mass Spectrometry(ICP-MS,SCIEX ELAN 6000,Perkin Elmer,Waltham,USA).Each treatment was replicated three times.
Data analysis
The control treatment compared the effects of Pb stress on the seedlings,while Pb+SA treatment investigated the effects of different concentrations of SA.For each parameter,the mean values of 10 or 3 replications and one standard deviation of each treatment are presented.The significance of results between different treatments was determined using an analysis of variance with SPSS 18.0 for a completely randomized design using the effects of SA concentration and treatment time with a repeated measure.
Results
The concentration of SA and treatment exposure times significantly affected all the parameters measured in this study and there were significant interactions between these effects for all variables,except for POD and total chlorophyll(Table 1).
Effects of SA on biomass accumulation
When comparing Ck with the Pb+SA 0 treatment,exposure to Pb significantly inhibited the accumulation of leaf,stem,and root biomass.SA treatments increased different parts of the biomass as compared with Pb-stressed seedlings only(Pb+SA 0).The greatest increases were found at 10.0 mmol L-1,and the duration order was 30>20>10 days(Fig.1).
Effects of SA on lipid peroxidation and membrane permeability
Pb-induced oxidative stress and membrane injury in L.olgensis leaves were estimated in terms of MDA content and relative electrical conductivity,respectively.Compared with Ck,significant increases in MDA content and relative electrical conductivity were found.SA addition dramatically decreased both values,and the greatest decreases were found at 10.0 mmol L-1.In terms of decreasing MDA contents, the order was 10>20>30 days,while for relative electrical conductivity it was 20>10>30 days(Fig.2;Table 1).
Effects of SA on POD activities
When comparing Ck with the Pb+SA 0 treatments,POD activities in L.olgensis leaves were reduced by Pb stress;however,SA supplementation increased POD activities of Pb-stressed leaves,with the peak value occurring at 10.0 mmol L-1.The order was 20>10>30 days.There was no significant interaction between treatment exposure times and SA concentration(Fig.3;Table 1).
Effects of SA on soluble protein content
Compared with non-stressed plants(Ck),Pb stress inhibited protein biosynthesis and accumulation,and decreased the soluble protein content of leaves.SA treatments increased soluble proteins compared with Pb-alone treatments(Pb+SA 0),with the peak value occurring at 10.0 mmol L-1.The order was 30>20>10 days,with the effects of SA concentration and day of analysis being significant(Fig.4;Table 1).
Effects of SA on photosynthetic pigment content
Pb stress resulted in declines in chlorophyll and carotenoid that increased with Pb exposure time.Chlorophyll and carotenoid contents were higher in SA-treated leaves than those treated with Pb alone,and the effects of SA concentration and treatment exposure time were significant.The 10.0 mmol L-1SA treatment was the most effective,and the duration order of chlorophyll and carotenoid contents were 20>10>30 days and 10>30>20 days,respectively.There was no significant treatment exposure time×SA concentration interaction.Pb signi ficantly decreased chlorophyll ratios,as did SA,with the highest decrease occurring at 10.0 mmol L-1,and the order was 30>20>10 days(Fig.5;Table 1).
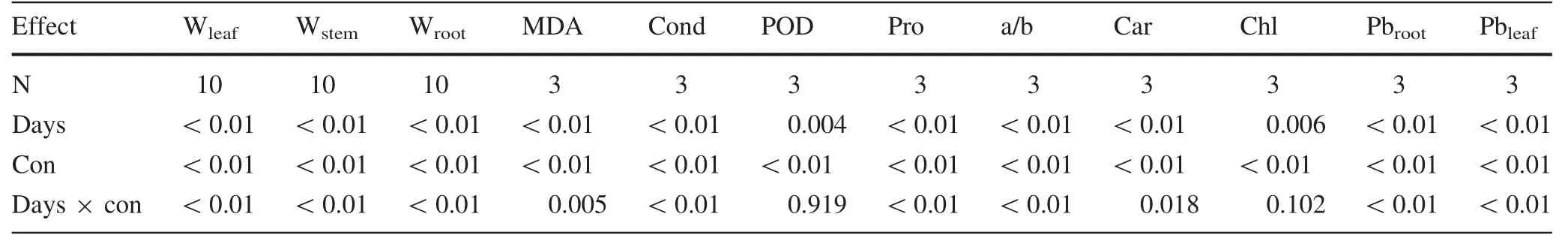
Table 1 P values of the effects of treatment times(Days),concentrations of SA(Con)and their interactions on biomass and physiological parameters
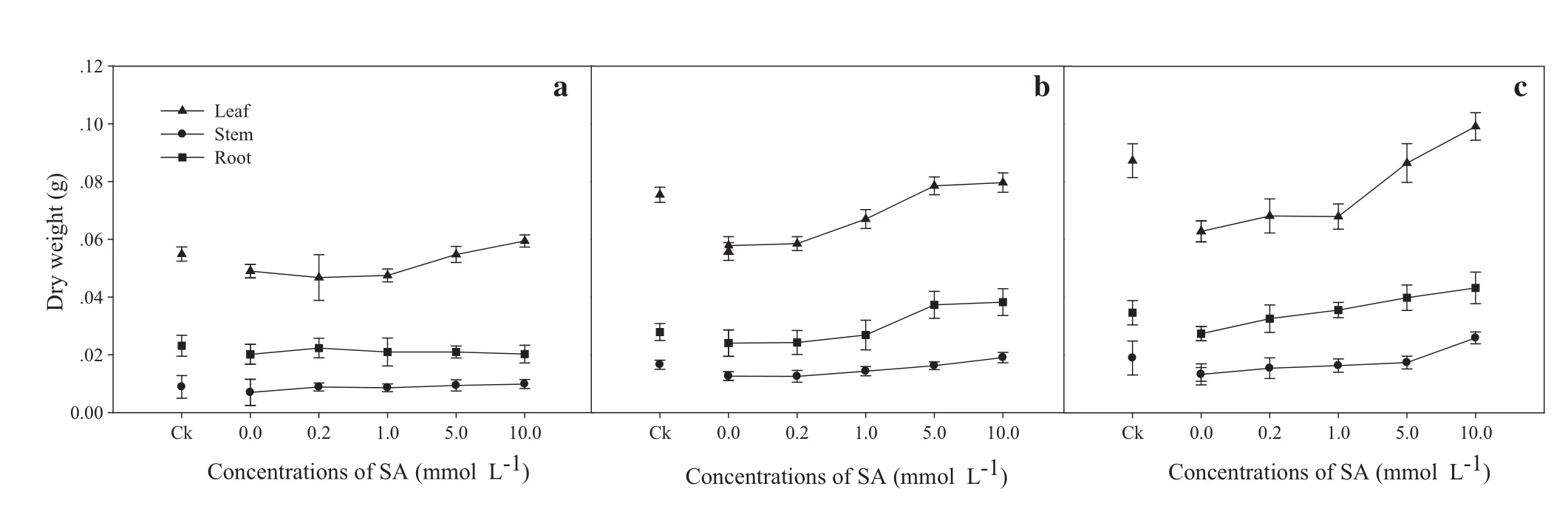
Fig.1 Leaf,stem and root dry weights of Larix olgensis seedlings induced by different concentrations of SA at a 10,b 20 and c 30 days.Ck is a control without the SA and Pb treatments.The mean values of 10 replications and standard deviation(SD)are presented
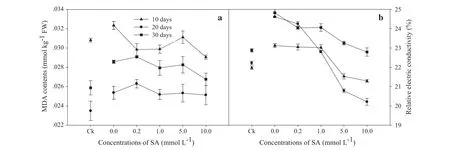
Fig.2 a MDA contents and b relative electrical conductivity of Larix olgensis leaves induced by different concentrations of SA and different treatment times.Ck is a control without SA and Pb treatments.The mean values of three replications and standard deviation(SD)are presented.FW in the y-axis means fresh weight
Effects of SA on Pb accumulation
Pb stress in soils significantly increased its uptake in fine roots and leaves,where greater Pb concentrations were observed in the roots.SA facilitated Pb uptake in both the roots and leaves.The concentrations in fine roots significantly increased as the SA concentration rose and peaked at 10.0 mmol L-1.The Pb concentrations in leaves increased and then decreased,and were highest at 5.0 mmol L-1.The order of Pb uptake in fine roots and leaves was 20>10>30 days in both,with the effects of treatment exposure time being significant(Fig.6;Table 1).
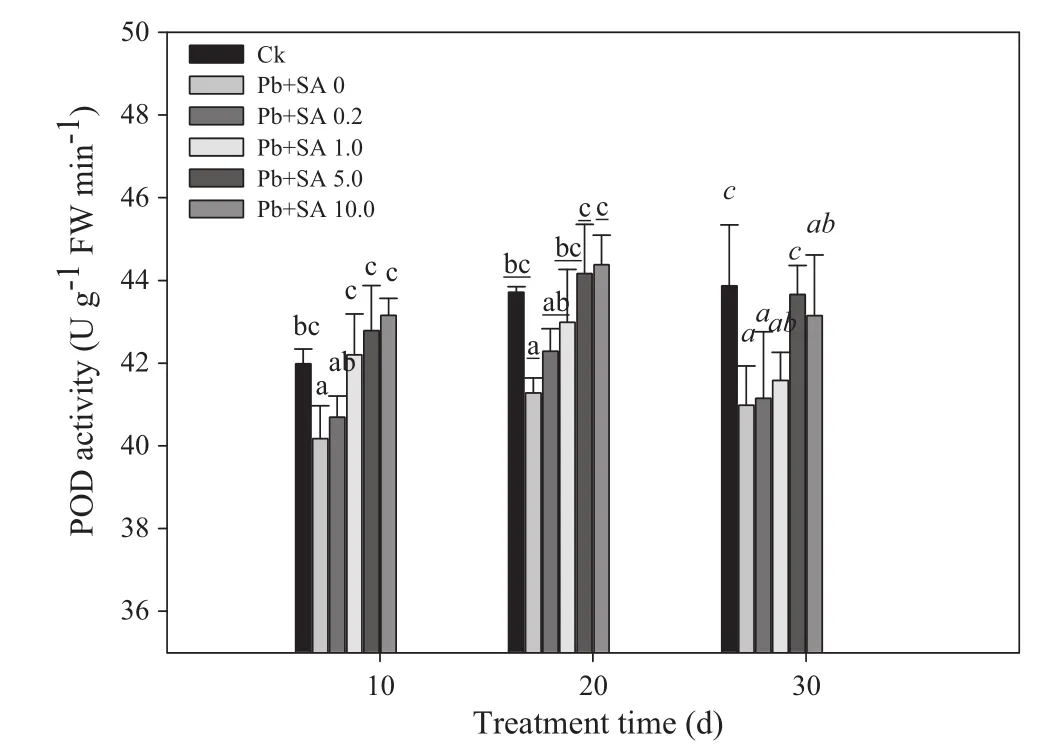
Fig.3 POD activities of Larix olgensis leaves treated with different concentrations of SA and different treatment times.Data are means±1 SD(standard deviation)of three replications.Ck is a control without the SA and Pb treatments.Values followed by the same letter for the same parameter are not statistically different at P<0.05 by ANOVA(DUNCAN).FW in the y-axis means fresh weight
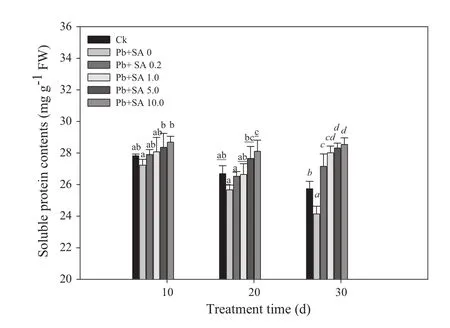
Fig.4 Soluble protein content of Larix olgensis leaves treated with different concentrations of SA and different treatment times.Data are means±1 SD(standard deviation)of three replications.Ck is a control without the SA and Pb treatments.Values followed by the same letter for the same parameter are not statistically different at P<0.05 by ANOVA(DUNCAN).FW in the y-axis means fresh weight
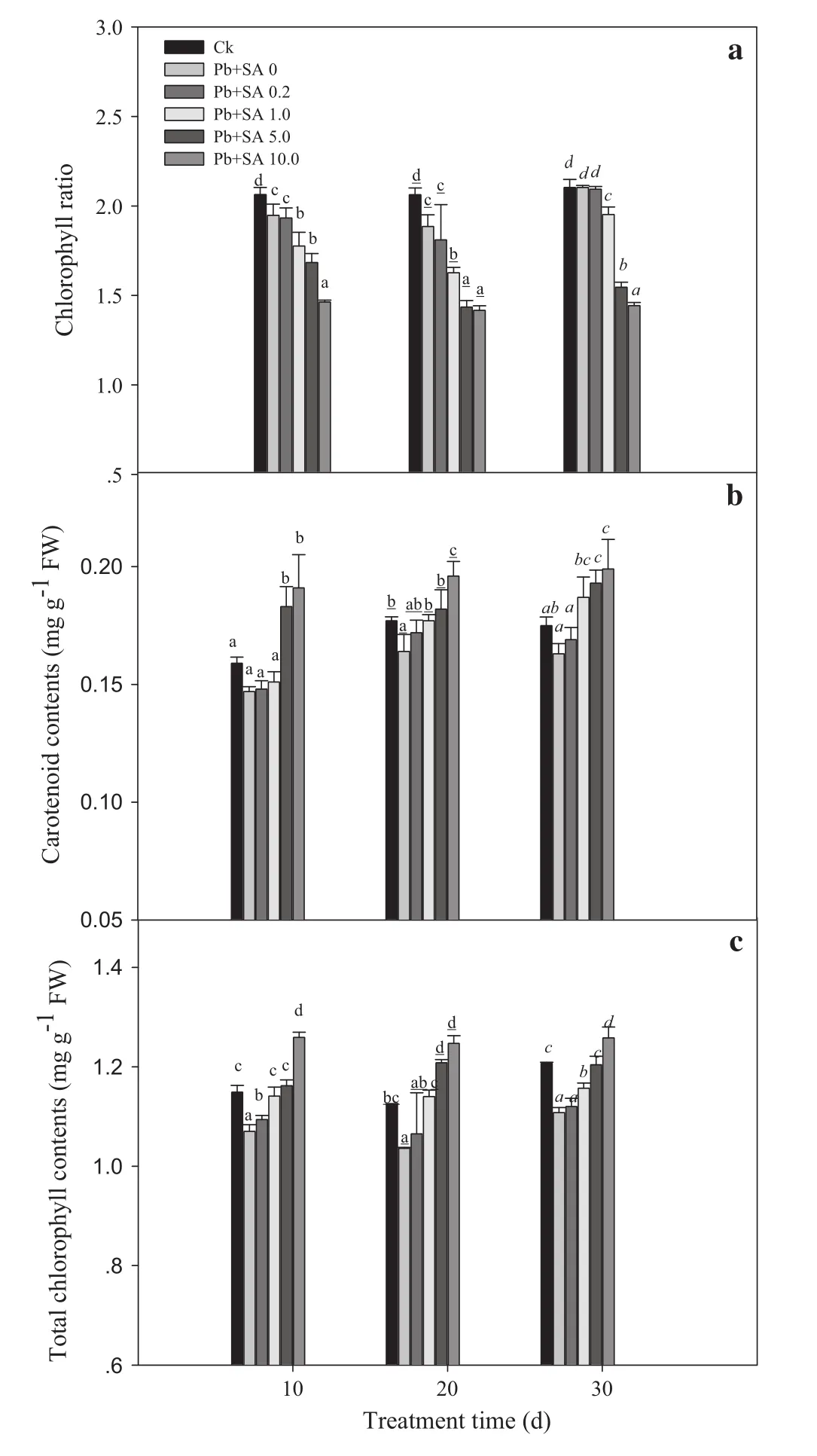
Fig.5 a Chlorophyll ratios,b carotenoid contents,and c total chlorophyll contents of Larix olgensis leaves induced by different concentrations of SA and different treatment times.Data are means±1 SD(standard deviation)of three replications.Ck is a control without the SA and Pb treatments.Values followed by the same letter for the same parameter are not statistically different at P<0.05 by ANOVA(DUNCAN).FW in the y-axis means fresh weight
Discussion
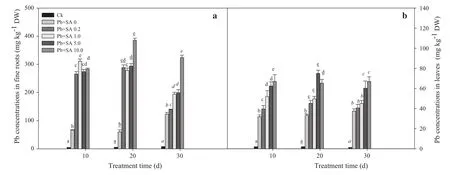
Fig.6 Pb accumulation;roots and in a fine roots and b leaves of Larix olgensis treated with different concentrations of SA and different treatment times.Data are means±1 SD(standard deviation)of three replications.Ck is a control without the SA and Pb treatments.Values followed by the same letter for the same parameter are not statistically different at P<0.05 by ANOVA(DUNCAN).DW in the y-axis means dry weight
Stress in plants is measured by a number of morphological and physiological indicators(He et al.2016;Li et al.2013;Yang et al.2012).We found that SA application reversed measures of plant stress in L.olgensis exposed to Pb,supporting our hypothesis that exogenous SA could improve the tolerance of this important forestry species to Pb by mediating physiological and biochemical responses.A similar role was con firmed in Iris lacteal var.chinensis(Fisch.)Koidz.(Wang et al.2010).Stoyanova and Doncheva(2000)also reported that succinate can take part in the formation of metal-succinate complexes in the roots of pea plants(Pisum sativum L.,cv.citrine),thus reducing Zn toxicity.However,Doncheva et al.(2005)found that succinate addition did not reduce the inhibitory effect of manganese(Mn)on plant growth,chlorophyll fluorescence parameters,or chloroplast structure of the pea(Pisum sativum,cv.citrine)plants and did not contribute to Mn tolerance.
Heavy metals,especially at high concentrations,have negative effects on plants(Roberts et al.2014).Sharp decreases in seed germination and growth rates have been observed with increasing metal concentrations(Hu et al.2012;Shu et al.2012),and were also demonstrated in our study.Srivastava et al.(2004)showed that cadmium-induced reduction of plant biomass could induce inhibition of irreversible growth and was correlated with the decrease in cadmium-induced root activity(RA).Reduced RA,in turn,weakened nutrient absorption and interfered with enzyme activities related to plant growth(Li et al.2014).The harmful effects of Pb on plant growth and function vary between plant species.For example,150 mg L-1Pb significantly inhibited the rate,energy and index of germination and biomass in Chenopodium album L.,whereas such an inhibition occurred at the higher rate of 300 mg L-1Pb for Salsola passerine Bunge where Pb was mainly precipitated in the cell wall of the apoplast(Hu et al.2012).
Measures of physiological and metabolic plant stress in this study included MDA and relative electrical conductivity,POD activity and soluble protein and chlorophyll contents.A permselective cell membrane regulates the transportation and exchange between intracellular and extracellular materials in order to maintain stability for optimized physiological activity.The changes in MDA content and relative electrical conductivity caused by Pb stress are in agreement with previous studies(Cao et al.2005;Gallego et al.1996;Shakoor et al.2014).The increase in membrane permeability in Pb-treated plants may be due to the metal reacting with phospholipids on the cell membrane,generating orthophosphate or pyrophosphate that changes the membrane structure.It may be possible that Pb is absorbed by pectic acid on the cell wall,destroying the elasticity,plasticity,and physiological functions of the wall(Cao et al.2005).
Antioxidant enzymes in plants regulate the cellular redox homeostasis.They maintain ROS and peroxide at low levels to protect cellular membranes(Yuan et al.2013),in which POD uses guaiacol as an electron donor,and participates in lignification,ethylene biosynthesis,defense,and wound-healing(Bhaduri and Fulekar 2012;Shakoor et al.2014).POD activity under Pb stress is unclear because it was shown to decrease in Avicennia marina Forsk.(Zhong et al.2010)but increase in Zea mays L.(Cao et al.2005)and Festuca arundinacea Schreb.(Hu et al.2015).Our results demonstrated that 100-mg kg-1Pb decreased the POD activities in L.olgensis leaves,which was in excess of the plant’s tolerance extreme and is in agreement with the results of Zhong et al.(2010).However,we found that POD activities decreased with increasing Pb exposure time,which is counter to the results of Hu et al.(2015)who found that POD activity in Festuca arundinacea was enhanced after 24 and 48 h of Pb exposure.Our experiment showed that SA induced an increase in POD activity of Pb-stressed L.olgensis leaves,which may have contributed to the rapid removal of rapid oxygen species(ROS),resulting in improved resistance.In other studies,different concentrations of salicylic acid have been shown to enhance the POD activity of cucumber seedlings and induce new POD isozymes(Fodor et al.1997).
Soluble protein is an important osmolyte and is not specifically bound to membranes.The changes in soluble protein content of Pb-stressed L.olgensis leaves are consistent with studies of Bechmeria nivea(L.)Gaud.(Liu et al.2007)and Zea mays(Cao et al.2005).Under heavy metal stress,some plants synthesize proteins to complex metal ions.The increase in soluble protein also elevates cell osmotic concentrations and levels of functional protein that facilitate normal cell metabolism(Cao et al.2005).These increasing effects were also observed in cucumber and alfalfa under low temperatures(Jia 2007;Zhang 2010).It is clear that plants exhibit diverse responses to adverse environmental conditions.Our research showed that SA treatments resulted in a distinct increase in soluble protein content of Pb-stressed L.olgensis leaves,but this was in contrast to studies which found that soluble protein content in Bechmeria nivea(L.)exposed to 5-mg L-1cadmium decreased by high doses(3.1 mg g-1)of oxalic acid(Li et al.2014).
Chlorophyll plays a decisive role in forest productivity and carotenoid is important in scavenging ROS and preventing membrane lipid peroxidation(Li et al.2014).We found that Pb caused a dramatic breakdown in chlorophyll and carotenoid contents in L.olgensis leaves,which is consistent with studies on Brassica napus L.(Ali et al.2015;Shakoor et al.2014).However,Shu et al.(2012)and Hu et al.(2012)report contrasting and complex effects of Pb on chlorophyll:at low concentrations chlorophyll A was significantly suppressed in Salsola passerine Bunge but remained constant at higher concentrations.Chlorosis of Chenopodium album was observed at 300 mg L-1Pb,but was not in Salsola passerine Bunge.It appears,therefore,that the effects of Pb concentration and exposure times on chlorophyll contents are specific.In our experiment,SA treatments enhanced pigment synthesis in Pb-stressed L.olgensis leaves,indicating that SA inhibited the damage of Pb to chloroplasts;this has also been demonstrated in Pbstressed Iris lactea var.chinensis treated with oxalic and citric acids(Wang et al.2010),Sesbania drummondii Rydb.treated with EDTA,DTPA(diethylene triamine pentoacetic acid)and citric acid(Ruley et al.2006),and Iris lacteal var.chinensis growing in Pb mine tailings treated with citric acid and EDTA(Han et al.2013).A beneficial role of oxalic and citric acids was found in cadmium-stressed Boehmeria nivea(L.)Gaud.(Li et al.2014).
The effects of heavy metal stress on chlorophyll ratios are unclear.Some studies have revealed that the decline of chlorophyll A was more significant than that of chlorophyll B under heavy metal stress(Hu et al.2012;Pandey and Sharma 2002),while others studies report contradictory results(Gajewska et al.2006).In this study,the effect of Pb stress on chlorophyll ratios varied with exposure time and this may be due to differential response mechanisms to heavy metal stress.Effects of organic acids on the chlorophyll ratios in plants under Pb stress vary with acid concentration.Chen and Zhang(2005)found that,while low concentrations of oxalic acid increased the ratio in wheat,the ratios decreased under higher concentrations.An impeding effect of SA on chlorophyll ratios of Pbstressed L.olgensis leaves was found;however,the same concentrations of oxalic and citric acids increased the ratio in cadmium-stressed leaves(Song et al.2014).
Pb concentrations in plant organs also affect tolerance to polluted environments.Our finding about Pb uptake in L.olgensis organs was in accordance with previous studies(Bai et al.2015;Qiao et al.2012).However,Li et al.(2014)found that ion exchange sites on root cell walls that normally prevent metal ions from entering into tissues were the primary site of heavy metal access.We found that under stress conditions,a stimulating effect of Pb uptake in plants was SA concentration-dependent;this effect has also been reported in Boehmeria nivea(L.)Gaud.(Li et al.2014).In our experiment,SA facilitated Pb uptake in Pbstressed fine roots and leaves,a finding which agrees with studies of Brassica napus L.and Medicago sativa L.treated with EDTA(López et al.2005;Zaier et al.2010).Exogenous applications of other organic acids to heavy metal polluted soils have also been shown to increase toxin uptake.For example,cadmium absorption in above and below-ground parts of Halimione portulacoides(L.)Aellen was induced by citric and oxalic acids(Duarte et al.2007),and SA applications at a high Mn concentration resulted in an increase in uptake in Pisum sativum,cv.citrine roots,accompanied by a decrease in Mn translocation to leaves and stems compared with Mn-treated pea plants(Doncheva et al.2005).
Conclusions
High concentrations of lead in the soil are detrimental to the growth of L.olgensis through its impact on measures of plant stress in leaves.Pb treatment inhibited plant biomass,enhanced lipid peroxidation and electrolyte leakage,decreased POD(peroxidase)activities,and the synthesis of soluble proteins and pigments.Exogenous application of SA reversed the adverse effects of Pb-stress on seedlings.Thus,planting L.olgensis in combination with naturally occurring oramended SA could beusefulin the afforestation of Pb-contaminated soils.
AcknowledgementsThe authors thank Dr.Guoyou Chen(Heilongjiang Academy of Agricultural Sciences)for his assistance in Pb determination of leaf and fine root samples.
 Journal of Forestry Research2018年6期
Journal of Forestry Research2018年6期
- Journal of Forestry Research的其它文章
- Black locust(Robinia pseudoacacia L.)as a multi-purpose tree species in Hungary and Romania:a review
- The impact of the environmental factors on the photosynthetic activity of common pine(Pinus sylvestris)in spring and in autumn in the region of Eastern Siberia
- Osmoregulators in Hymenaea courbaril and Hymenaea stigonocarpa under water stress and rehydration
- Effect of nitrogen levels on photosynthetic parameters,morphological and chemical characters of saplings and trees in a temperate forest
- Free amino acid content in trunk,branches and branchlets of Araucaria angustifolia(Araucariaceae)
- Pilot study on the effects of elevated air temperature and CO2 on artificially defoliated silver birch saplings
