Translocator protein ligand, YL-IPA08, attenuates lipopolysaccharide-induced depression-like behavior by promoting neural regeneration
Xiao-Ying Zhang , Li-Ming Zhang Wei-Dong Mi, , Yun-Feng Li
1 Anesthesia and Operation Center, Chinese PLA General Hospital, Beijing, China
2 State Key Laboratory of Toxicology and Medical Countermeasures, Beijing, China
Abstract Translocator protein has received attention for its involvement in the pathogenesis of depression. This study assessed the effects of the new translocator protein ligand, YL-IPA08, on alleviating in flammation-induced depression-like behavior in mice and investigated its mechanism of action. Mice were intracerebroventricularly injected with 1, 10, 100 or 1000 ng lipopolysaccharide. The tail-suspension test and the forced swimming test confirmed that 100 ng lipopolysaccharide induced depression-like behavior. A mouse model was then established by intraventricular injection of 100 ng lipopolysaccharide. On days 16–24 after model establishment, mice were intragastrically administered 3 mg/kg YL-IPA08 daily. Immunohistochemistry was used to determine BrdU and NeuN expression in the hippocampus. YL-IPA08 effectively reversed the depression-like behavior of lipopolysaccharide-treated mice, restored body mass, increased the number of BrdU-positive cells, and the number and proportion of BrdU and NeuN double-positive cells. These findings indicate that YL-IPA08 can attenuate lipopolysaccharide-induced depression-like behavior in mice by promoting the formation of hippocampal neurons.
Key Words: nerve regeneration; YL-IPA08; hippocampus; dentate gyrus; lipopolysaccharide; neuroin flammation; depression; translocator protein;neural regeneration
Introduction
Depression is a chronic, severe, widespread and burdensome psychiatric illness. Unfortunately, the pathophysiological mechanism underlying depression and anxiety remains obscure. Over the past two decades, evidence has accumulated to show that inflammation can contribute to the etiology of clinical depression in co-morbid accompanying diseases(Smith, 1991; Maes, 2009; Raison and Miller, 2011; Beumer et al., 2012; Berk et al., 2013). Components that mediate inflammation in the immune system can participate or be involved in the pathology of depression (Slavich and Irwin,2014). Inflammation can in turn induce behavioral performance changes, including low mood, anhedonia, social-behavioral withdrawal and psychomotor retardation, which are the initial symptoms of depression (Lotrich, 2015).Knock-out or down-regulation of cytokines can exert anti-depression-like effects (Lawson et al., 2013a). These results led to potential therapies for clinical depression targeting in flammation (Catena-Dell’Osso et al., 2011).
In the central nervous system, translocator protein (TSPO)is mainly located in the outer membrane of glial cell mitochondria and acts as a mediator in the translocation of cholesterol from the outer to the inner mitochondrial membrane, which is the rate-limiting step in the synthesis of neurosteriods, including allopregnanolone (Nothdurfter et al.,2012; Hatty and Banati, 2015). Moreover, TSPO participates in inflammation and regulates microglial activation (Karlstetter et al., 2014). Chronic stress and depression induce a decrease in TSPO density in the brain and peripheral organs in rodents (Milenkovic et al., 2015; Wang et al., 2015; Zhang et al., 2016). Moreover, TSPO ligand, PK 11195, demonstrated significant neuroprotective effects by inhibiting microglial activation and cytokine accumulation (Leaver et al.,2012). Allopregnanolone is one of the most important neurosteroids and alleviates neuroin flammation and contributes to neural regeneration in many rodent models (Wang et al.,2010; Nezhadi et al., 2016). Overexpression of TSPO and increased allopregnanolone levels reversed lipopolysaccharide(LPS)-induced in flammation and neural impairment (Wang et al., 2016a). Thus, TSPO is a promising target for anti-depressant and anxiolytic drugs, but its specific mechanism of action remains to be determined.
To search for new TSPO ligands for the therapeutic treatment of stress-related disorders, a series of novel compounds was designed and synthesized. Among these compounds, N-ethyl-N-(2-pyridinyl methyl)-2-(3,4-ichlorophenyl)-7-methylimidazo [1,2-a] pyridine-3-acetamide hydrochloride (YL-IPA08) was identified for its high TSPO af finity and strong anti-depressant and anxiolytic-like effects in the absence of tolerance and withdrawal liabilities. The current study first investigated whether intracerebroventricular LPS could induce depression-like behavior in mice, and then tested the hypothesis that YL-IPA08 could attenuate in flammation involved in depression-like behavior. We also examined hippocampal neurogenesis after LPS and YLIPA08 administration.
Materials and Methods
Animals
One hundred and twenty specific-pathogen-free adult male C57BL/6 mice weighing 18–22 g and aged 6–8 weeks were obtained from the Beijing SPF Animal Technology Company,Beijing, China (Animal license No. SCXK 2016-0002). All mice were housed with controlled temperature (23 ± 1°C), humidity(45%), and lighting (12 hours per day). Food and water were available ad libitum unless specified. All procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8thedition). The experimental procedures were approved by the Institutional Committee on Animal Care and Use of the Academy of Military Medical Sciences (approval No. IACUC.20094). All efforts were made to minimize animal suffering and reduce the number of animals used for the experiments.
Group assignment
Sixty mice were randomly assigned into five groups and administrated with 0, 1, 10, 100, or 1000 ng LPS (Sigma-Aldrich, St. Louis, MO, USA) by intracerebroventricular microinjection (n = 12 per group; Table 1). Tail suspension and forced swimming tests were performed 24 hours after LPS administration to determine the optimal dose of LPS to induce depression-like behavior.
An additional sixty mice were assigned into four groups:control, LPS (100 ng), YL-IPA08, and LPS + YL-IPA08 (n =15 per group; Table 1). Two weeks after BrdU injection (day 1), mice were treated with LPS by intracerebroventricular injection (day 16).
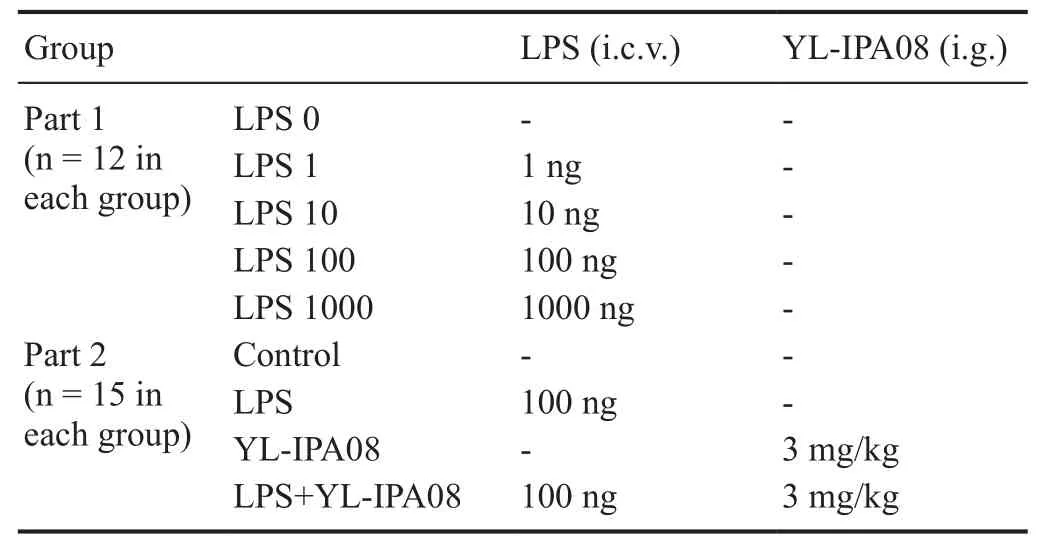
Table 1 Group assignment
Figure 1 Chemical structure of YL-IPA08.
Intervention
YL-IPA08 (purity ≥ 99%) (a selective TSPO ligand that has entered phase II clinical trials) was synthesized by the Department of Medicinal Chemistry in the Academy of Military Medical Sciences, Beijing, China (Figure 1). Bromodeoxyuridine (BrdU) and LPS were purchased from Sigma-Aldrich.BrdU was dissolved in 0.9% saline containing 2.5% dimethyl sulfoxide. For evaluation of neurogenesis, BrdU (100 mg/kg)was administered intraperitoneally three times with 3-hour intervals two weeks before LPS administration.
For the intracerebroventricular injection of LPS, mice were anesthetized by intraperitoneal injection of Avertin (200 mg/kg; Sigma) and placed on a stereotactic apparatus (Kopf Instruments, Tujunga, CA, USA) for stereotactic injection of LPS. Injection was made through a hole drilled in the skull into the lateral ventricle using the following the coordinates of the mouse brain atlas (Franklin and Paxinos, 2008) (in mm): 0.5 posterior, +1.0 lateral and 2.0 ventral from bregma.The injection speed was set at 0.667 μL/min and the needle was left in place for 1 minute following injection.
LPS was dissolved in artificial cerebrospinal fluid and administered by intracerebroventricular injection in a volume of 2 μL. The artificial cerebrospinal fluid vehicle contained 140 mM NaCl, 3.0 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2, and 1.2 mM Na2HPO4, adjusted to pH 7.4. YL-IPA08 (3 mg/kg) was administered by intragastric gavage at a volume of 2 mL/kg from 8:00 to 9:00 a.m., daily from the third week to the end of the experiment (days 16–24). The dose and route of administration are based on our previous study (Zhang et al.,2014b) and preliminary exploration. Behavioral tests were performed 60 minutes after YL-IPA08 administration (day 17).
Body weight of mice in all four groups was determined daily using electronic scales (Scientech, Boulder, CO, USA). One week after behavioral tests, the mice were euthanized by CO2asphyxiation followed by transcardial perfusion with ice-cold phosphate buffered saline (PBS) (day 24). The brains were immediately removed, fixed in 4% paraformaldehyde for 48 hours, cryoprotected in 30% sucrose at 4°C for 48 hours and then processed for BrdU/NeuN histological analysis.
Behavioral experiments Open field test
To evaluate whether the reversion of depression-like behavior by YL-IPA08 depends on affecting locomotor activity,the number of line crossings and rearings 24 hours after LPS administration was assessed as described in our previous studies (Qiu et al., 2013; Zhang et al., 2016). Mice were placed in the corner of a plastic box (36 cm × 29 cm × 23 cm; XR-XZ301, Xinruan Technology Co., Shanghai, China)in which the base was divided into equal sectors and recorded for 5 minutes. The number of crossings (all four paws placed into a new square) and rearings (both front paws raised from the floor) were counted by an observer who was blind to the group allocation.
Tail suspension test
The tail suspension test was performed as previously described (Steru et al., 1985), with minor modifications. The mice were suspended for 6 minutes from the top of the apparatus (Biowill Co., Ltd., Shanghai, China) using adhesive tape placed approximately 1 cm from the tip of the tail. The duration of immobility during the last 4 minutes of the 6 minutes was measured. The mice were judged to be immobile when they hung passively without moving. YL-IPA08 was administered 60 minutes before the test.
Forced swimming test
The forced swimming test was performed following the protocol of Porsolt et al. (1978), with minor modifications.All of the mice received a single YL-IPA08 administration.Sixty minutes after drug administration, the mice were individually placed in cylindrical containers (diameter 12 cm,height 20 cm, containing 10 cm of water maintained at 25°C;Biowill Co., Ltd.) for 6 minutes. The duration of immobility during the last 4 minutes of the 6 minutes was recorded.The mice were considered to be immobile when they floated motionless, only making movements necessary to keep their heads above the water.
Immunohistochemistry
Immunohistochemistry was performed as described in our previous report (Li et al., 2009). After behavioral testing,mice that received intraperitoneal injections of BrdU were deeply anesthetized and transcardially perfused with 0.9%NaCl. Brains were then removed and fixed for 24 hours in 4% buffered formalin before cryoprotection in 30% sucrose in PBS. For immuno fluorescent staining of BrdU, free- floating brain sections were incubated in 2× standard saline citrate/50% formamide at 65°C for 2 hours, and then in 2 N HCl at 37°C for 30 minutes and 0.1 M boric acid (pH 8.5)for 10 minutes before blocking with PBS-plus for 60 minutes. Brain sections were incubated for 1 day in cold PBS-plus containing both rat anti-BrdU antibody (1:200; Abcam,Cambridge, MA, USA) and mouse anti-NeuN antibody(1:1000; Chemicon, Temecula, CA, USA). After rinsing with PBS, the sections were incubated with Red-X-conjugated goat anti-rat IgG and FITC-conjugated goat anti-mouse IgG(both at a dilution of 1:200; Jackson, MS, USA) in PBS for 2 hours before mounting with Vectashield (Vector Laboratories, Burlingame, CA, USA). The sections were analyzed using a confocal laser-scanning microscope (Zeiss LSM510,Thornwood, NY, USA). BrdU-positive cells were counted using a modified stereological protocol. In brief, every sixth section throughout the entire hippocampus was analyzed.All BrdU-labeled cells in the granular cell layer and hilus were counted through a 60× objective lens to distinguish individual cells and multiplied by six, to record as the total number of labeled cells in the dentate gyrus. At least fifty BrdU-positive cells in the DG were randomly identified from each animal to determine the percentage of newborn neurons among the BrdU-labeled cells.
Statistical analysis
Data are represented as the mean ± SEM. Data were analyzed using GraphPad Prism 6 software (Graphpad Prism Institute Inc., La Jolla, CA, USA) by an observer who was blind to the experimental protocol. The dose effect of LPS was analyzed using one-way analysis of variance with Dunnett’s test for multiple comparisons. All other measures were analyzed using two-way analysis of variance with Bonferroni test for multiple comparisons. When the two-way interaction P value was < 0.05, post-hoc analysis using Fisher’s protected least significant difference test was employed to test differences among means.
Results
Effect of intracerebroventricular injection of LPS on depression-like behavior in mice
To test the hypothesis that central LPS would induce depression-like behavior, tail suspension and forced swimming tests were employed following intracerebroventricular treatment of LPS. In the tail suspension test, a single intracerebroventricular injection of LPS increased immobility 24 hours after treatment (LPS main effect, F4,52= 7.961, P <0.001; Figure 2A). Doses of 100–1000 ng significantly increased immobility (P100ng< 0.001, P1000ng< 0.0001 vs. vehicle group; Dunnett’s test; Figure 2A). Likewise, in the forced swimming test, a single intracerebroventricular injection of LPS increased immobility 24 hours after treatment (LPS main effect, F4,53= 3.619, P < 0.05; Figure 2B). Doses of 10–100 ng significantly induced depression-like behavior(P10ng< 0.001, P100ng< 0.0001 vs. control group; Dunnett’s test; Figure 2B). These results indicated that a single intra-cerebroventricular injection of LPS could induce depression-like behavior in C57 mice.
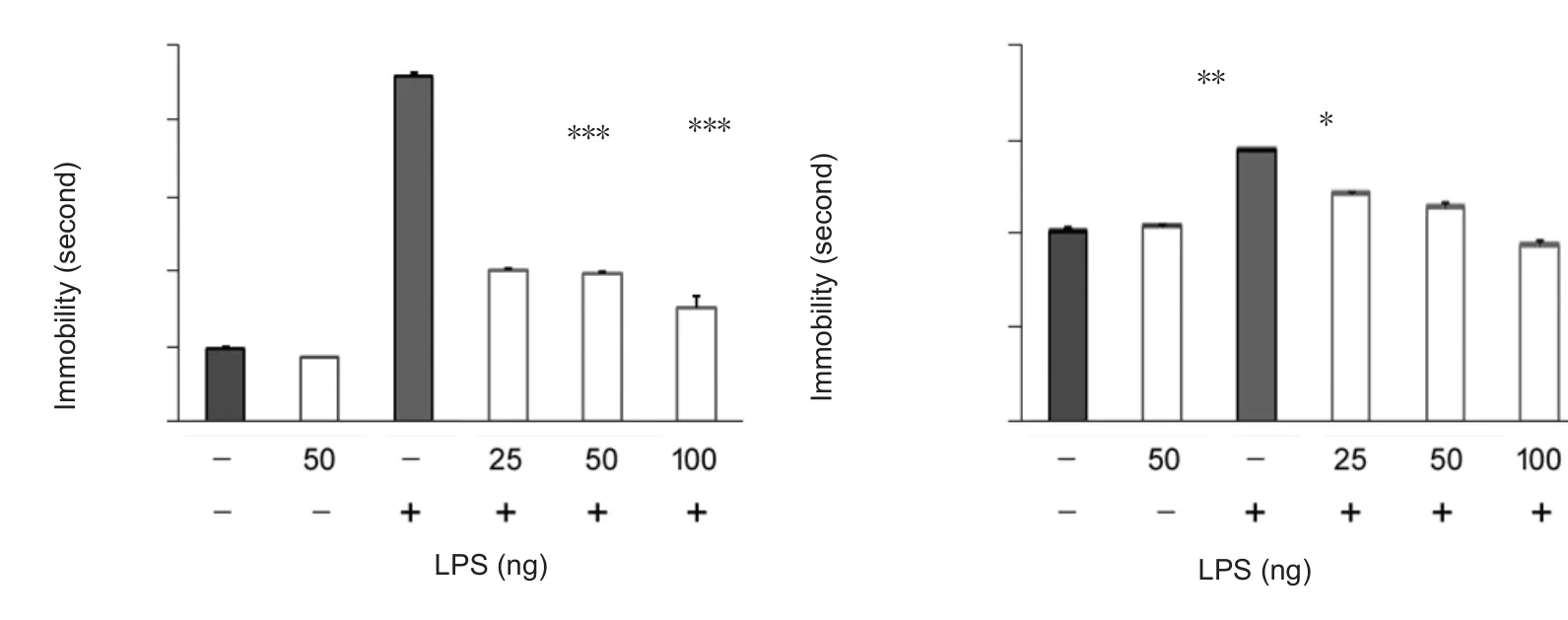
Figure 2 Intracerebroventricular LPS induced depression-like behavior in mice.

Figure 3 YL-IPA08 attenuated 100 ng LPS-induced depression-like behavior and sickness response in mice.
Anti-depression-like effect of YL-IPA08 in mice presenting with LPS-induced depression-like behavior
Following the LPS dose-effect experiment, 100 ng LPS was chosen as the dose to induce depression-like behavior and the effects of LPS and YL-IPA08 on locomotor activity were tested. As shown in Figure 2A, neither LPS nor YL-IPA08 changed the number of line crossings or rearings in the open field test (FLPS= 1.248, FDFO= 0.3903, FLPS×DFO= 0.2423, for line crossings, P > 0.05; FLPS= 0.976, FDFO= 1.5102, FLPS×DFO= 1.836, for rearings, P > 0.05; Figure 3A, B), indicating that differences in behavioral performance in other tests was not because of differences in locomotor activity.
YL-IPA08 protected mice from LPS-induced depression-like behavior in the tail suspension test and the forced swimming test. YL-IPA08 attenuated the LPS-induced increase in immobility in the tail suspension test (FLPS= 9.623,P = 0.0038; FDFO= 19.24, P = 0.0199; FLPS×DFO= 14.186, P =0.0001; Figure 3C) and the forced swimming test (FLPS=9.623, P = 0.0038; FDFO= 19.24, P = 0.0199; FLPS×DFO= 14.186,P = 0.0001; Figure 3D).
Changes in body weight were analyzed to assess a sickness response induced by LPS. As expected, mice lost body weight 24 hours following LPS treatment, which was reversed by YL-IPA08 (FLPS= 8.749, P = 0.036 FDFO= 7.55, P =0.0192; FLPS×DFO= 17.55, P = 0.0002; Figure 3E).
Effects of LPS and YL-IPA08 on neurogenesis in the hippocampus
Many studies have shown that inflammation reduces neurogenesis and that this reduction might be involved with depression-like behavior. We used immuno fluorescence his-tology to label BrdU- and NeuN-positive cells in the dentate gyrus to measure neurogenesis (Figure 4A, B). As shown in Figure 4, the number of BrdU-positive cells was reduced in the LPS group (FLPS= 14.56, P = 0.0051; Figure 4C) as was the number of cells co-labeled with BrdU and NeuN (FLPS=18.85, P = 0.0022; Figure 4D). The percentage of BrdU- and NeuN-positive cells among total BrdU-positive cells was also reduced (FLPS= 13.78, P = 0.0051; Figure 4E). YL-IPA08 alleviated these reductions (FYL-IPA08= 8.313, P = 0.0360,FYL-IPA08×LPS= 8.122, P = 0.037 for BrdU+cells; FYL-IPA08= 8.211,P = 0.005, FYL-IPA08×LPS= 6.323, P = 0.022 for BrdU+/NeuN+co-labeled cells; FYL-IPA08= 5.987, P = 0.0462, FYL-IPA08×LPS=7.021, P = 0.0356, for the percentage of co-labeled BrdU-and NeuN-positive cells among total BrdU+cells; Figure 4C–E). These data indicated that YL-IPA08 attenuated the impairment of hippocampal neurogenesis induced by LPS.
Discussion
In flammation in the central nervous system can cause depression-like behavior in many rodent models (Kojima et al., 2014;Byrne et al., 2015; Kiecolt-Glaser et al., 2015). TSPO as a modulator that participates in the pathology of neuroin flammation also plays an important role in the development of depression,but the mechanism remains unclear. This study tested the hypothesis that TSPO ligand, YL-IPA08, could attenuate lipopolysaccharide-induced depression-like behavior in mice.
LPS acts as a bacterial Toll-like receptor 4 ligand and can activate the innate immune response. LPS administered by systemic injection, intracerebral microinjection or chronic infusion induces inflammation in rodents. LPS can result in accumulation of pro-inflammatory cytokines and neuronal cell death via apoptosis (Bossù et al., 2012; Huang et al., 2012; Zhang et al., 2015; Wang et al., 2016b; Yang et al., 2017). It is known that cytokines disrupt neuronal functions and cause many mental disorders. In the current study, intracerebroventricular injection of LPS was used to establish an in flammation-induced depression-like behavior mouse model. Compared with peripheral inflammation,central in flammation is more involved with depression and dominates the effect of peripheral in flammation, which will affect an animal’s performance in behavioral tests. Furthermore, TSPO has been reported to play an important role in microglial activation and expression of pro-inflammatory cytokines (Liu et al., 2014). Studies showed that TSPO ligands such as PK11195 and XBD173 could target to TSPO to exert a broad in fluence on the in flammatory signaling in microglia (Nothdurfter et al., 2012a; Karlstetter et al., 2014a;Scholz et al., 2015). These knowledges support our hypothesis that the amelioration of TSPO ligand YL-IPA08 on the immune response elicited by LPS was attributed to the effect of TSPO, giving that the YL-IPA08 alone had no significant effect on depression-like behaviors or in flammatory markers compared with the control group.
The tail suspension and forced swimming tests are two widely used screening tests for the detection of anti-depressive effects in mice (Castagne et al., 2011). In this study, intracerebroventricular injection of 100 ng LPS induced depression-like behavior in both tests without changing locomotor activity. The difference in dose-dependence between the two models may result from differences in test sensitivity.
LPS-induced body weight loss indicated a sickness response, which is consistent with our previous study (Zhang et al., 2015). Body weight change is a clinical symptom in depression patients (Krimpuri et al., 2018), and body weight loss is considered part of the sickness response of many mental disorders (Lawson et al., 2013b). However, this impairment was remarkably attenuated by treatment with YLIPA08. This effect might be related to neurosteroid biosynthesis in which TSPO participates.
Over the past twenty years, neurosteroids have been considered potential treatments for many mental disorders, including anxiety and depression, and impaired neurosteroid synthesis contributes to the pathology of these mental impairments (Pinna et al., 2006). Clinical research also discovered decreased levels of neurosteroids in plasma, serum, and cerebrospinal fluid of depression patients. Of these neurosteroids,the progesterone metabolite allopregnanolone is a potent positive modulator of the action of γ-aminobutyric acid (Nin et al., 2011; Zorumski et al., 2013; Comenencia-Ortiz et al.,2014). Importantly, neuroactive steroids elicit anxiolytic and anti-depressant properties in many different animal models(Eser et al., 2006, 2008; Brinton, 2013; Jahn, 2016). Recently,the role of TSPO has drawn increased attention in the pathophysiology of depression-related disorders (Schüle et al., 2014;Maciukiewicz et al., 2015; Setiawan et al., 2015). The TSPO ligand, Ro5-4864, reduced microglial activation and neuronal apoptosis induced by quinolinic acid (Ryu et al., 2005). R05-4684 and another ligand, PK11195, exerts an anti-in flammatory effect in many animal models of acute inflammation,including arthritis (Water field et al., 1999), pleural exudation(Torres et al., 2000) and in flammation in the central nervous system (Ryu et al., 2005; Veiga et al., 2005), indicating anti-infl ammatory properties in addition to regulation of depression and stress. YL-IPA08, as a new TSPO ligand with high TSPO affinity, exerts potent antidepressant-like effects (Zhang et al., 2014a, b), which is consistent with the in flammation-induced depression-like model used in this study. The dose of YL-IPA08 was chosen as a therapeutic dose according to our previous study (Zhang et al., 2014a). YL-IPA08 attenuated LPS-induced depression-like behavior without changing locomotor activity, indicating that the decrease of immobility in the behavioral tests was not a false-positive phenomenon resulting from stimulation of the central nervous system, further verifying the anti-depression effect of YL-IPA08. In addition, compared with the control group, YL-IPA08 alone did not exert an anti-depression effect. Combined with previous studies detailing the anti-depressant effect of YL-IPA08 and the depression-like effect induced by LPS, TSPO may participate in in flammation-induced depression-like behaviors, and YL-IPA08 may exert an anti-depressant effect by alleviating the in flammation. However, the molecular mechanism for this needs to be determined.
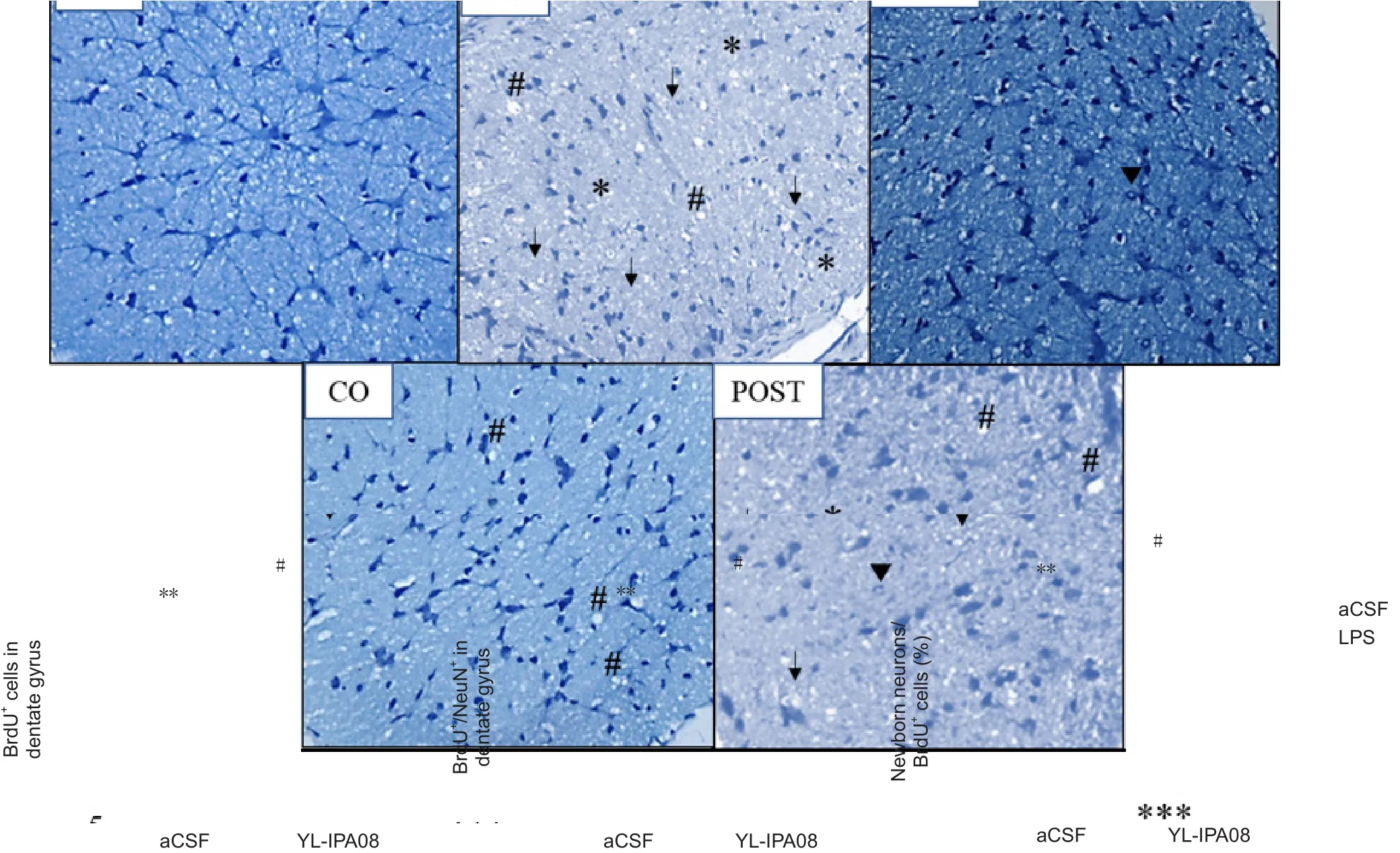
Figure 4 YL-IPA08 (a selective translocator protein ligand) attenuates the reduction in neurogenesis caused by 100 ng LPS.
One of the most significant brain regions involved in the pathology of depression is the limbic system (Anthes, 2014;Hoogenboom et al., 2014). The prefrontal cortex, the amygdala and the hippocampus are three brain regions within the limbic system, and are the most clearly altered brain regions in depression (Anthes, 2014; Hoogenboom et al., 2014).Adult hippocampal neurogenesis was demonstrated to be intimately involved with emotion, and impaired hippocampal neurogenesis contributes to the development of depression (Masi and Brovedani, 2011; Eisch and Petrik, 2012).Moreover, dysfunctional hippocampal neurogenesis could be caused by neuroin flammation (Chugh et al., 2015; Vay et al., 2016). Furthermore, anti-depressants protect neural cell regeneration in the hippocampus (Chugh et al., 2015;Vay et al., 2016). Allopregnanolone, a product downstream of TSPO, might modulate neurogenesis in rodents (Brinton and Wang, 2006; Charalampopoulos et al., 2008). Therefore,we hypothesized that TSPO ligand YL-IPA08 may be benefi cial for the improvement of in flammation-induced reduction in neural regeneration by increasing subsequent synthesis of allopregnanolone and neurogenesis. To address our hypothesis, we measured the effect of YL-IPA08 on hippocampal neurogenesis. Intracerebroventricular LPS decreased hippocampal neurogenesis, while YL-IPA08 obviously ameliorated the impaired hippocampal neurogenesis, suggesting that TSPO could participate in neuroin flammation-induced depression-like behavior, which is consistent with previous studies (Brinton and Wang, 2006; Charalampopoulos et al.,2008; Wang et al., 2016a). Thus, these results lead to the idea that reversal of impaired neurogenesis plays an important role in the neuroprotection effect of YL-IPA08. Importantly,we observed depressive-like behaviors in mice exposed to LPS ten days before BrdU/NeuN analysis. The timing of depressive-like behaviors in LPS-mice seems to be coincident with the loss of mature newborn neurons. Interestingly,LPS mice showed decreases in both BrdU+cells and BrdU+/NeuN+cells as well as depressive-like behaviors. One explanation may be that LPS impaired neurogenesis in the hippocampal dentate gyrus. This idea is supported by the acute strong inflammation, including cytokine accumulation,which induces immune reactions against neuronal bodies and glial cells for the survival and conditioning of neurons to regenerate severed nerves (Dubový et al., 2013; Molinos et al., 2015). The damaged neural cells lead to anxiety and depressive-like behaviors (McHugh et al., 2004). These results indicate a possible cause-and-effect relationship between the LPS-impaired neurogenesis and the LPS-induced depression-like behavior. Anti-depressants may exert their behavioral effects by increasing neurogenesis in the hippocampus(Banasr et al., 2006). In particular, YL-IPA08 in LPS mice not only reduced the loss of BrdU+cells, but also relieved the depressive-like behaviors. This neural protective effect of YLIPA08 is consistent with our hypothesis.
In summary, the TSPO ligand, YL-IPA08, attenuated LPS-induced depression-like behavior In C57 mice and YLIPA08 neuroprotection may involve hippocampal neurogenesis. This study might provide a new strategy for depression treatment. A limitation of this study is a lack of measurement of neurosteroids inside and outside of mitochondria.
Author contributions: XYZ conceived the study, carried out the study execution and data analysis and contributed to the manuscript draft. LMZ participated in the study design, the construction of recombinant lentiviruses and the draft of manuscript. WDM and YFL contributed to the study design, data analysis and manuscript revision. All authors approved the final version of this paper.
Conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 8167050047. The funder had no role in the study design, data collection or analysis; the decision to publish; or the preparation of the manuscript.
Institutional review board statement: This study was approved by the Institutional Committee on Animal Care and Use of Academy of Military Medical Sciences (approval No. IACUC.20094).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers: Jigar Pravinchandra Modi, Florida Atlantic University, USA; Simone Molz, Contestado University, Brazil.
Additional file: Open peer review reports 1, 2.
- 中国神经再生研究(英文版)的其它文章
- Restoration of an injured lower dorsal ascending reticular activating system in a patient with intraventricular hemorrhage
- Taurine protects against retinal and optic nerve damage induced by endothelin-1 in rats via antioxidant effects
- SIRT1 facilitates amyloid beta peptide degradation by upregulating lysosome number in primary astrocytes
- Cognitive deficits and Alzheimer-like neuropathological impairments during adolescence in a rat model of type 2 diabetes mellitus
- Enriched environment elevates expression of growth associated protein-43 in the substantia nigra of SAMP8 mice
- Achyranthes bidentata polypeptide protects dopaminergic neurons from apoptosis induced by rotenone and 6-hydroxydopamine