Restoration of an injured lower dorsal ascending reticular activating system in a patient with intraventricular hemorrhage
The ascending reticular activating system (ARAS) plays a key role in the control of arousal and awareness for consciousness(Paus, 2000; Zeman, 2001; Van der Werf et al., 2002; Weiss et al., 2007; Siposan and Aliu, 2014). It is well known that the ARAS originates from the reticular formation (RF) of the brainstem, and connects to the cerebral cortex via intralaminar nuclei (ILN) of thalamus, hypothalamus and direct pathways to the cerebral cortex (Paus, 2000; Zeman, 2001; Van der Werf et al., 2002; Yeo et al., 2013; Jang and Kwon, 2015). The hypothalamus is involved in the regulation of sleep and awareness which is associated with the main timekeeper of consciousness(Lin, 2000; Lin et al., 2011). By contrast, ILN is related to arousal of cortical and subcortical regions (Purpura and Schiff, 1997;Van der Werf et al., 2002). Therefore, precise evaluation of each component of the ARAS is important for assessment and intervention of patients with impaired arousal or awareness.However, exact responsibility of each pathway of the ARAS in the regulation of consciousness remains a topic of interest and concern.
Diffusion tensor tractography (DTT) has enabled reconstruction of each component of the ARAS (Edlow et al., 2012; Yeo et al., 2013; Jang and Kwon, 2015). As a result, several recent studies using DTT have reported lesion of the ARAS in patients with brain injury (Edlow et al., 2013; Jang et al., 2014, 2015a;Jang and Lee, 2015). However, relatively little is known about restoration or recovery of an injured ARAS (Jang and Yeo,2015).
In the current study, using DTT, we report on a patient with intraventricular hemorrhage who showed restoration of a damaged lower dorsal ARAS between the RF and ILN, which was demonstrated on DTT.
A 50-year-old female patient underwent brain computed tomography (CT) guided stereotactic drainage of intraventricular hematoma using the right frontal approach in the neurosurgery department (Figure 1A). Four weeks after onset, a patient was transferred to the department of rehabilitation medicine. The patient exhibited mild cognitive impairment and mild motor weakness in both upper and lower extremities, however, she did not show impairment of consciousness; Glasgow Coma Scale:15 (4 weeks), Coma Recovery scale: 23 (4 weeks) and Mini Mental State Examination: 19 (4 weeks) (Teasdale and Jennett,1974; Ross et al., 2003; Lovstad et al., 2010). Six age-matched control subjects (two males; mean age: 52.0 years, range: 48–56 years) with no history of neurologic disease were randomly recruited for the control group. The study protocol was approved by the Institutional Review Board of Yeungnam University Hospital, Republic of Korea (YUH-12-0421-O60).
Diffusion tensor imaging (DTI) data were collected at 4 weeks and 4 months after stroke onset using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips., Ltd., Best, The Netherlands) with single-shot echo-planar imaging.
Sixty seven contiguous slices were collected parallel to the anterior commissure-posterior commissure line for 32 non-collinear diffusion sensitizing gradients. Acquired imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed matrix = 192 × 192; field of view = 240 × 240 mm2;repetition time = 10,726 ms; echo time = 76 ms; parallel imaging reduction factor = 2; echo planar imaging factor = 49; b =1000 s/mm2; number of excitations = 1; and a slice thickness of 2.5 mm.
Analysis of DTT data was performed using Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL v5.0; www.fmrib.ox.ac.uk/fsl). Fiber tracking for the reconstruction of neural pathway was performed using a probabilistic tractography method based on a multifiber model.
The reconstruction of the ARAS was determined by seed regions of interest (ROI) and the target ROI. For the lower ventral ARAS between RF and hypothalamus, a seed ROI was given on the RF of the pons, and the target ROI was given at the hypothalamus including the mammillary body on the upper midbrain (Jang and Kwon, 2015). For the lower dorsal ARAS between RF and ILN, a seed ROI was given on the RF of the pons, and the target ROI was given on the ILN of the thalamus at the level of the commissural plane (Yeo et al., 2013). To analyze the connectivity of the ILN, the seed ROI was given at the ILN at the level of the commissural plane (Yeo et al., 2013). Out of 5000 samples generated from the seed voxel, results for contact were visualized at a minimum threshold of 1 streamlined through each voxel for analysis. In this study, we measured the fractional anisotropy value (FA), mean diffusivity value (MD),and tract volume (TV) for the lower dorsal and ventral ARAS.We defined an abnormal value when the DTT parameter values showed a deviation of more than two standard deviations from normal control values.
On 4-week DTT, the TV value in both hemispheres of the lower dorsal ARAS was decreased by more than two standard deviations of those of normal control subjects (Table 1). By contrast, on 4-month DTT, the TV values in both hemispheres were within two standard deviations of those of normal control subjects. In the lower ventral ARAS, FA, MD, and TV values at 4 weeks and 4 months after stroke onset were similar to those of the normal control subjects. On 4-week DTTs, both lower dorsal ARAS in the patient were thinner compared with normal control subjects; in contrast, both lower dorsal ARAS had become thicker on 4-month DTT. Regarding the neural connectivity of the ILN, neural connectivity to the basal forebrain and cerebral cortex at 4 weeks and 4 months after stroke onset was similar to that in the normal control subjects (Figure 1B).
In the current study, using DTT, we evaluated the lower dorsal and ventral ARAS, and cerebral connectivity of ILN in a patient who showed intact consciousness following intraventricular hemorrhage. According to our findings, on the 4-week DTT, the TV of the lower dorsal ARAS in both hemispheres was decreased compared with that in the control group, however, the values of FA and MD were similar to those of the control group. In addition, the FA, MD, and TV values of the lower ventral ARAS and connectivity of ILN were not different from those of the control group. The FA value represents the degree of orientation and integrity of white matter microstructures,while the MD value indicates the water diffusion magnitude(Assaf and Pasternak, 2008). The TV is determined by number of voxels contained within a reconstructed neural pathway (Seo and Jang, 2013). Therefore, the decreased TV without changes in FA and MD values appears to indicate injury of the lower dorsal ARAS (Assaf and Pasternak, 2008; Seo and Jang, 2013).In addition, the increment of TV for both ARAS between RF and ILN on 4-month DTT indicates restoration of the injured lower dorsal ARAS. The thickening of both dorsal ARAS at 4 weeks and 4 months after stroke onset appears to coincide with the results of DTT parameters. In contrast, the patient did not show impairment of consciousness despite the injury to the lower dorsal ARAS. The hypothalamus is responsible for regulation of awareness and ILN of the thalamus for arousal of cortical and subcortical regions (Purpura and Schiff, 1997; Lin,2000; Van der Werf et al., 2002; Lin et al., 2011). In addition,the level of activation of ILN of the thalamus is correlated with the level of arousal (Purpura and Schiff, 1997; Van der Werf et al., 2002). Therefore, our results suggest that preservation of integrity of the lower dorsal ARAS even in case of partial injury might be enough to show intact consciousness when the lower ventral ARAS and the cerebral connectivity of the ILN are intact.
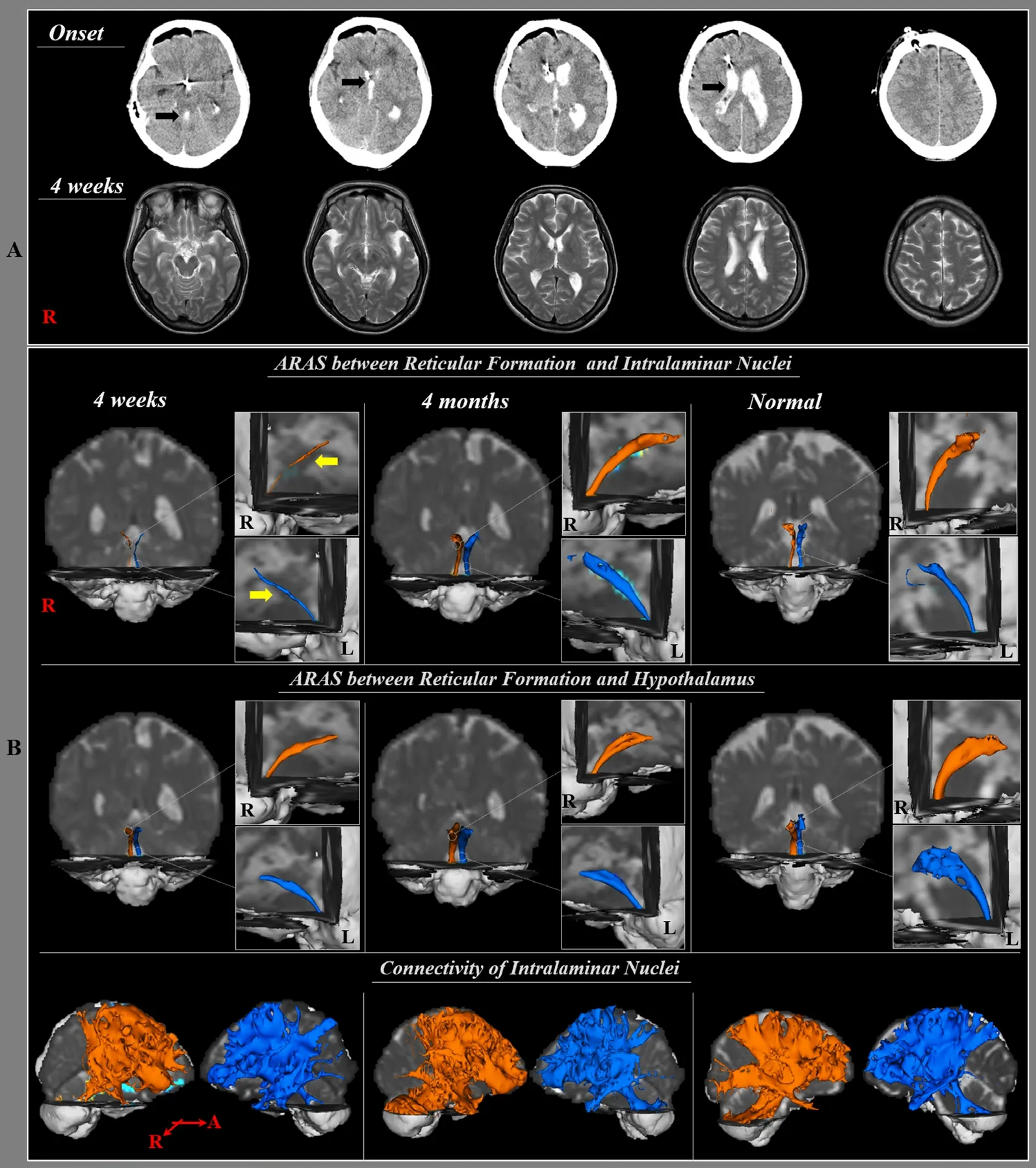
Figure 1 Brain computed tomography (CT) and diffusion tensor tractography (DTT) of a 50-year-old female patient with intraventricular hemorrhage who showed restoration of a damaged lower dorsal ARAS between the reticular formation(RF) and intralaminar nuclei.
Since introduction of DTI, a few studies have reported on recovery of the ARAS in patients with brain injury (Jang et al.,2015b, 2016; Jang and Lee, 2015). Jang et al. (2015b) reported on an injured dorsal ARAS in a patient with traumatic brain injury who exhibited severe alertness deficits at onset time.At 40 months after stroke onset, the patient showed intact consciousness with recovery of the injured dorsal ARAS. Subsequently, Jang and Lee (2015) reported on the recovery of an injured ARAS with recovery of consciousness in a patient with hypoxic-ischemic brain injury. They suggested that the increment of TV of the dorsal ARAS and neural connectivity of the ILN were in agreement with recovery of consciousness; GCS of the patient was recovered from 6 to 12 points. Jang et al. (2016)reported on a patient with traumatic brain injury and hypoxic ischemic brain injury who showed recovery of the ARAS with the recovery from a vegetative state to a minimally conscious state following shunt operation for hydrocephalus and rehabilitation. The patient did not show significant change in the lower ARAS, however, increased neural connectivity between the thalamic intralaminar nucleus and other brain structures including cerebral cortex, basal forebrain, and hypothalamus was observed in both hemispheres on postoperative DTT. However,no study has reported on intact arousal with injury of the lower dorsal ARAS and the restoration of the dorsal ARAS by assessment of the whole component of the ARAS.
In conclusion, restoration of an injured lower dorsal ARAS with intact consciousness was demonstrated in a patient with intraventricular hemorrhage. We believe that DTT analysis of the whole component of the ARAS would be helpful in the evaluation of consciousness impairment after brain injury. Our results suggest the importance of preservation of the lower dorsal ARAS between the pontine RF and ILN even in case of partial injury for maintenance of consciousness. Several limitations of DTI should be considered. First, regions of fiber crossing and complexity can interrupt full reconstruction of neural pathway (Yamada et al.,2009). Second, we could not precisely define the location of ROIs because of the small and cramped size of brainstem nuclei. Lastly, further studies involving a larger number of patients should be encouraged because this is a case study.

Table 1 Comparisons of diffusion tensor imaging parameters for the lower ventral and dorsal ascending reticular activating systems between a patient and normal control subjects
Sung Ho Jang, Sang Seok Yeo,*
Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Republic of Korea(Jang SH)
Department of Physical Therapy, College of Health Sciences,
Dankook University, Cheonan, Republic of Korea (Yeo SS)
*Correspondence to: Sang Seok Yeo, PhD, eangbul@hanmail.net.
orcid: 0000-0003-3873-9516 (Sang Seok Yeo)
Accepted: 2018-07-11
doi: 10.4103/1673-5374.238719
Author contributions: Conception and design of the study, fundraising, data acquisition, manuscript development and writing: SHJ; manuscript development, data acquisition, manuscript writing and authorization: SSY. Both SHJ and SSY approved the final version of this paper for publication.
Conflicts of interest: The authors declare that they have no conflicts of interest.Financial support: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF)funded by the Ministry of Education, Science and Technology (NRF-2015R1D1A1A01060314). The funding body played no role in the study conception design, in the collection, analysis and inter pretation of data, in the preparation and writing of the report, and in the decision to submit the article for publication.
Institutional review board statement: This study was approved by the Institutional Review Board of Yeungnam University Hospital, Republic of Korea(approval No. YUH-12-0421-O60).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers: Takatoshi Hara, The Jikei University School of Medicine, Japan; Han-A Park, University of Alabama, USA.
Additional file: Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- The unfolded protein response signaling and retinal Müller cell metabolism
- Sequencing of high-efficacy disease-modifying therapies in multiple sclerosis: perspectives and approaches
- Targeting prion-like protein spreading in neurodegenerative diseases
- Cadmium-induced neurotoxicity: still much ado
- Analysis of the traf ficking system in blood-brain barrier models by high content screening microscopy
- Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death