Analysis of the traf ficking system in blood-brain barrier models by high content screening microscopy
The blood-brain barrier (BBB) and drug delivery: In our modern societies, the prevalence of nervous system disorders is increasing in relation to the aging population. To overcome this problem, several interesting pharmacons and biomolecules are introduced and tested each day. The biggest obstacle to delivering drugs into the brain parenchyma is the presence of the BBB (Toth et al., 2011).The BBB is a regulatory interface, which limits free transport of large and small molecules between the systemic circulation and the central nervous system. This regulation enables proper neuronal function and protection from outside toxic insults as well as maintenance of a stable ionic homeostasis. The morphological basis of the BBB is the monolayer of brain endothelial cells (BECs) in the cerebral microvessels. The adult human brain contains more than 600 km of capillaries, which differ fundamentally from other endothelial interfaces in the body. The tight inter-endothelial junctions connecting the cells reduce paracellular permeation of ions and other small hydrophilic solutes as well as larger molecules. Consequently, molecules have to enter the brain via transcellular transport mechanisms, but this access is also subjected to restrictive control. The metabolic and enzymatic barrier, formed by a unique expression pattern of enzymes and efflux pumps at the luminal membrane of BEC, limits BBB penetration of lipophilic drugs and other xenobiotics. Thus, the supply of essential nutrients to brain cells is tightly controlled via the vesicular-mediated transcellular transport mechanism. Transcytosis of larger molecules such as peptides and proteins are initially endocytosed by absorptive- and receptor-mediated mechanisms and then transcytosed via subcellular vesicles. This regulated vesicular transport is also known as absorptive- and receptor-mediated transcytosis (Abbott et al.,2010). In addition to essential supplements, cell surface receptors are considered a potential gate for targeted delivery of large drugs to the brain. In the last decade, several publications have focused on the transferrin receptor as a target for bispecific antibodies and nanoparticles with pharmaceutical effect (Freskgård and Urich,2017). Additionally, the cross-talk among endothelial cells and neighboring cells such as astroglia, pericytes, microglia, and neurons should be mentioned, which induce a unique barrier phenotype in BEC. This interaction is important for drug delivery, as it is known to affect expression of tight junction molecules, receptors,and transporters, as well as in fluence the subcellular vesicular system (Abbott et al., 2010). In this paper, we introduce high content screening microscopy as an approach to analyze the subcellular vesicular structure and the traf ficking system of the BBB in vitro. The method is particular useful to describe and compare differences between different culture set-ups.
In vitro BBB models: Even though live in vivo investigation methods, such as intracranial 2-photon microscopy on rodents has become a standard approach, they do not provide the necessary resolution to obtain detailed information on traf ficking of receptor and subcellular vesicles across the BBB. Meanwhile, in vivo models based on free- floating sections (Villaseñor and Collin, 2017) and in vitro transwell models of BBB have proven to be a valuable tool for studying subcellular traf ficking of receptors and their cargos during receptor-mediated transcytosis using high-resolution microscope setups. These models are quite useful for efficient and inexpensive screening of novel drug candidates. Another beneficial aspect of in vitro models is the reduction in number of animal experiments needed. Therefore, in the past thirty years, several primary and immortalized cell models have been developed for BBB studies (Toth et al., 2018). In our laboratory we established the primary porcine in vitro BBB models for these research purposes. Porcine cells possess also favorable features for the pharmaceutical industry,since their genome, anatomy, physiology, and disease progression of the pig reflect the human biology. Furthermore, these BECs are capable of forming relatively tight endothelial barriers, even when grown in monoculture as compared to the models from other species. As porcine brains are a common by-product of the meat industry, they constitute an easily accessible source of BECs, minimizing the number of animals needed for the experiments, and providing a high purification yield from one porcine brain (Nielsen et al., 2017). The receptor expression and paracellular tightness of endothelial cells in commonly used BBB models are well described,though their vesicular structures and transport regulation are poorly investigated (Toth et al., 2011). Earlier assumptions on subcellular traf ficking in BEC were primarily based on observations in epithelial cells. Epithelial cells also form a polarized barrier, but they have different molecular structure and morphology, for example the elongated lateral domain structure present in epithelial cells is absent in BEC (Fung et al., 2018). In our recent work, we have outlined a process to characterize the vesicular apparatus in BEC,which is important for receptor-mediated transcytosis and therefore drug delivery to the brain. We have analyzed in detail how astrocytes in fluence the vesicular composition of BEC and we are confident that these data will lead to a more reliable information to delivering drugs across the BBB (Toth et al., 2018).
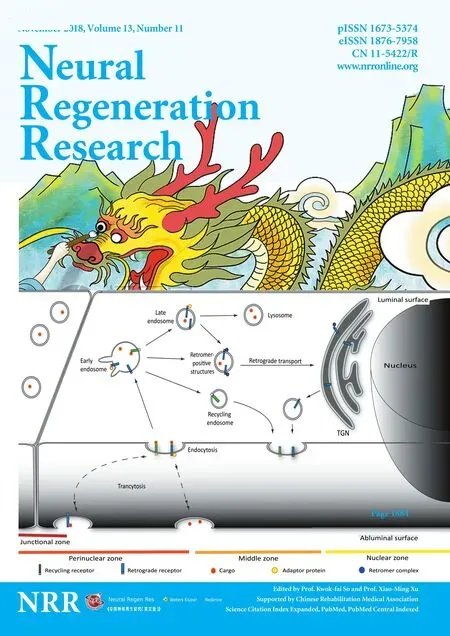
High content screening microscopy for characterization of the vesicular system: Transcytosis across brain capillaries is a complex process. It involves general and specific adaptor proteins, and subcellular vesicles known as the endo-lysosomal system (Toth et al.,2018). The endo-lysosomal system itself is highly complex, but it can be divided into the following types of vesicles: the trans-Golgi network, retromer positive structures, lysosomes, and several types of endosomal vesicles including early, recycling, and late endosomes (Figure 1) (Huotari and Helenius, 2011). The quantity of these endosomes and vesicles varies from cell type to cell type as they have different functions within the cells. Between these vesicles,several cytosolic adaptors and protein complexes regulate and guide the traffic of transmembrane receptors (Bonifacino, 2014). Early endosomes, located near the cell membrane, ful fill the role of a sorting station; receiving receptors and cargo from almost all types of endocytosis. The internalized extracellular fluid as well as part of the membrane, is recycled back to the cell surface from early endosomes via recycling endosomes. The remaining portion of early endosomes mature into late endosomes and finally fuse with lysosomes (Huotari and Helenius, 2011). Alternatively, retrograde receptors and certain ligands are transported toward the trans-Golgi network. In this process, the retromer complex is responsible for formation of the retromer positive structures and for direction of the transport. Retrograde transport in BEC is particularly interesting since retrograde receptors are considered potential targets for receptor mediated drug delivery. For instance, the cation independent mannose-6-phosphate receptor is capable of travelling from the luminal to the abluminal membrane in BEC (Siupka et al., 2017).

Figure 1 Vesicular transport in brain endothelial cells.
Due to the involvement of the endo-lysosomal system in receptor-mediated drug delivery, its description in BEC has high importance. We recently introduced a method in which we analyzed and classified the intra-endothelial vesicles in detail. Applying high content screening microscopy and specific markers for the vesicular structures, we are able to provide a horizontal map of the endo-lysosomal system in primary porcine BEC (Toth et al., 2018).Microscopy-based high content screening is an emerging technology that has numerous applications in cell-biology. The advantage of this method is that it enables large-scale screening of defined structures in order to extract statistically relevant observations. These data can be further analyzed to pinpoint differences that are invisible to the human eye (Boutros et al., 2015). During our investigation, we were provided with several parameters of each vesicle type and grouped them further based on their distance from the cell nucleus and the intercellular junctions (Figure 1). With the help of this method, we were able to compare in vitro BBB models in which primary BEC were grown on Transwell inserts alone (monoculture), and together with astrocytes (co-culture models) (Toth et al., 2018). By quantifying the vesicular structures, we observed that the presence of astrocytes resulted in significant down regulation of late and recycling endosomes, while early endosomes, lysosomes and retrograde positive structures were increased. Subsequently,by staining the tight junction and nucleus, we were able to further segment the cytosolic area into perinuclear, middle, process and junction zones and to investigate the quantitative and qualitative changes of the vesicles in these individual zones. Our observations provide a useful tool in achieving further mechanical knowledge of the transcytotic process in BEC.
Conclusion and further perspectives: Characterization of the traf ficking system of the BBB is of high value in elucidating and reflecting on disease process and species differences in BEC. Furthermore, examination of the vesicular system with high content screening microscopy has an enormous potential to investigate and compare the endo-lysosomal system of different in vitro BBB models. We are currently in the process of comparing the vesicular traffi cking system of immortalized and primary in vitro BBB models.We are particularly interested to reveal whether immortalized cellbased models have the same capacity and vesicular system as primary cell-based models. Detailed characterization of the transport system is important in order to better understand the necessary mechanisms for more efficient drug delivery across the BBB and to select the optimal model for the experiments of interest.
This study was supported by the Research Initiative on Brain Barriers and Drug Delivery funded by the Lundbeck Foundation (Grant No. 2013-14113).
Andrea E. Toth, Morten S. Nielsen*
Department of Biomedicine, Faculty of Health, Aarhus University,Aarhus, Denmark
*Correspondence to: Morten S. Nielsen, PhD, Cand.scient,mn@biomed.au.dk.
orcid: 0000-0001-9863-9694 (Morten S. Nielsen)
Accepted: 2018-07-02
doi: 10.4103/1673-5374.239435
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers: Isaac G. Onyango, Gencia Biotechnology, USA; Ahmed Alhusban, Jordan University of Science and Technology, Jordan.
Additional file: Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Restoration of an injured lower dorsal ascending reticular activating system in a patient with intraventricular hemorrhage
- Taurine protects against retinal and optic nerve damage induced by endothelin-1 in rats via antioxidant effects
- SIRT1 facilitates amyloid beta peptide degradation by upregulating lysosome number in primary astrocytes
- Cognitive deficits and Alzheimer-like neuropathological impairments during adolescence in a rat model of type 2 diabetes mellitus
- Enriched environment elevates expression of growth associated protein-43 in the substantia nigra of SAMP8 mice
- Achyranthes bidentata polypeptide protects dopaminergic neurons from apoptosis induced by rotenone and 6-hydroxydopamine