Achyranthes bidentata polypeptide protects dopaminergic neurons from apoptosis induced by rotenone and 6-hydroxydopamine
Su Peng, Li Xu, Jin-Yu Ma, Xiao-Song Gu, Cheng Sun
Key Laboratory for Neuroregeneration of Jiangsu Province and Ministry of Education, Co-Innovatioin Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
Abstract It has been well documented that Achyranthes bidentata polypeptides (ABPPs) are potent neuroprotective agents in several types of neurons. However, whether ABPPs protect dopaminergic neurons from apoptosis induced by neurotoxins is still unknown. This study was designed to observe the effect of ABPPk, a purified fraction of ABPPs, on apoptosis of dopaminergic neurons. SH-5YHY cells and primary dopaminergic neurons were pre-treated with ABPPk (25, 50, or 100 ng/mL) for 12 hours. Cells were then exposed to 6-hydroxydopamine(50 or 150 μM) or rotenone (50 or 200 μM) for 36 hours to induce cell apoptosis. Our results demonstrate that ABPPk markedly increased viability in SH-SY5Y cells and primary dopaminergic neurons, decreased lactate dehydrogenase activity and number of apoptotic dopaminergic neurons, elevated mitochondrial membrane potential, and increased Bcl-2/Bax ratio. These findings suggest that ABPPk protects dopaminergic neurons from apoptosis, and that ABPPk treatment might be an effective intervention for treating dopaminergic neuronal loss associated with disorders such as Parkinson’s disease.
Key Words: nerve regeneration; Achyranthes bidentata polypeptides; neuroprotection; cell apoptosis; neurotoxin; mitochondrial dysfunction;cell viability; Bcl-2/Bax; neural regeneration
Introduction
To test this hypothesis in this study, we pre-treated dopaminergic neurons with ABPPk and then subjected the cells to exogenous insults induced by neurotoxic agents including rotenone and 6-hydroxydopamine (6-OHDA). Our objective was to determine whether ABPPk protects dopaminergic neurons from apoptosis induced by neurotoxins.
Materials and Methods
ABPPk isolation and purification
Achyranthes bidentata blume roots were purchased from a local Chinese medicine store, and identified by Professor Haoru Zhao from China Pharmaceutical University (Nanjing, China). The crude ABPP extraction procedure has been previously described (Shen et al., 2008). ABPPk fraction was purified by high performance liquid chromatography (Cheng et al., 2014; Yu et al., 2014).
Cell culture and treatment
Human dopaminergic SH-SY5Y cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA,USA), and cultured in Dulbecco’s modified Eagle’s medium(Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco), and incubated in a humidified atmosphere with 5% CO2at 37°C. Primary rat midbrain dopaminergic neurons were prepared from newborn Sprague-Dawley rat (P0) brains, as described elsewhere (Gandhi et al., 2009). SH-SY5Y cells and primary midbrain dopaminergic neurons were treated with 6-OHDA (50 or 150 μM; Sigma-Aldrich, St. Louis, MO, USA) or rotenone(50 or 200 μM; Sigma-Aldrich) for 36 hours with or without 12 hour-pretreatment of ABPPk (25, 50, or 100 ng/mL).
Cell viability assay
Cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Cells were seeded into 96-well plates and treated with 6-OHDA (50 or 150 μM) or rotenone (50 or 200 μM) for 36 hours with 12 hour-pretreatment of ABPPk (25, 50, or 100 ng/mL). Subsequently, the medium was removed. MTT( final concentration 500 μg/mL; Sigma-Aldrich) was added and incubated at 37°C for 4 hours. After incubation, cells were lysed in sodium dodecyl sulphate (20%) at 37°C for 20 hours. Absorbance at 570 nm was measured using a microplate reader (BioTek, Winooski, VT, USA).
Terminal deoxynucleotidyltransferase-mediated-uridine triphosphate nick-end labeling (TUNEL) staining of apoptotic cells
TUNEL analysis was performed using a kit (Roche, Penzberg,Germany). Brie fly, after fixation for 1 hour in 4% paraformaldehyde at room temperature, cells were permeabilized for 2 minutes on ice. After thoroughly washing, 1500 U/mL DNase 1 (100 μL) was added and incubated for 20 minutes. TUNEL mixture solution (500 μL) was then added, and cells incubated in the dark for 60 minutes. Images were obtained using a phase contrast microscope (Zeiss, Oberkochen, Germany).
Lactate dehydrogenase (LDH) assay
LDH activity in cell culture medium was measured as previously described (Wang et al., 2014). Brie fly, cell culture medium was collected and treated with LDH assay reaction mixture (Jiancheng, Nanjing, China) for 30 minutes at room temperature in the dark. Absorbance was measured using a microplate reader (BioTek) at 490 nm. According to the manufacturer’s instructions, the cell death ratio (%) was calculated by: (Absorbancesample– Absorbanceblank) / (Absorbancemax– Absorbanceblank) × 100%, with Absorbancemaxreferring to the absorbance value of the positive group. The cell death ratio was expressed as LDH release.
Mitochondrial membrane potential analysis
Mitochondrial membrane potential was examined using a commercial kit (ab113852; Abcam, Cambridge, MA, USA),with tetramethylrhodamine, ethyl ester used as a specific dye for mitochondria. The experiments were performed in strict accordance with the manufacturer’s instructions. Briefly,culture medium was removed and the cells incubated in working solution for 20 minutes. After incubation, working solution was evaporated and phosphate-buffered saline added. Fluorescence density was analyzed by microplate spectrophotometry (BioTek). Images were captured using a fluorescence microscope (Zeiss).
The father was furious() . If the only reason you wanted to know how much money I make is just so you can borrow some to buy a silly toy or some other nonsense, then you march yourself straight to your room and go to bed. Think about why you re being so selfish. I work long, hard hours everyday and don t have time for such childish games.
Western blot assay
Cell protein extraction was performed as previously described (Liu et al., 2016). Brie fly, cells were homogenized in ice-cold tissue lysis buffer and the lysates centrifuged at 12,000 r/min for 15 minutes at 4°C. Supernatants reflected total cell protein extraction, in which protein concentration was measured using a commercial kit (Bio-Rad). Samples were boiled at 100°C for 5 minutes in Laemmli buffer. Prepared samples were subjected to western blot assay, as previously described (Sun et al., 2014). The primary antibodies used were: anti-B-cell lymphoma 2 (Bcl2), anti-Bcl2 associated X protein (Bax), and anti-β-actin. They were all rabbit polyclonal antibodies obtained from Cell Signaling Technology (Beverley, MA, USA). Membranes were incubated with primary antibodies (1:1000) at 4°C overnight. After washing, membranes were incubated with secondary antibody(1:10,000; Abcam) at room temperature for 1 hour.
Statistical analysis
Data are presented as the mean ± SEM, and were analyzed using SPSS 19.0 software (IBM, Armonk, NY, USA). Statistical significance was calculated by one-way analysis of variance followed by Bonferroni post hoc test. A value of P < 0.05 was considered statistically significant.
Results
ABPPk improved cell viability in injured dopaminergic neurons

Figure 1 ABPPk improved cell viability detected by MTT assay in the presence of 6-OHDA and rotenone.
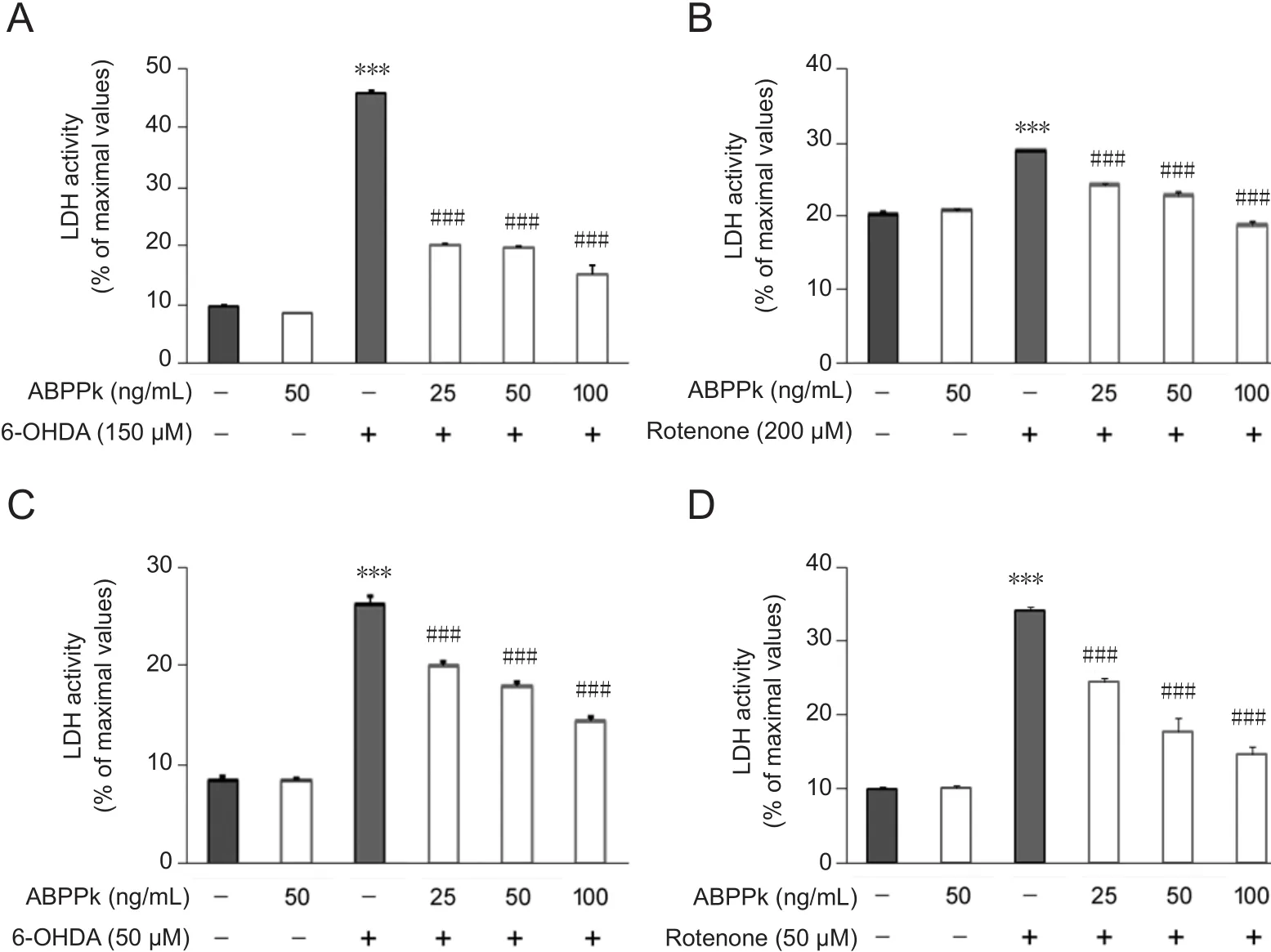
Figure 2 ABPPk attenuated LDH activity measured by a microplate reader in 6-OHDA- or rotenonetreated cells.
To evaluate the potential protective role of ABPPk in dopaminergic neurons, SH-SY5Y cells were pretreated with ABPPk at different concentrations and then exposed to rotenone or 6-OHDA. We found that application of 6-OHDA or rotenone significantly reduced cell viability, while ABPPk-treated cells were resistant to exogenous insult (P < 0.01,P < 0.001; Figure 1A, B). Meanwhile, we also used primary rat dopaminergic neurons to further examine the protective role of ABPPk. Similarly, exposure of 6-OHDA or rotenone dramatically reduced cell viability, whereas application of ABPPk rescued these reductions in cell viability (P < 0.001;Figure 1C, D). Interestingly, the observed protective role of ABPPk against neurotoxic insult was dose-dependent.
ABPPk attenuated LDH activity in insulted dopaminergic neurons

Figure 3 TUNEL assays showing cell apoptosis in SH-SY5Y cells and primary dopaminergic neurons following ABPPk treatment.
To further confirm the protective role of ABPPk, LDH activity was examined. We found that LDH activity in the culture medium of SH-SY5Y cells was significantly increased by 6-OHDA or rotenone (P < 0.001; Figure 2A, B). As expected, this increase was largely counteracted by ABPPk (P <0.001; Figure 2A, B). Next, we determined whether similar protective effects of ABPPk were recapitulated in primary dopaminergic neurons. Indeed, pretreatment with ABPPk significantly reduced released LDH activity in the presence of 6-OHDA or rotenone (P < 0.001; Figure 2C, D).
ABPPk attenuated cell apoptosis in insulted dopaminergic neurons
We next determined whether ABPPk protects cells from apoptosis using the TUNEL assay. Briefly, TUNEL staining recognizes damaged DNA including double- and single-stranded DNA breaks (Gavrieli et al., 1992). In SHSY5Y cells, exposure to 6-OHDA significantly induced cell apoptosis, as shown by an increase in TUNEL-positive cells(P < 0.001; Figure 3A). However, ABPPk largely attenuated cell apoptosis induced by 6-OHDA (P < 0.001; Figure 3A).Similar results were also observed in SH-SY5Y cells treated with ABPPk or rotenone alone (P < 0.001; Figure 3B).
In primary dopaminergic neurons, incubation of 6-OHDA or rotenone also induced cell apoptosis, while ABPPk application significantly protected these cells from apoptosis (P <0.001; Figure 3C, D).
ABPPk improved mitochondrial function (mitochondrial membrane potential) in insulted dopaminergic neurons
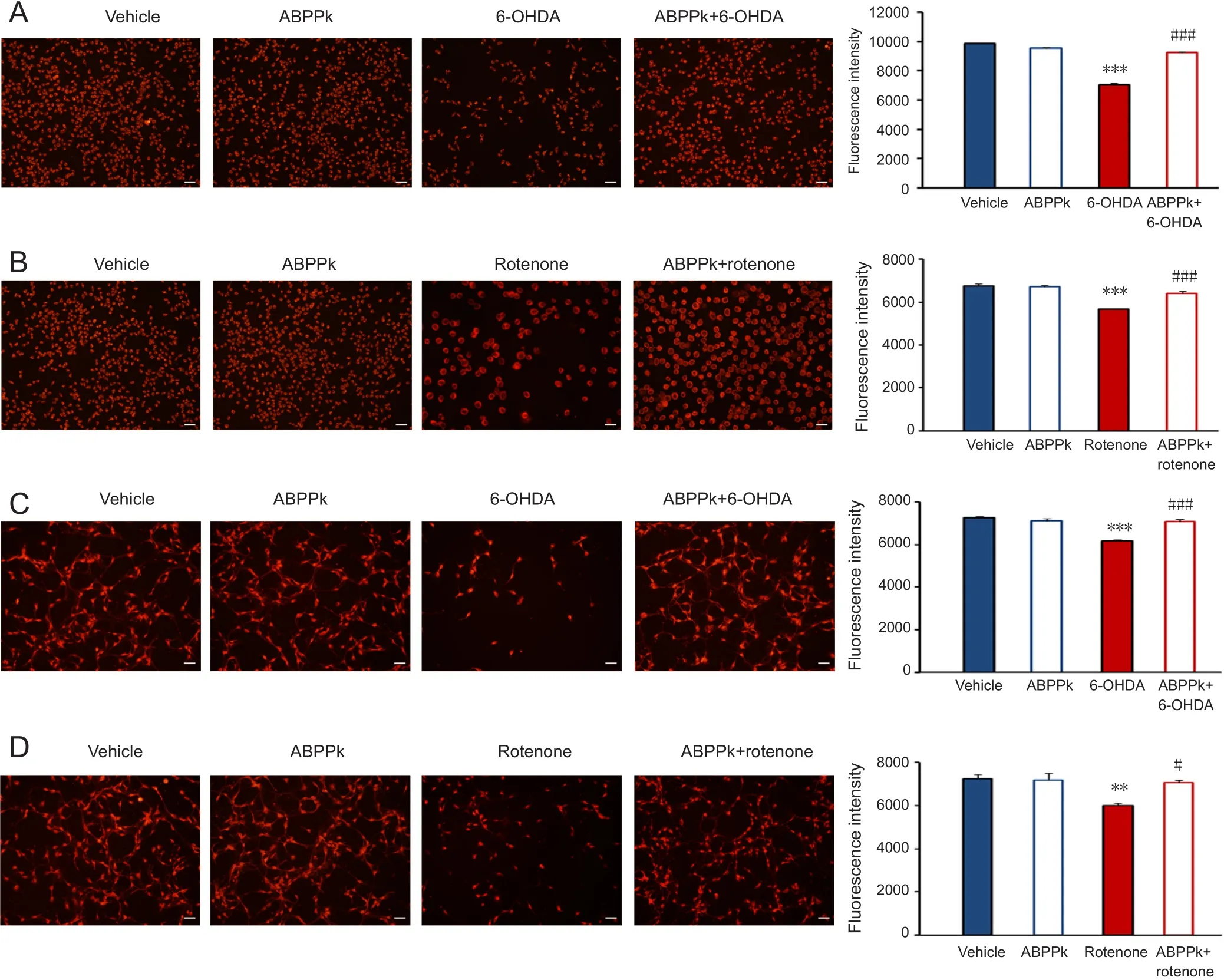
Figure 4 ABPPk improved mitochondrial membrane potential in SH-SH5Y cells and primary dopaminergic neurons.
Mitochondrial dysfunction has been proposed to play a major role in PD pathogenesis, and can be induced by both exogenous and endogenous neurotoxins (Schapira and Gegg,2011). Numerous evidence has shown that rotenone and 6-OHDA are two potent inducers of mitochondrial dysfunction (Glinka and Youdim, 1995; Glinka et al., 1996; Sherer et al., 2003; Panov et al., 2005). Therefore, we determined whether ABPPk improves mitochondrial dysfunction induced by 6-OHDA or rotenone. In SH-SY5Y cells, exposure to 6-OHDA or rotenone greatly impaired mitochondrial capacity. While application of ABPPk attenuated this impairment (P < 0.001; Figure 4A, B). Moreover, in primary dopaminergic neurons, impaired mitochondrial capacity induced by 6-OHDA or rotenone was also attenuated by ABPPk (P <0.05, P < 0.001; Figure 4C, D).
ABPPk increased Bcl-2/Bax in SH-SY5Y cells and primary dopaminergic neurons
Bcl-2 is a major anti-apoptotic protein and Bax is a pro-apoptotic factor. The Bcl-2/Bax ratio has been shown to determine cell fate (Mignard et al., 2014). Thus, we measured Bcl-2 and Bax protein levels by western blot assay.In SH-SY5Y cells, ABPPk alone showed no effect on Bcl-2 and Bax protein levels (P < 0.01; Figure 5A, B). Further,when cells were exposed to 6-OHDA or rotenone, Bcl-2 was reduced while Bax was enhanced. Consequently, Bcl-2/Bax ratio was dramatically reduced (Figure 5A, B). However,pretreatment of ABPPk reversed these changes in Bcl-2/Bax ratio. Similarly, in primary dopaminergic neurons, ABPPk pretreatment counteracted the effects of 6-OHDA and rote-none on Bcl-2/Bax ratio (P < 0.05, P < 0.01; Figure 5C, D).
Figure 5 ABPPk increases Bcl-2/Bax ratio detected by western blot assay in injured cells.
Discussion
In this study, SH-SH5Y cells and primary dopaminergic neurons were pretreated with ABPPk, the most active fraction isolated from the traditional Chinese medicine, Achyranthes bidentata. Our results show that ABPPk markedly protects cells from apoptosis induced by 6-OHDA or rotenone. Moreover, our findings show that ABPPk stimulates the Bcl-2/Bax ratio in insulted dopaminergic neurons. Altogether, these data indicate that ABPPk has a potent neuroprotective role in dopaminergic neurons and might be used as an intervention against PD.
PD is the second most common neurodegeneration disorder, affecting over four million people, and with pronounced loss of dopaminergic neurons in the substantia nigra. Over 90% of PD cases do not have an identified genetic cause, and environmental factors likely contribute to the disease. 6-OHDA and rotenone are two types of chemicals with selective destroying roles on dopaminergic neurons.Thus, these two chemicals are widely used for generating PD models both in vitro and in vivo (Qiu et al., 2016). Here, we used 6-OHDA and rotenone to treat SH-SY5Y and primary dopaminergic neurons and create an in vitro model of PD.Indeed, exposure to these two neurotoxins greatly reduced cell viability. Of note, low dosage of 6-OHDA and rotenone was used for the primary dopaminergic neurons, suggesting that primary dopaminergic neurons are vulnerable to these exogenous neurotoxins. This conclusion is in agreement with a previous study (Aime et al., 2015).
Extensive studies of Achyranthes bidentata suggest that it has multiple physiological functions including adipogenesis inhibition, anti-oxidative stress, promotion of osteogenic differentiation, and chondrocyte proliferation (Tie et al.,2013; Oh et al., 2014; Suh et al., 2014). Saponins and polysaccharides are considered the two main constituents of Achyranthes bidentata that are responsible for its pharmaceutical efficacy. Recently, we prepared a polypeptide extraction from Achyranthes bidentata blume (i.e., ABPP) and found that ABPP exhibits neurotrophic and neuroprotective actions in several types of neurons (Shen et al., 2008; Shen et al., 2013). To obtain the most potent fraction of ABPP,we separated crude ABPP by high performance liquid chromatography, with one fraction (namely ABPPk) showing the greatest neuroprotective action both in vitro and in vivo(Cheng et al., 2014; Yu et al., 2014). In the present study, we focused on ABPPk and its potential application for treating diseases associated with neuronal loss.
Since dopaminergic neuronal loss is a main causative factor for developing PD, we hypothesized that ABPPk might play a beneficial role against PD by preventing neuronal apoptosis.To test this hypothesis, SH-SY5Y cells were treated with ABPPk, followed by 6-OHDA or rotenone. As expected, ABPPk treatment significantly rescued cells from apoptosis. Similarly, 6-OHDA or rotenone-induced cytotoxicity was greatly attenuated by ABPPk in primary dopaminergic neurons. These findings are consistent with previous studies showing potent neuroprotective efficacy of ABPP in several different types of neurons injured by various toxic agents or myocardial ischemia (Shen et al., 2008, 2010, 2011, 2013; Yu et al., 2014).Moreover, we previously demonstrated that ABPPk can enhance neuronal growth in vitro and promote peripheral nerve regeneration after crush injury in vivo (Cheng et al., 2014).It should be noted that these findings, together with our current data, are all obtained from cell models. Therefore,to further ascertain the potential application of ABPPk for treating diseases associated with neuronal loss, such as PD,in vivo PD animal models should be used.
In summary, purified ABPPk fraction exhibits potent neuroprotective effects on dopaminergic neurons, suggesting that ABPPk might be a potential drug for treating neuronal loss associated with diseases such as PD. Consequently, we have an ongoing project investigating the effect of ABPPk using an in vivo PD model. The structure of ABPPk is still unknown. Thus, future research will be focused on the structure of ABPPk, which will shed light on the molecular mechanisms underlying its neuroprotective role. Moreover,recombinant ABPPk will be produced by genetic engineering to pave the way for its clinical application.
Author contributions: XSG conceived the study and provided ABPPk.CS designed the study, analyzed data and wrote the manuscript. SP, LX and JYM performed the experiments. All authors approved the final version of the paper.
Conflicts of interest: The authors declare that they have no competing financial interests.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81471037, 81770841; a grant from the Basic Research of Jiangsu Education Department of China, No. 14KJA180006;a grant from the Six Talent Summit Project of Jiangsu Province of China,No. SWYY-051; a grant from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China. The funders had no involvement in the study design; data collection, analysis, and interpretation;paper writing; or decision to submit the paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers: Kun-Che Chang, Stanford University, USA; Puneet Bagga, University of Pennsylvania Perelman School of Medicine, USA.
- 中国神经再生研究(英文版)的其它文章
- The unfolded protein response signaling and retinal Müller cell metabolism
- Sequencing of high-efficacy disease-modifying therapies in multiple sclerosis: perspectives and approaches
- Targeting prion-like protein spreading in neurodegenerative diseases
- Cadmium-induced neurotoxicity: still much ado
- Analysis of the traf ficking system in blood-brain barrier models by high content screening microscopy
- Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death