Optimization of the Gram Staining Method Based on Superparamagnetic Magnetic Nanobeads
Yuren Feng,Zihe Ren,Fuhua Wu,Annika Reichert,Yun Lu
School of Environment,Tsinghua University,Beijing,100084,China
Keywords Magnetic nanobeads Gram staining Adsorption
Abstract Gram staining is a widely used method for bacterial identification,where an alcohol burner is used as the fixed step,which requires a high level of proficiency,while the open flame is a potential fire hazard.A rapid,convenient and alternative method is also available that does not require a microscope or alcohol burner.This method utilizes the adsorption capacity of magnetic nanobeads.Ferriferous oxide beads are made by means of the co-precipitation method and adsorb bacteria in suspension liquid after being coated on the surface.The staining process omits the “heat”step and the need for a microscope,and distinguishes bacteria through the observation of the color of the liquid.According to our results,the behavior of this magnetic material performs well with various types of microbes.In particular,the color is clear and is easily distinguishable.
1 Introduction
Gram staining is a widely used staining and identification method in bacteriology.Given that the refractive index of bacteria is similar to the surrounding air,it is nearly impossible to observe bacterial structure under a microscope without staining.Identification can only be achieved after staining.This is because the cytoplasm in its entirety is colored,and the detailed structure of bacteria can be clearly observed with the naked eye.In order to achieve the “ fixed”step which immobilizes bacteria on the slide,the traditional the Gram staining method requires an alcohol burner to dry bacteria by heat and a microscope to examine the bacteria,which increases the operational complexity.Microbe concentrations in the flooding liquid on the slide decrease due to the temperature of the flame,which is difficult to control and produces uncertain results.Moreover,the timing used for the fixed step is only determined by the intuition and experience of the operator.
Microbial smear is also randomly made.Therefore,a balanced or uniform pattern formation of dry bacteria on a slide is difficult to achieve.These factors can lead to inaccuracy,which could subsequently influence results.A Japanese study found that coated magnetic nanobeads can adsorb bacteria in water(Akikazu and Takashi,2012).In addition,a Norwegian study also found that nanoscale polystyrene beads can be used as an alternative method to identify different microbes(Yazdankhah et al.,2001),and this suggests that effective microbial immobilization can be achieved through the utilization of their adsorption onto such nanobeads.Thus,an alcohol burner is no longer necessary to achieve the same objective.Sizemore et al.(1990)provided an alternative method to stain bacteria using fluorescence labels;nevertheless,the method still requires the use of a microscope.However,bacteria can be identified without using a microscope through a chemical reaction following the disruption of the cell wall by observing the color of the cytosol released.A comparison between the traditional method and the alternative optimization method is provided in Table 1.
Nanobeads possess unique qualities,such as good affinity,dispersibility and superparamagetism,that are also able to carry organic material after being coated,and are widely used in the biochemical,medical and materials science fields.Conditions of preparation are convenient and the fabrication cost is low,while synthesis can be achieved in a common laboratory.Such self-manufactured nanobeads can be used in situations where the physical condition requirements of beads are low,such as microbial adsorption experiments conducted during student research training exercises.Li et al.(2012)reported that coated nanobeads had a higher adsorption rate compared to non-coated nanobeads;thus,this study attempted to self-manufacture coated nanobeads to con firm their adsorption capacity.
The process optimization of this study used nanobeads as the main body of adsorption and synthesized ferriferous oxide through the co-precipitation(CPT)method in order to preserve nanobeads over time.A dispersing agent was added in case nanobeads reunited.According to the chemical method,nanobeads were coated with silicon(Si)to enlarge their adsorption area.The culture solution was therefore adsorbed and then the nanobeads were stained.Staining conditions were optimized to achieve a more accurate,easy and rapid process of identification.
2 Materials and methods
2.1 Materials
We obtained iron(II)chloride tetrahydrate(FeCl2·4H2O)(analytical reagent(AR)grade)and iron(III)chloride hexahydrate(FeCl3·6H2O)(AR grade)from the XiLong Chemical Engineering Ltd.,China;ammonia(NH3)and ethyl orthosilicate(TEOS)were from the Beijing Chemical Factory(China);anhydrous ethanol(also referred to as absolute ethanol)and tetramethylhydroxide(TMAH)were from the Beijing Tong Guang Fine Chemical Co.,China.For the instruments required for this experiment,we used a fourier-transform infrared spectrometer(FTIR)from Thermo Fisher Scientific(USA);a nanoparticle size and zeta potentiometer from Beckman Coulter Inc.;a DJ1C-40 electric stirrer from Longer Pump Co.,Ltd;a precise peristaltic pump BT100-2J loaded with a YZ1515X and a Vortex 3000 Oscillator from Wiggens Co.
The experimental bacteria and the dye were manufactured in our laboratory and at the Environmental Microbiology Laboratory of Tsinghua University.
2.2 Methods
Manufactured magnetic beads:
Fe3O4beads were manufactured using the CPT method,where the main chemical equation used is as follows:
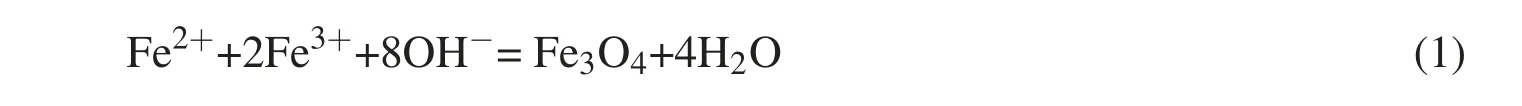
This study applied the knowledge and optimization experience obtained by Zhang(2004)and Li(2012).Following their suggestions,we separately prepared 0.05 mol/L FeCl2and 0.15 mol/L FeCl3solutions at a 1:1 ratio,which provided 50 mL of each solution that was then stored in a three neck flask at room temperature(25°C)under N2protection.The solutions were stirred while NH3was added(dropwise)until it reached a pH level of 10.Following this,the solutions were mixed in a water bath at 70°C for 30 min until fully cured Fe3O4particles were obtained.Nanobeads were then rinsed in ionized water to achieve a neutral pH level under an external magnetic field.Magnetic nanobead powder was made following drying.

Table 1 Comparison between the traditional method and the optimized method.
Dispersion and coating:
Nanobeads were suspended in water in a conical-shaped bottle(5 mg/mL suspension),which was added with the same mass fraction as the TMAH solution(Lu et al.,2006).Following this,the solution was mixed for 1 h while adding a NH3mixture of TEOS,which was prepared at a 2:1 ratio(Ikner et al.,2011).After mixing,the conical bottle was placed into an external magnetic field.Nanobeads were then first rinsed with ethanol and then with ultrapure water.We determined that nanobeads were clean when no visible solids were observed in the waste liquid.The nanobeads were then dried in an oven to generate powder.
Bacterial Cultivation:
An inoculation loop was used to transfer the original bacterial culture solution by streaking the culture dish plate.Following this,the dish was placed into a thermostatic laboratory incubator at 37°C for 24 h for cultivation.A single colony was selected and transferred to a 50 mL centrifuge tube in a lysogeny broth(LB)medium(5 mL),and the tube was then placed in a incubator set at a temperature of 37°C,at 180 RPM,for 18 h.The bacterial suspension stage was then assumed complete.
Staining and identification:
We weighed out 2 mg of Fe3O4/SiO2magnetic beads into a 5 mL centrifugal tube,and then added 1.2 mL of the bacterial cultivation solution and fully washed the solution after the shaking procedure was complete.Following this,we added 100µL of crystal violet and stained beads in the dye solution on a rotary instrument for 60 s,after which we washed the nanobeads in ultrapure water three separative times before adding 100µL iodide fluid and repeating the procedure,namely,staining nanobeads in the dye solution on a rotary instrument for 30 s before washing in ultrapure water three separate times.The next step was to add a 100µL 95%ethanol solution,which was oscillated and decolonized on a rotary instrument for approximately 20 to 25 s before washing to the colorless stage.Following this,we diluted the safranin solution to a one-quarter concentration of the original solution and added 100µL before shaking the dye for 30 s and washing it to nearly colorless.Finally,we added 2 mL of anhydrous ethanol(absolute ethanol)and oscillated the solution to observe whether we could determine the presence of dark purple coloring in the clear liquid that indicated the presence of Gram positive bacterium.Red or pink coloring indicated the presence of Gram negative bacterium,while no color indicated no bacterium.
3 Experimental results
The diameter of magnetic beads is an important physical index by which its properties can be determined.When the magnetic beads are prepared by means of CPT,any small changes in reaction parameters can significantly affect the bead diameter;thus,it is important to determine a suitable set of reaction conditions.Using the knowledge obtained by a previous study(Ikner et al.,2011),changes in conditions,such as concentrations of Fe2+/Fe3+ion ratios,NH3and dispersants,will result in different particle sizes.
Fe2+/Fe3+ion ratio concentration effect:
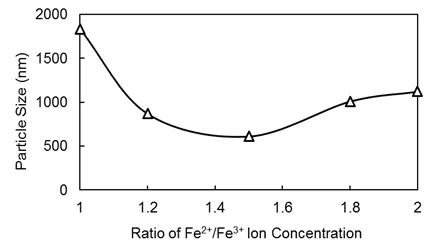
Fig.1 Effects of the Fe2+/Fe3+ratio concentration on particle size.
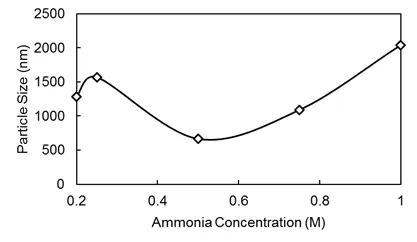
Fig.2 Effects of the ammonia concentration on particle size.
In this study,the stoichiometric ratio of the equation was set at the upper limit.Given that some Fe2+is oxidized in the air,the lower limit of Fe2+is the same as Fe3+.We gradually increased Fe3+input ratios,namely,1:1,1.2:1,1.5:1,1.8:1 and 2:1,and then examined the particle size of magnetic beads for all five ratios under the same experimental conditions(see Fig.1).
Ammonia concentration effect:
The NH3concentration determined the instantaneous rate of the OH-concentration in the solution,namely,a high concentration results in the formation of ferric hydroxide(Fe(OH)3)precipitation,while a low concentration prolongs the reaction time,increasing the possibility of Fe2+oxide and NH3metamorphism,subsequently influencing the magnetism of the nanobeads.In our experiment,we selected 0.2,0.25,0.5,0.75 and 1 mol/L to observe the effect of changing NH3concentrations on the particles of the magnetic nanobeads(see Fig.2).
Dispersant concentration effect:
Without a dispersant,our self-manufactured magnetic nanobeads would be difficult to disperse and,therefore,would be prone to agglomerate,subsequently increasing the particle size.TMAHis an excellent dispersant.In our experiment,we used 0%,1%,2%,4%and 8%TMAH to compare the effects of changing dispersant particle concentration on the magnetic nanobeads(see Fig.3).
Results showed that the particle size was smallest when the Fe3+/Fe2+ion concentration ratio was 1.5:1 and the NH3concentration was 0.5 mol/L.The quantity of the dispersant and particle size con firmed a negative correlation;however,a decrease in particle size did not continue when the mass fraction was greater than 1%.Thus,the particle size was smaller when the fraction was 1%.Under such reaction parameter conditions,the particle size of magnetic nanobeads can reach approximately 10∼102nm.
Cost analysis:
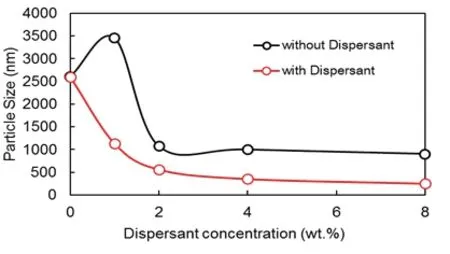
Fig.3 Effects of the dispersant concentration on particle size.
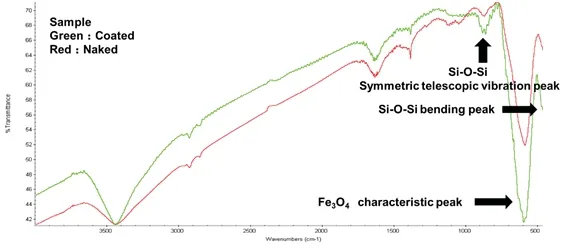
Fig.4 Infrared spectrum of naked and coated magnetic beads.
It is necessary to make a cost analysis of self-manufactured magnetic nanobeads in a laboratory environment.The total cost was divided into four parts:chemical cost,instrument cost,dye cost and other costs.The chemical cost was as follows:500 g FeCl2cost$3 USD(1 USD=∼6.0 RMB).The remaining cost conversions followed the same pattern,namely,500 g FeCl3cost$6.50 USD,500 g NH3cost$1.67 USD,500 g anhydrous ethanol(absolute ethanol)cost$1.8 USD,100 g tetramethylammonium hydroxide cost$13.3 USD,500 g ethyl silicate cost$5 USD.A one-time manufacturing process requires 1 g,2 g,2.9 g,0.2 g,0.1 g and 3 g of the aforementioned chemicals,respectively.Thus,the cost of the chemicals combined is$0.017/g USD.Additionally,25 g of crystal violet cost$2.50 USD,although the cost could be compounded when purchasing the standard size solution(1250 mL).The standard safranin dye cost is$4.17 USD,and 500 ml of Gram’s iodine cost$3.33 USD.The cost of the dyeing liquid for one experiment is$0.003 USD.The three neck flask is the only instrument cost;however,after approximately four to six usages,the inner surface became stained with residue,which was difficult to remove,requiring it to be replaced.The remaining equipment does not require any input costs.Therefore,one three neck flask that can be utilized an average of five times at a cost of$13.3 USD will cost$2.67 USD per experiment.
As it pertains to dye and water costs,a single preparation produces 5 g±0.2 g nanobeads,which costs approximately$3.33 USD,while the identification of microbes requires 2 mg/each time,and,taking loss and error into account,the one-time identification cost would therefore be$0.003 USD,which is a cost-effective alternative.
FT-IR Result Analysis:
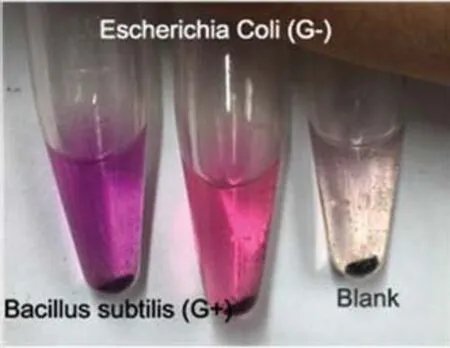
Fig.5 Staining results of model bacteria.
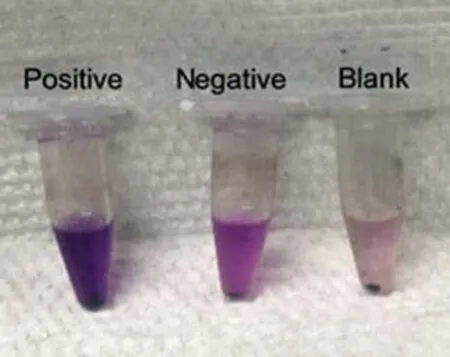
Fig.6 Staining results of other:Positive,Bacillus subtilis(G+);Negative,Escherichia coli(G-);Blank,no bacteria present.
Fig.4 provides a near-infrared spectra of Fe3O4coated by SiO2.This figure shows that the bending peak of Si-O-Si is 4 460 cm-1,the characteristic peak of Fe3O4is 580 cm-1,and the symmetric telescopic vibration peak of Si-O occurred at wavenumber 800 cm-1.The characterization of the infrared spectrogram indicated that SiO2had been successfully coated to the Fe3O4magnetic nanobead surface through a chemical reaction.
4 Staining results
Model bacteria staining result:
For our experiments,we selected two model bacteria,namely,the well-developed X3(positive,not sequenced)and the X5(negative,Klebsiella pneumoniae,Klebsiella root subsp.Root MGH 78578),and they were joined to the blank group(with microbes using equal amounts of ultrapure water),while the remaining steps were the same.Dyeing was conducted according to the above process in accordance with the X3,X5,blank group order from left to right.Results are provided in Fig.5.
In Fig.5,the left flask displays a purple color,the middle displays a red color,and the right flask displays a very pale pink color.In view of the fact that magnetic nanobeads may potentially adsorb the safranin dye solution,we concluded that the left flask was Gram positive,the middle flask was Gram negative and the right flask contained no bacteria.This is consistent with known facts and con firms that this experimental staining test method can produce effective discrimination results by means of color alone.
Other bacterial staining result:
A study by Qiu et al.(2006)indicated that the magnetic nanobeads have the capacity to adsorb various bacteria,such as Escherichia Coli,etc.Relevant experiments have stained some common microbes,such as E.Coli(G-),Bacillus subtilis(G+),Staphylococcus aureus(G+)and Enterococcus Faecalis(G+).Staining results were consistent with known facts,and they con firm the accuracy of this method.Results are shown in Fig.6.
Blind experiment staining result:
In order to apply this method to current scientific research,it is necessary to simulate real-world conditions and conduct tests under blind experiments.Namely,bacteria were randomly selected from the microbe pool(not excluding the potential of repeating the same bacteria),and the selection scheme was recorded.Bacteria were sent to testers who were not informed as to the content of samples(blind),and the samples were then identified according to the above process.The final test results were then compared to the selection scheme to verify the accuracy and feasibility of this method.One test result is provided in Fig.7.

Fig.7 Staining results from the blind experiment:No.1,Bacillus subtilis(G+);No.2,Escherichia Coli(G);No.3,Enterococcus Faecalis(G+);No.4,blank.
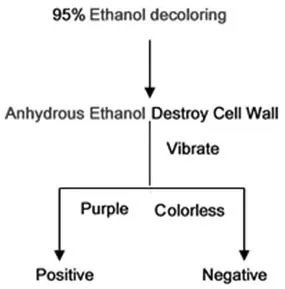
Fig.8 Optimized staining process(bacteria con firmed).
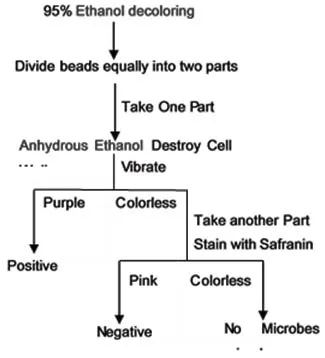
Fig.9 Optimized staining process(bacteria unknown).
5 Discussion and optimization
Blank group color phenomenon:
The phenomenon of the very pale pink color in the blank is due to the adsorption effect of magnetic nanobeads on the dye solution and the long settling time of the dye solution caused precipitation,whereby the small dye particles block the magnetic nanobead layer and affect color rendering.It is difficult to wash out dyed liquid particles using only a limited number of washings,which consequently led to the very pale pink color of the blank control group.The crystal violet that adsorbed onto the magnetic beads will be removed during the decolorizing step.Furthermore,given that the very pale pink color does not affect the dyeing conditions of bacterial resumption on the magnetic beads,we did not find it necessary to remove the color.In this study,we provided two optimization steps,using a 0.45 mm filter membrane to filter the dye solution,removing solid particles,and increasing the washing time to clean dyes more effectively.In addition,reducing dye concentrations,which reduces the amount of dye molecules entering into the centrifuge tube,can also diminish the influence of dye adsorption on color.
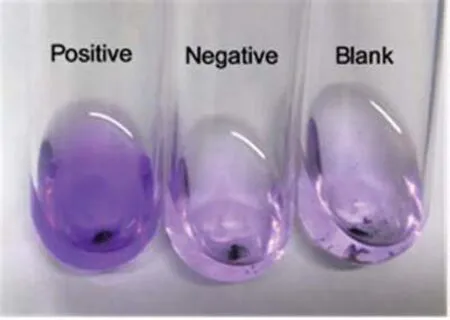
Fig.10 Staining results from optimized steps.
Dye Optimization
An effective way to determine whether bacteria are present in culture liquid is to observe the transparency daily.This study provides a faster detection method when the minimum value of bacterial concentration is detectable(con firmed)in the culture liquid.Previous steps are the same as the process discussed above,namely,after the decolorizing step,omit the re-dyeing step,directly add anhydrous ethanol(absolute ethanol)to destroy cell walls and allow the out flow of cytoplasm.Following this,observe the color.If the color is dark purple,it is G+(Gram positive),if colorless,it is G-(Gram negative).There are also processes designed to test culture liquid when uncertain whether bacteria are alive.Two specific processes are shown in Fig.8 and Fig.9.Test results are shown in Fig.10.
Optimization of weighing nanobeads:
According to tester feedback,weighing nanobeads accurately on a milligram scale is difficult.However,we provide a method to measure nanobeads on a miniscule scale.Because the diameter of nanobeads are between 10∼102nm,they belong to colloidal dispersion,which can disperse stably in water.Testers can convert weight measures into volume by mixing nanobeads with water.For instance,if 2 mg of nanobeads are required,100 mg of nanobeads should be weighed out and mixed with 1000 mL of water and stirred until it forms a colloidal solution.Following this,absorb 20 mL of the solution using a pipette.By pouring out water under external magnetic control,the correct weight of nanobeads will remain in the container.
6 Conclusions and expectations
The experiment conducted by this study was convenient and easy to carry out,and it can be run without the need of a fire source or electricity.Moreover,its overall cost is low,its experimental duration is short and it requires less instrumentation to perform.Furthermore,results from the experiment were clear and easy to analyze,and the magnetic nanobeads can be reused after washing.It is also a suitable technique to use to introduce and perhaps popularize in teaching experiments designed for college students.
This method can be applied to scientific research by designing a reagent kit for the same experimental purpose.The magnetic nanobeads and requisite dyeing liquid can be packaged into several disposable doses,and scientists can familiarize themselves with this new method by following a complementary instruction book.
Acknowledgments
This work was supported by the National Key R&D Program of China(2016YFC0401405)and the National Key R&D Program of China for International Science&Innovation Cooperation Major Project between Governments(2016YFE0118800).
 Journal of Environmental Accounting and Management2018年3期
Journal of Environmental Accounting and Management2018年3期
- Journal of Environmental Accounting and Management的其它文章
- Impact of Climate Change Disclosure on Financial Performance:An Analysis of Indian Firms
- Estimates of the Effectiveness for Urban Energy Conservation and Carbon Abatement Policies:The Case of Beijing City,China
- The Evaluation of Forest Cultural Value Based on WTP:A Case Study in Diebu County of Gansu Province in China
- Audit Judgment Performance:The Effect of Performance Incentives,Obedience Pressures and Ethical Perceptions
- A Simulation Approach to Understanding The Effect of Mimicry on Prey’s Flourishing When Predators Decline Due to Environmental Disturbance
- Modeling of a Small Scale Wind Turbine for Water Pumping Process:Case Study
