Shenxian Shengmai Oral Liquid for treatment of slow arrhythmia: a randomized controlled trial
Jing Li, Chen Zhao, Jing Chen*, Yan-Wei Xing, Ke Qian, Zhi Liu, Jing-Bo Zhai, Yang Shi
1Key Laboratory of Chinese Internal Medicine of MOE and Beijing, BUCM, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China. 2Tianjin University of Traditional Chinese Medicine, Tianjin, China. 3Guang’an Men Hospital, China Academy of Chinese Medical Sciences, Beijing, China. 4Shandong Buchang Pharmaceutical Co.,Ltd., Shandong, China.
Abstract Background: Slow arrhythmia, a common clinical condition, has a higher incidence in the elderly population. Shenxian Shengmai Oral Liquid has been shown to improve the symptoms of patients with slow arrhythmia in many clinical studies and systematic reviews. Sixty participants with slow arrhythmia will be randomized to treatment group (Shenxian Shengmai Oral Liquid) and control group (Shenxian Shengmai Oral Liquid Placebo) in a 2:1 ratio. This clinical trial is a pilot study to compare the effects of Shenxian Shengmai Oral Liquid and the control groups; to analyze the effect of Shenxian Shengmai Oral Liquid for slow arrhythmia.
Keywords: Slow arrhythmia, Shenxian Shengmai Oral Liquid, Clinical trial
Background
Slow arrhythmia is characterized by a heart rate of <60 beats/min in resting and awake states [1]; it is a common clinical disease, with a high incidence in the elderly population [2]. The progress of modern medicine in the development of treatment for slow arrhythmia has been slow. Generally, atropine, salbutamol sulfate tablets,aminophylline, and isopreterenol are used for clinical treatment [3, 4]. However, these drugs are unsuitable for long-term use as they cause adverse reactions and have a short duration of efficacy. Therefore, a pacemaker is the most common treatment method for slow arrhythmia [5,6].
The symptoms of slow arrhythmia are palpitation,chest discomfort, syncope, severe palpitation, and vertigo in traditional Chinese medicine (TCM). Shenxian Shengmai Oral Liquid, produced by the Shandong Pharmaceutical Co., Ltd [7], is the first patented Chinese medicine. Shenxian Shengmai Oral Liquid includes Ginseng Radix Et Rhizoma Rubra (Hongshen),Epimedii Folium (Yinyanghuo), Psoraleae Fructus(Buguzhi), Lycii Fructus (Gouqizi), Ephedrae Herba(Mahuang), Asari Radix Et Rhizoma (Xixin), Salviae Miltiorrhizae Radix Et Rhizoma (Danshen), and Hirudo(Shuizhi) [8]. It can invigorate heart and kidney and promote blood circulation for removing blood stasis [9].
Chinese medicine is a favorable option for the treatment of slow arrhythmia. In recent years, clinical trials and post-marketing evaluation have been conducted,and Chinese medicine has gradually been recognized and accepted by the world. But the Chinese medicine effective constituents are complex. Thus, their use is often questioned [10, 11].
Shenxian Shengmai Oral Liquid has been shown to improve the clinical symptoms of slow arrhythmia by many clinical studies and systemic reviews [12-14].Therefore, this study was designed to test the efficacy and safety of Shenxian Shengmai Oral Liquid in the treatment of slow arrhythmia.
Methods
Objectives
To design a pilot study to investigate the efficacy and safety of Shenxian Shengmai Oral Liquid in the treatment of slow arrhythmia.
Trial design
Research type.The study will be a multicenter, double blind, placebo parallel control, randomized controlled clinical trial, with a study term of 12 weeks.
Allocation ratio.Sixty participants will be randomized to treatment group and control group. Participants will be recruited from two Chinese cities, Beijing and Tianjin.
The treatment group: Shenxian Shengmai Oral Liquid,n = 40.
The control group: placebo, n = 20.
Recruitment.The hospital has launched a campaign of television commercials and leaflets to recruit participants.
Screening of participants.The inclusion criteria for the study are participants diagnosed with slow arrhythmia.The primary outcome index includes Holter. Whether the heart rate rises according to the holter [15]. The secondary outcome indices include: evaluation of TCM syndrome and score of single TCM symptoms. The safety indices include: general medical examinations such as temperature, heart rate, blood pressure, and breathing rate;regular blood and urine tests; electrocardiogram; liver function (ALT, AST, r-GT, Tbil, and ALP) and renal function tests (BUN and creatinine); and determination of incidence of adverse reactions.
The information that will be captured is shown in Table 1.

Table 1 Data capture

×:Item required to be assessed during a different period. ICF: Inform consent form.
Interventions
Study Interventions. The participants will be randomly assigned to two groups in a 2:1 ratio. All the study drugs will be orally administered for 12 weeks.
The treatment group will receive Shenxian Shengmai Oral Liquid at 20 ml twice daily.
The control group will receive Shenxian Shengmai Oral Liquid Placebo at 20 ml twice daily.
Concomitant medications.Western medicine and Chinese medicine will be forbidden for treatment of arrhythmia during the follow-up period. Coronary heart disease, blood pressure, and lipid-lowering, and anti-platelet drugs will be unchanged. Throughout the study, the investigators will record the use of all concomitant medications in detail on the case report form.
Randomization and blinding.The statisticians will use the SAS 9.1.3 software to generate random numbers according to the randomization method. The patients will be randomly assigned into the experimental and control groups by using a computer-based random number generator in a 2:1 ratio.
The study will have a double-blind design; the participants, investigators, and statisticians will all be blind to the group allocation. We will produce Shenxian Shengmai Oral Liquid and Placebo. The placebo will be identical to the real medication in shape, size, taste, color,and packages. The packages for both the dummy agents and real drugs will be identical. Drug packaging will be executed by the personnel responsible for blind coding and personnel from the sponsor unit, neither of whom will have a relationship with the trial.
Sample size.Sample size was based on the study-related literature, experts’ opinions, and previous study results[16, 17]. The significance level will be α = 0.05, and the power will be (1-β) = 0.8. Adjusting for 10% loss, we will need a sample size of 40 participants in treatment group and 20 participants in control group [18]. Thus, the total number of participants will be 60.
Outcome measures
Outcome indices. Holter; Evaluation of TCM symptoms;Single TCM symptoms.
Indices of safety.Vital signs, such as temperature, heart rate, blood pressure, and breathing; Regular blood and urine tests; electrocardiogram, liver function (ALT, AST,r-GT, Tbil, and ALP) and renal function (BUN and creatinine) test; Incidence of adverse reactions.
Inclusion and exclusion criteria.
These are presented in Table 2.
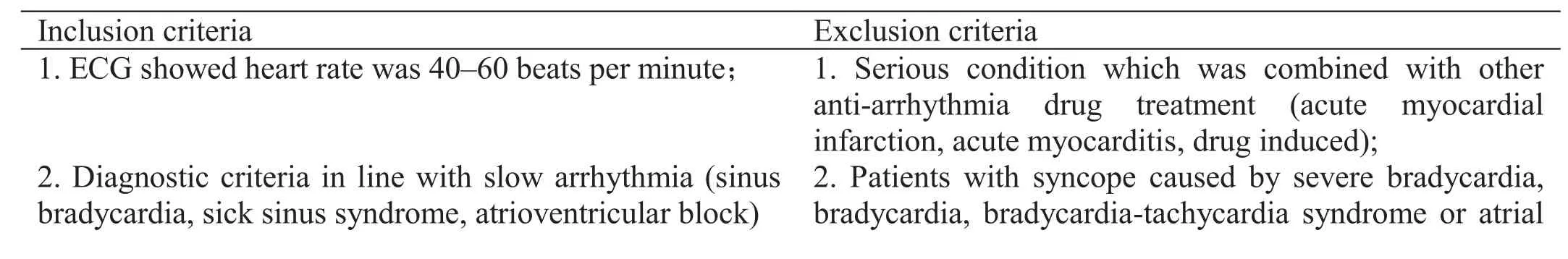
Table 2 Inclusion and exclusion criteria
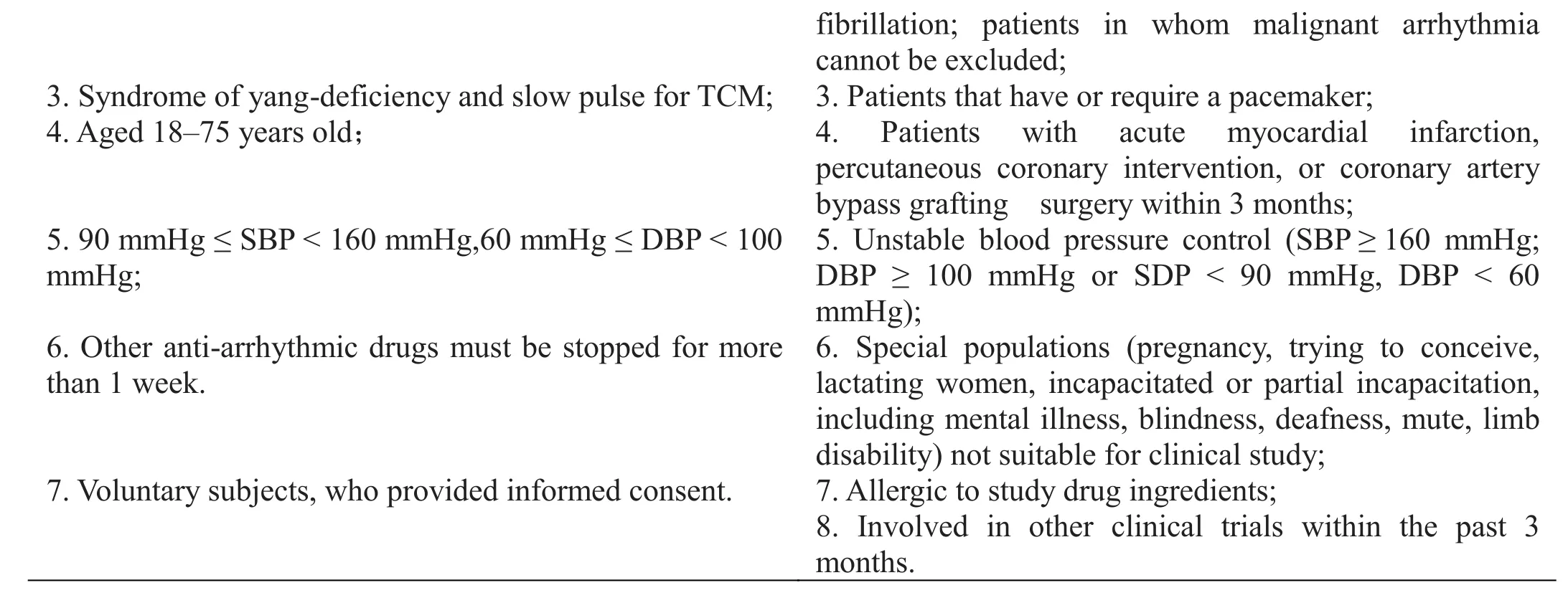
fibrillation; patients in whom malignant arrhythmia cannot be excluded;3. Syndrome of yang-deficiency and slow pulse for TCM; 3. Patients that have or require a pacemaker;4. Aged 18–75 years old; 4. Patients with acute myocardial infarction,percutaneous coronary intervention, or coronary artery bypass grafting surgery within 3 months;5. 90 mmHg ≤ SBP < 160 mmHg,60 mmHg ≤ DBP < 100 mmHg;5. Unstable blood pressure control (SBP ≥ 160 mmHg;DBP ≥ 100 mmHg or SDP < 90 mmHg, DBP < 60 mmHg);6. Other anti-arrhythmic drugs must be stopped for more than 1 week.6. Special populations (pregnancy, trying to conceive,lactating women, incapacitated or partial incapacitation,including mental illness, blindness, deafness, mute, limb disability) not suitable for clinical study;7. Voluntary subjects, who provided informed consent. 7. Allergic to study drug ingredients;8. Involved in other clinical trials within the past 3 months.
Adverse events.An adverse event will be defined as any untoward medical occurrence that may present itself during the conduct of the study, which may or may not have a causal relationship with the study procedures.
Severe adverse events.A serious adverse event will be defined as an adverse event resulting in death, a lifethreatening adverse experience, hospitalization, or significant disability/incapacity. Severe adverse events will be reported to the institutional review board and/or independent ethics committee and the principal investigator within 24h [19].
Ethics committee. The study received approval by China Ethics Committee of Registering Clinical Trial in 26 January, 2016.
Ethics statement. Researchers are responsible to ensure that the study should conduct in accordance with the principles of the Declaration of Helsinki. Participants entirely voluntarily give their written, informed consent prior to any study procedures and they can voluntarily withdraw from the study for any reason. Parents or guardians are informed the risks and benefits of the study if the participants had difficulty in decision-making. Each patient will be identified with a unique random number and the private data must be preserved by researches in order to maintain confidentiality.
Ethical approval. The study protocol, informed consent form (ICF), and other research documents were approved by China Ethics Committee of Registering Clinical Trial in January 26, 2016. [ChiECRCT- 20150054].
Inform consent form.ICF must be reviewed and approved by ethics committees before study. Researchers should inform participants of the relevant information in comprehensible language. ICF must be signed and dated by the participants or their representatives. Participants and representatives should be given an adequate time to read the information. ICF should be preserved by researchers and participants independently.lower cost, and fewer adverse events. Thus, these Chinese medicines have slowly been accepted for clinical use [20,21].
Shenxian Shengmai Oral Liquid has been shown to improve the clinical symptoms of slow arrhythmia in patients by many clinical studies. However, they also can lead to adverse reactions, such as gastrointestinal discomfort and liver damage [22, 23]. The quality of Chinese medicine clinical trials is varied, which makes international acceptance difficult.
This is a proposed pilot study to compare the effects of Shenxian Shengmai Oral Liquid and the control groups;to analyze the effect of Shenxian Shengmai Oral Liquid for slow arrhythmia; to identify the characteristics of the treatment; and to improve the quality of clinical trials of Chinese medicine for future research.
Registration number: ChiCTR-IPR-16007835.
Discussion
In recent years, in Chinese medicine, the treatment for slow arrhythmia is well established, an increasing number of treatments have been revealed with lower side effects,
Acknowledgements
The funding support of the Ten Thousand People Plan and Natural Science Foundation of China are gratefully acknowledged.
 TMR Modern Herbal Medicine2018年2期
TMR Modern Herbal Medicine2018年2期
- TMR Modern Herbal Medicine的其它文章
- Acupuncture for hyperlipidaemia in adults: A systematic review and meta-analysis
- A meta-analysis of randomized controlled trials of Yiyiren Decoction in the treatment of rheumatoid arthritis
- Chinese medicine in the treatment of intracranial vascular stenosis in 1 case
- Clinical evaluation of Xiaoyao Jieyu prescription in the treatment of persistent postural-perceptual dizziness
- Metabolites identification and quantification of antcin H in mice tumors after oral administration of the anticancer mushroom Antrodia camphorata
- Progress of Alzheimer’s disease related glucose metabolism regulating hormones and a research perspective in nootropics of herbal medicine
