Metabolites identification and quantification of antcin H in mice tumors after oral administration of the anticancer mushroom Antrodia camphorata
Zi-Wei Li, Shuai Ji, Bin Li, Shuang Wang, Yew-Min Tzeng, Xue Qiao*, Min Ye*
1State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing,China. 2Department of Life Science, National Taitung University, Taitung, Taiwan. 3Department of Pharmaceutical Analysis, Xuzhou Medical University, Xuzhou, China
Abstract Objective: Antrodia camphorata (AC), a precious medicinal mushroom in Taiwan, is popularly used for adjuvant cancer therapy. This paper aims to clarify the metabolites which are present in tumor tissues after oral administration of AC in Sarcoma-180 tumor-bearing mice, as well as their contents in tumors. Methods: Tumors of Sarcoma-180 tumor-bearing mice were obtained at 1 h and 4 h after oral administration of AC extract, and the metabolites in the tumor homogenate samples were characterized using UHPLC-orbitrap/MS analysis. Then, a fully validated LC-MS/MS method was developed for quantitative analysis of the most abundant compounds in tumor tissues, namely (25R/S)-antcin H. Results:A total of 33 compounds were characterized in tumor homogenate samples including 28 prototypes of triterpenoids and 5 metabolites. Among them, (25R)-antcin H and (25S)-antcin H had the highest contents of 2.03 and 0.66 μg/g tumor tissues for the 1 h group, and 2.04 and 0.59 μg/g tumor tissues for the 4 h group, respectively. It was obvious that(25R)-antcin H had higher tumor affinity than (25S)-antcin H, since the content of (25R)-antcin H was lower than that of(25S)-antcin H in AC extract (P < 0.01). Conclusion: Triterpenoids can enter tumor tissues after oral administration of AC. Particularly, (25R)-antcin H showed higher exposure to tumor than (25S)-antcin H. These compounds could contribute to the anticancer activities of AC.
Keywords: Antrodia camphorata, Antcin H, Metabolite, Anticancer, LC-MS/MS
Introduction
Antrodia camphorata(AC), also known asAntrodia cinnamomea, is a medicinal fungus with a long history of use in Taiwan. It is reported to possess a lot of bioactivities including anticancer, anti-inflammatory,hepatoprotective, and antioxidant activities [1-8]. During the past decades, AC has attracted many attentions as a“complementary alternative medicine” due to its potent anticancer effects, and it could provide effective and less-toxic therapies for various types of cancers [9-10].For example, AC extracts could inhibit the growth of xenografted tumors such as non-small cell lung cancer and estrogen receptor positive breast cancer [11-12], and showedin vitrocytotoxicities against ovarian carcinoma cells, HL-60 premyelocytic leukemia cells, and MCF-7 breast cancer cells [13-15]. In addition, anticancer activities of the commercial readily accessible AC products have been revealed [16–17]. Several effective anticancer compounds have been discovered from AC, so far, and (25R/S)-ergostane and Δ7,9/Δ8-lanostane triterpenoids were identified as the major bioactive constituents [18]. Yehet al.obtained three ergostane triterpenoids and five lanostane triterpenoids from AC with cytotoxicity against HT-29 human colon cancer cells[19]. Dehydroeburicoic acid, a major lanostane triterpenoid in AC, could damage the mitochondria of human glioblastoma cells and inhibit cell viability of premyelocytic leukemia cells [20-21].
In our previous study, metabolism and pharmacokinetics of AC and its major triterpenoids in healthy Sprague-Dawley rats were investigated by ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry(UHPLC-qTOF/MS) analysis, and (25R/S)-ergostanes were found to be the most bioavailable compounds in rats plasma after oral administration of ethanol extract of AC.They were usually metabolically stablein vivo[22].However, metabolism of AC and its chemical constituents in pathological animal models, such as tumor-bearing mice, remains unclear. On the other hand, tumor affinity,namely concentrations in tumor tissues, is highly critical for the clinical use of anti-tumor agents [23]. Up to now,tumor affinity of AC and its chemical constituents have never been investigated.
In this study, a UHPLC-orbitrap/MS method was used to comprehensively profile the metabolites of AC extract(ACE) in the tumor tissues of sarcoma-180 tumor-bearing mice after oral administration. Furthermore, an LC-MS/MS method was fully validated to simultaneously quantify (25R/S)-antcin H in tumor tissues.
Methods
Chemicals and reagents
The 18 reference compounds were isolated from AC by the authors [24-25]. They included 15 ergostane triterpenoids, namely (25S)-antcin K (E1), (25R)-antcin K(E2), antcamphin E (E4), antcamphin F (E6), antcamphin K (E7), antcamphin L (E9), antcin F (E12), (25S)-antcin C (E13), (25R)-antcin C (E15), (25R)-antcin H (E16),(25S)-antcin H (E17), (25R)-antcin I (E19), (25S)-antcin I(E20), (25S)-antcin B (E21) and (25R)-antcin B (E22),and 3 lanostane triterpenoids, namely dehydrosulphurenic acid (L1), sulphurenic acid (L2) and 15α-acetyl-dehydrosulphurenic acid (L3). The purities were >95% as determined by HPLC analysis. The internal standard (IS) ganoderic acid B was purchased from Zelang Co. Ltd. (Nanjing, China). Structures of reference compounds and IS are shown in Figure 1.
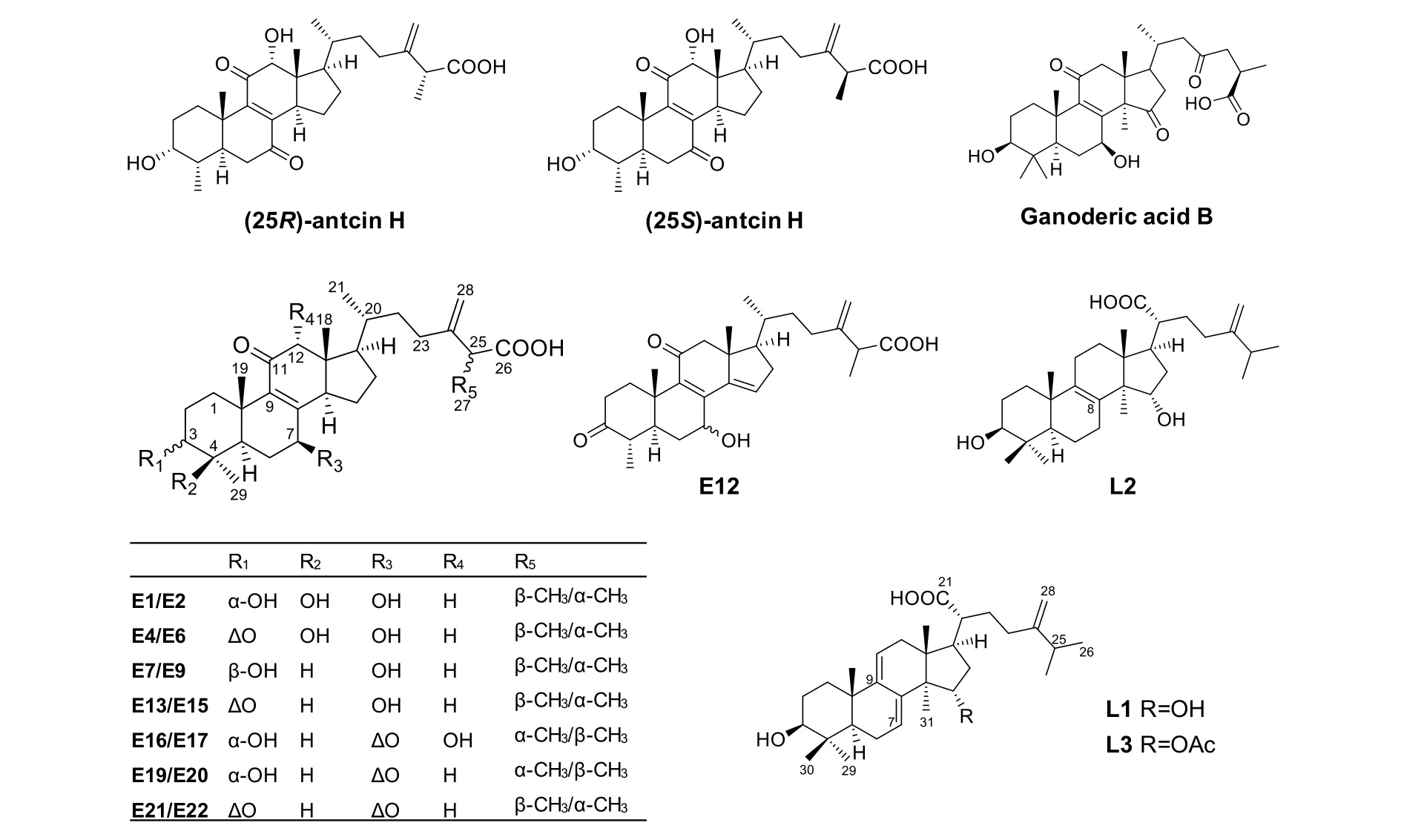
Figure 1 Structures of reference compounds and the internal standard
Medical-grade soybean oil was purchased from Maclin(Shanghai, China), and tween-80 was from Sigma (St.Louis, MO, USA). Acetonitrile, methanol, and formic acid (Mallinkrodt Baker, Phillipsburg, NJ, USA) were of HPLC grade. De-ionized water was purified by a Milli-Q system (Millipore, MA, USA). High-purity nitrogen(99.9%) and helium (99.99%) were purchased from Gas Supplies Center of Peking University Health Science Center (Beijing, China).
Preparation of ACE
The fruiting bodies of AC were cultivated by Professor Yew-Min Tzeng (National Taitung University, Taitung,Taiwan) in 2013. A voucher specimen (YMT 1002) was deposited at the Herbarium of School of Pharmaceutical Sciences, Peking University, Beijing, China. For drug sample preparation, AC fruiting bodies were powdered,and 49.91 g of the powder was extracted using 500 mL of ethanol by reflux heating (2 h × 5 times). The extracts were combined and evaporated to drynessin vacuumto produce 14.85 g of ACE.
Sarcoma-180 tumor-bearing mice model
Male ICR mice weighing 18-20 g were purchased from the Experimental Animal Center of Peking University Health Science Center. The mice were housed in a ventilated, temperature-controlled and standardized sterile animal room at 22-24 °C with a 12 h dark-light cycle, and they had free access to food and de-ionized water. S180 tumor cells (4.0 × 106per mouse) in 0.9%NaCl solution was injected subcutaneously into the right oxter of each mouse. After 10 days, when established tumors of approximately 1000 mm3were detected, mice were randomly divided into three groups (n = 8 for each group): control group, 1 h group, and 4 h group. All animals were fasted for 12 h before treatment. The animal facilities and protocols were approved by the Animal Care and Use Committee of Peking University Health Science Center, and the procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health).
Drug administration and sample collection
For oral administration, stable emulsion was prepared due to the poor solubility of ACE [26]. Briefly, 396 mg of ACE was infiltrated with 400 µL of soybean oil, mixed with 200 µL of tween-80, and then dispersed in 1600 µL of water to form stable emulsion. The final concentration of ACE in emulsion was 180 mg/mL.
Mice of the 1 h group and the 4 h group were orally administered with ACE emulsion at the dose of 10 mL/kg(equivalent to 6.16 g/kg of the crude drug). Mice of the control group were given an equal volume of the vehicle.The 1 h group and the 4 h group were sacrificed at 1 h and 4 h after administration, respectively, and the control group was sacrificed at 0 h. The tumors were collected and washed in PBS carefully to discard blood and muscle.Finally, they were accurately weighed and homogenized by a homogenizer after addition of 0.9% NaCl solution to obtain uniform concentration (1 mL/g tissue). The samples were stored at -20 °C until use.
Calibration standards, quality control, and internal standard stock solutions
The stock solutions of (25R)-antcin H (1 mg/mL),(25S)-antcin H (0.5 mg/mL) and ganoderic acid B (0.75µg/mL) were prepared in methanol and stored at -20 °C.These stock solutions were mixed and then serially diluted to obtain the calibration standard (CS) stock solutions (6.0, 2.0, 1.2, 0.4, 0.24, 0.08, 0.048, 0.016 and 0.0096 µg/mL for (25R)-antcin H; 3.0, 1.0, 0.6, 0.2, 0.12,0.04, 0.024, 0.008 and 0.0048 µg/mL for (25S)-antcin H).Quality control (QC) samples were prepared at three concentration levels based on linear ranges of the analytes and their predicted concentrations, namely 4.0 µg/mL(HQC), 0.2 µg/mL (MQC) and 0.02 µg/mL (LQC) for(25R)-antcin H, as well as 2.0 µg/mL (HQC), 0.1 µg/mL(MQC) and 0.01 µg/mL (LQC) for (25S)-antcin H.
Sample preparation
For metabolites identification, 500 µL of tumor homogenate was added into 1500 µL of methanol and centrifuged at 9000 rpm for 15 min. The supernatant was evaporated to dryness under a gentle flow of nitrogen gas at 35 °C, and the residue was resolved in 100 µL of methanol. After filtered through 0.22 µm nylon membranes, a 2-µL aliquot was injected for UHPLC-orbitrap/MS analysis.
For CS and QC samples for quantitative analysis, 100µL of CS or QC stock solution was added into 100 µL aliquot of blank tumor homogenate, followed by addition of 100 µL of IS solution and 100 µL of methanol. For unknown samples, a 100-µL aliquot of tumor homogenate was mixed with 100 µL of IS solution and 200 µL of methanol. The obtained mixed solutions were vortexed for 30 s and centrifuged at 9000 rpm for 15 min. The supernatant was evaporated to dryness under a gentle flow of nitrogen gas at 35 °C, and the residue was reconstituted in 100 µL of methanol. After filtered through 0.22 µm nylon membranes, a 5-µL aliquot was injected for HPLC-MS/MS analysis.
UHPLC-orbitrap/MS analysis for metabolites identification
A UHPLC Ultimate 3000 instrument coupled with a Q-Exactive hybrid quadrupole-orbitrap mass spectrometer (Thermo Fisher Scientific Inc., Waltham,MA, USA) was employed. The UHPLC was equipped with an on-line vacuum degasser, a quaternary pump, an autosampler, and a column compartment. An Acquity UPLC HSS T3 column (1.8 μm, 2.1 × 150 mm) equipped with a VanGuard precolumn (1.8 μm, 2.1 × 5 mm)(Waters, MA, USA) was used to separate the samples.The mobile phase consisted of acetonitrile containing 1%methanol (A) and water containing 0.2% formic acid (B).A linear gradient elution program was used as follows:0-5 min, 45% A; 5-6 min, 45%-55% A; 6-18 min, 55% A;18-23 min, 55%-60% A; 23-24 min, 60-95%; 24-30 min,95% A. The flow rate was 200 µL/min, and the column temperature was 40 °C. The mass spectrometer was connected to the UHPLC via an electrospray ionization(ESI) interface, and was operated in the negative ion mode. The parameters were as follows: spray voltage:-3.2 kV; sheath gas pressure: 35 arb; Aux gas pressure: 10 arb; capillary temperature: 350°C; heater temperature:300°C; scan mode: full MS (resolution 70000) and MS/MS (resolution 17500); normalized collision energy:35 eV; stepped normalized collision energy: 17.5, 35 and 52.5 eV; scan range:m/z100–1200. The multiple precursor ion function was used, where the two most abundant ions in the full scan mass spectra were selected to acquire MS/MS spectra for each cycle.
LC-MS/MS analysis for quantification of(25R/S)-antcin H
A Waters 2695 HPLC system coupled with a Waters ACQUITY TQD triple quadrupole mass spectrometer was used. Separation was achieved using an YMC-Pack ODS-A column (3.5 µm, 2.1 × 150 mm) equipped with a Zorbax SB-C18guard column (5 µm, 2.1 × 12.5 mm,Agilent, Waldbronn, Germany). The column temperature was 40 °C, and the flow rate was 200 µL/min. The mobile phase consisted of methanol (A) and water containing 0.1% formic acid (B). The following gradient elution program was used: 0-2 min, 60-65% A; 2-8 min, 65-74%A; 8-23 min, 74% A; 23-30 min, 74-100% A. The effluent was introduced into the mass spectrometer without splitting, and the mass spectrometer was operated in the negative ion mode. The parameters were as follows:capillary voltage: 2.8 kV; extractor voltage: 4.0 V; source temperature: 150°C; desolvation temperature: 450°C;desolvation gas flow: 600 L/h; cone gas flow: 50 L/h. The analytes were detected using the multiple reaction monitoring (MRM) scan mode. The MRM ion pair transitions, optimized cone voltages, and collision energies are listed in Table 1.
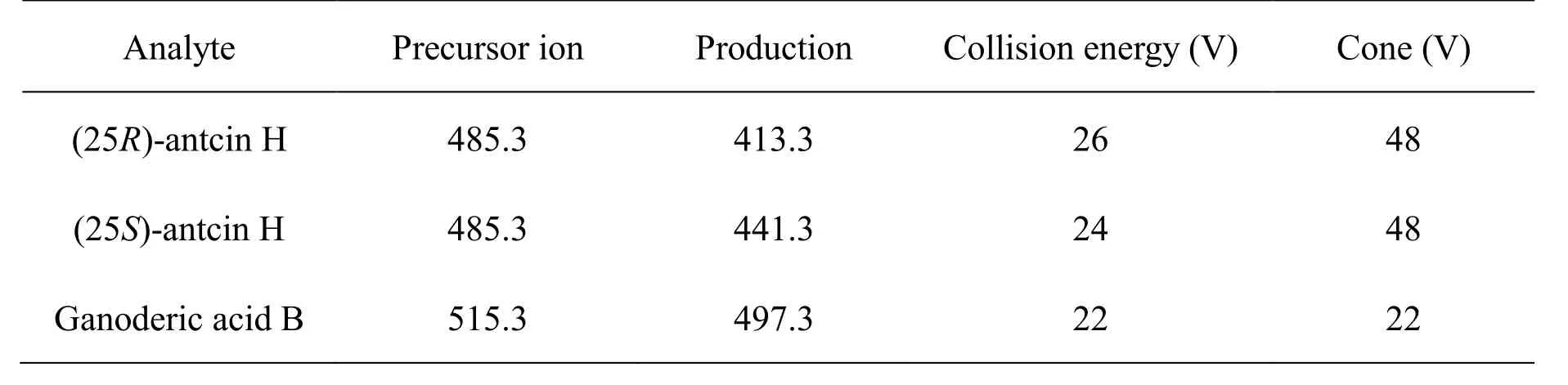
Table 1 Optimized MRM parameters for (25R)-antcin H, (25S)-antcin H, and ganoderic acid B (IS)
Results
Metabolites identification for AC in tumor tissues
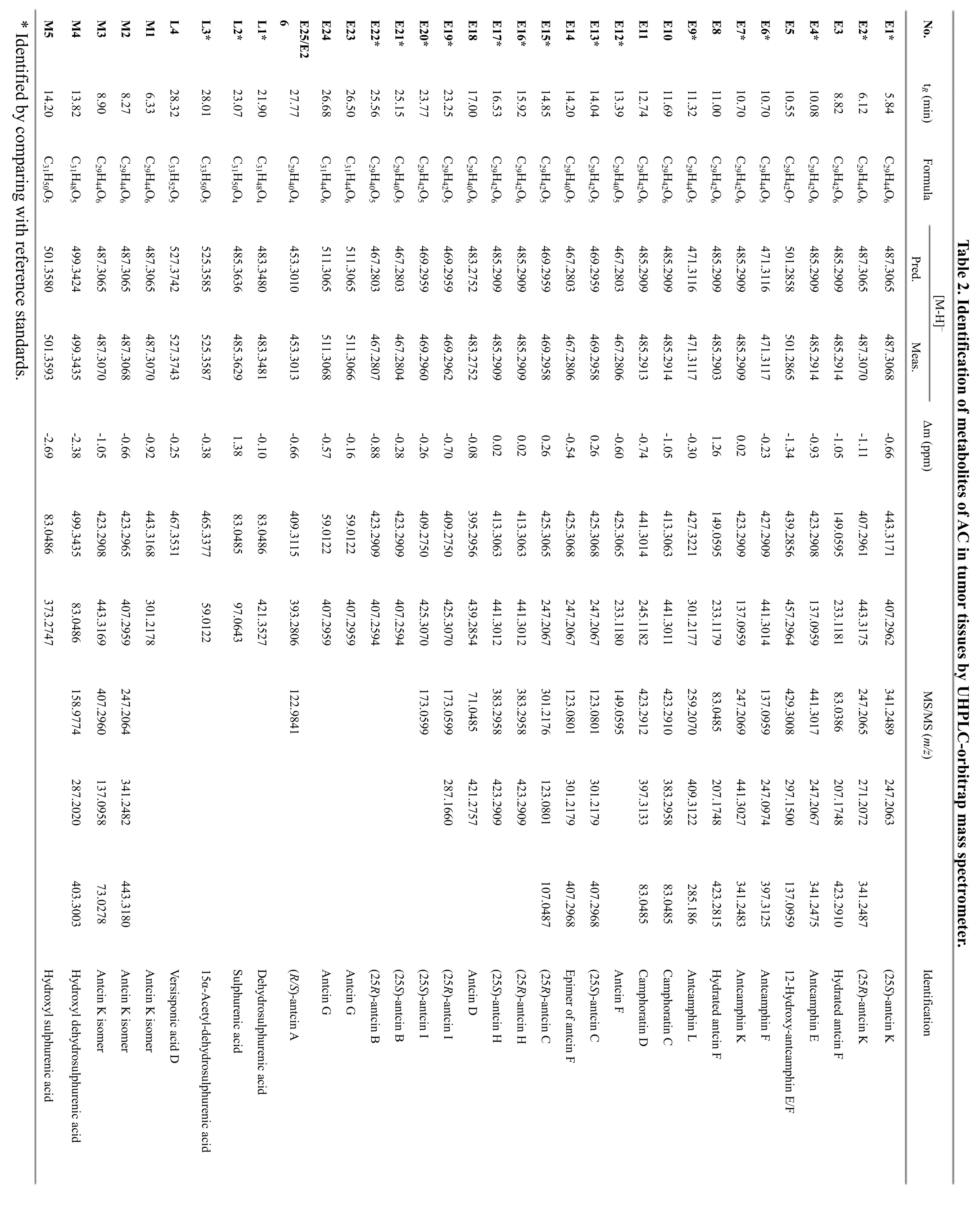
The chemical composition of ACE was firstly characterized through UHPLC-orbitrap/MS analysis. A total of 30 compounds were tentatively identified including 26 ergostane triterpenoids (E1–E26) and 4 lanostane triterpenoids (L1-L4) by comparing their MS and MS/MS data with previous reports (Figure 2). Among them, the structures of 18 compounds were confirmed by comparing with reference standards. It is obvious that(25R/S)-antcin K (E1/2), (25R/S)-antcin H (E16/17) and(25R/S)-antcin B (E21/22) were the most abundant components in ACE, which was consistent with our previous report [24].
The metabolites of ACE in tumor tissues of mice were then identified by UHPLC-orbitrap/MS analysis. The total ion chromatogram of chemical constituents in tumor tissues collected 1 h after oral administration is shown in Figure 2. A total of 33 compounds were detected, and 28 of them were present in ACE (E3 and E23 in ACE were not detected in tumor tissues). Accordingly, 5 metabolites(M1-M5) were detected, and their structures were tentatively characterized by analyzing their high-resolution MS spectra (Table 2). Metabolites M1-M3 were deduced to be the isomers of antcin K, since they showed the same [M-H]–ions as antcin K. M4 and M5, eluted at 13.82 min and 14.20 min, yielded [M-H]–ions atm/z499.3435 (C31H48O5) andm/z501.3593(C31H50O5), respectively. Their molecular weights were 16 Da greater than those of dehydrosulphurenic acid (L1)and sulphurenic acid (L2). Therefore, M4 and M5 were tentatively identified as monohydroxylated products of dehydrosulphurenic acid and sulphurenic acid,respectively.
As shown in Figure 2, the contents of most compounds were low in tumor tissues, especially those with retention times between 5 and 15 min. However, (25R/S)-antcin H had relatively high contents, and were the most abundant compounds in tumor tissues. Interestingly, there were three pairs of ergostane triterpenoids (E1/2, E16/17 and E21/22) with high contents in ACE, and only(25R/S)-antcin H (E16/17) showed high concentrations in tumor tissues, indicating they had high tumor affinity.
Method validation for quantitative analysis of(25R/S)-antcin H by LC-MS/MS
To determine the concentrations of (25R/S)-antcin H in the tumor tissues, we established a LC-MS/MS method with a triple quadrupole mass spectrometer as the detector.The separation of (25R)-antcin H and (25S)-antcin H was difficult, as they were a pair of epimers. Different chromatographic conditions (column, mobile phase,elution gradient) were tested (Figure 3). Finally, a YMC-Pack ODS-A (3.5 µm, 2.1 × 150 mm) column with methanol and water containing 0.1% formic acid as the mobile phase was chosen. For MS analysis, three pairs of MRM ion pair transitions were chosen. In addition, the methods were fully validated according to the Food and Drug Administration (FDA) guidelines for selectivity,linearity, accuracy, precision, recovery, matrix effect and stability [27].
?
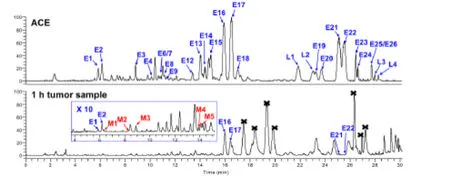
Figure 2 UHPLC-orbitrap/MS chromatograms of Antrodia camphorata extract (ACE) and tumor homogenate samples after oral administration of ACE (1 h group). Black cross mark, endogenous compounds in tumors.
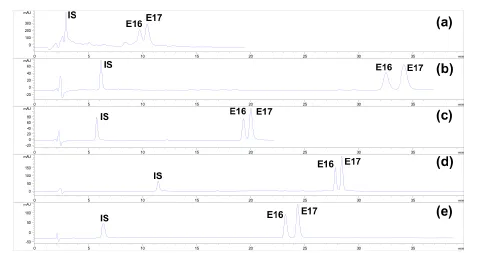
Figure 3 Optimization of the HPLC method for quantitative analysis of (25R/S)-antcin H. (a) SB-C18 (5 µm,4.6 × 250 mm) with acetonitrile and water containing 0.1% formic acid as eluent; (b) Symmetry C18 (5 µm,4.6 × 250 mm) with acetonitrile and water containing 0.1% formic acid as eluent; (c) YMC-Pack ODS-C18 (5µm, 4.6 × 250 mm) with acetonitrile and water containing 0.1% formic acid as eluent; (d) YMC-Pack ODS-C18 (5 µm, 4.6 × 250 mm) with acetonitrile and water containing 0.1% formic acid as eluent; (e)YMC-Pack ODS-C18 (3.5 µm, 2.1 × 150 mm) with methanol and water containing 0.1% formic acid as eluent.
Selectivity.The selectivity was evaluated by comparing chromatograms of blank and spiked tumor homogenate samples. The retention times of (25R)-antcin H,(25S)-antcin H and the IS were 18.76, 19.62 and 4.95 min,respectively (Figure 4), and no apparent interference was observed in the matrix. Both (25R)-antcin H and(25S)-antcin H could be detected using the two MRM ion pair transitions “485.33→441.30” and “485.33→413.28,and the two peaks were well resolved. Hence, selectivity of the method was sufficient for quantitative analysis of(25R/S)-antcin H.
Calibration and limit of detection.The calibration curves were constructed by plotting the ratio of mean peak areas of the samples to the IS against the concentration of each compound, and covered wide dynamic ranges (6.0–0.0096 µg/mL for (25R)-antcin H and 3.0–0.0048 µg/mL for (25S)-antcin H), as shown in Table 3. Both of the two calibration curves exhibited good linearity with correlation coefficients (r2) no less than 0.99. The limit of detection (LOD) was determined by injecting a series of standard solutions until the signal-to-noise ratios (S/N) were 3:1 and 10:1,respectively. As a result, the LODs for (25R)-antcin H and (25S)-antcin H were 0.0032 and 0.0048 µg/mL,respectively.
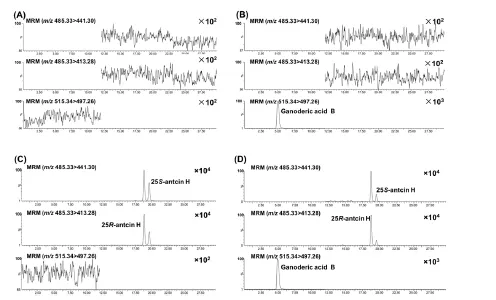
Figure 4 Representative MRM chromatograms of (25R)-antcin H, (25S)-antcin H, and ganoderic acid B (IS).(A) Blank tumor homogenate; (B) Blank tumor homogenate spiked with ganoderic acid B; (C) Blank tumor homogenate spiked with (25R)-antcin H and (25S)-antcin H; (D) Tumor homogenate samples obtained at 1 h after oral administration of ACE.
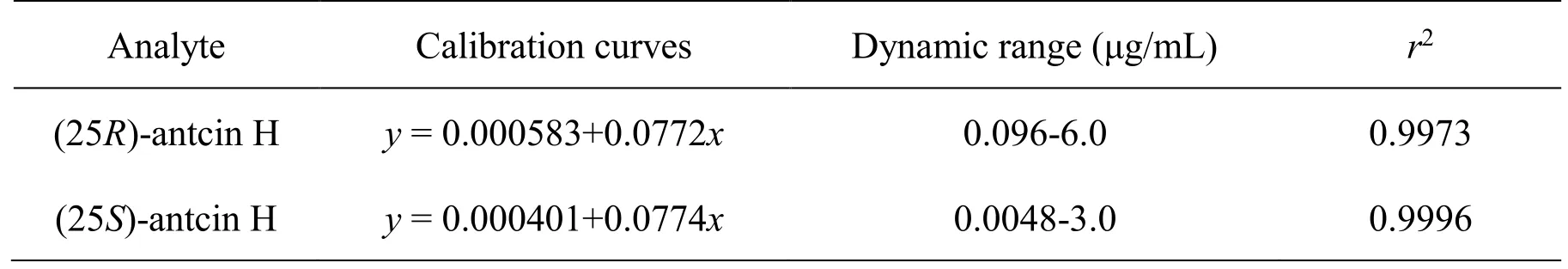
Table 3 Calibration curves for quantitative analysis of (25R/S)-antcin H.
Precision and accuracy.Intra- and interday precisions were determined by analyzing three different concentrations of QC samples (five replicates for intraday precision and three replicates for interday precision) for six times in the same day and three consecutive days,respectively. They were evaluated by relative standard deviations (RSD), which were less than 4.61% and 4.93%,respectively (Table 4). Intra- and inter-day accuracies were calculated as relative error (RE), and RE% =(measured concentration-nominal concentration)/nominal concentration × 100. As a result, the calculated accuracy values for intra- and inter-day accuracies were-12.96–12.71% and -8.48–8.25%, respectively. The above results demonstrated that the method had acceptable accuracy and precision, and met the FDA requirements for bioanalytical method validation.
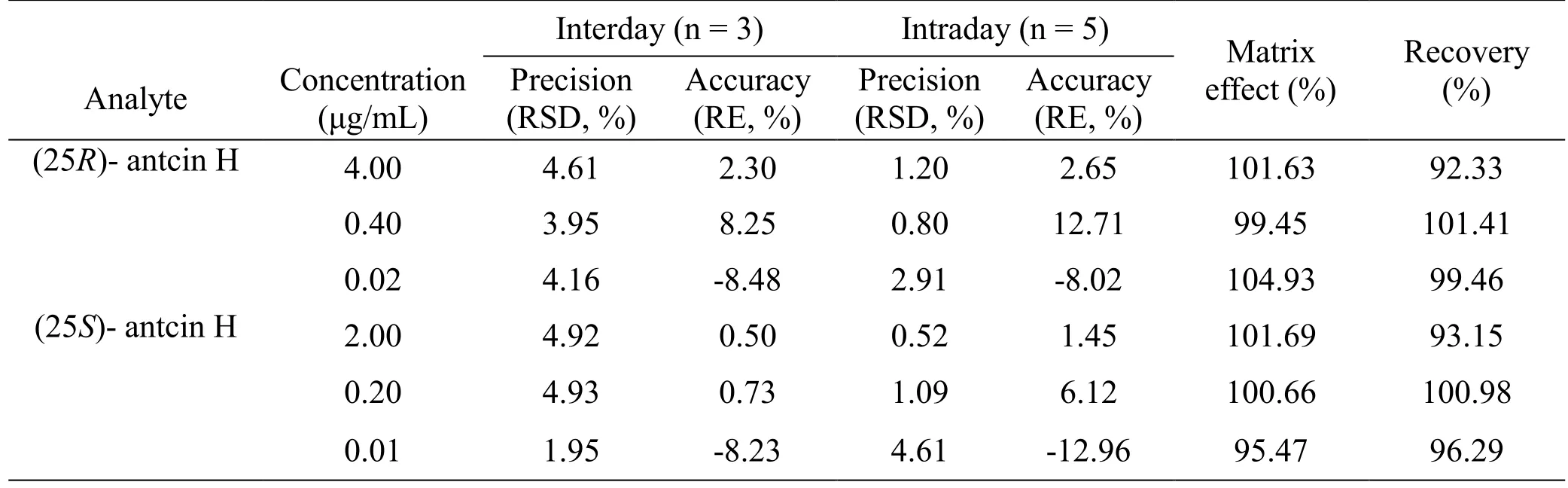
Table 4 Accuracy, precision, matrix effect, and recovery of the LC-MS/MS method
Recovery and matrix effect.The QC samples (HQC,MQC and LQC), as well as the blank tumor homogenate sample spiked QC solutions, were compared to determine the extraction recovery, which was calculated by the formula: recovery (%) = concentration found/concentration spiked × 100%. As a result, the extraction recoveries for (25R/S)-antcin H were between 92.33% and 101.41% (n = 5). The matrix effect was determined by comparing the signals produced by the same QC concentration in methanol and in blank tumor homogenate. The matrices were pretreated following the routine method, and were reconstituted with the same QC solution. As shown in Table 4, the obtained matrix effect ranged from 95.47% to 104.93% (n = 3), indicating that matrix effects were not significant for (25R/S)-antcin H.Stability. The stability test was conducted using HQC and LQC samples after 2 h storage at room temperature for short-term stability, and after 7 days storage at -20 °C for long-term stability. As shown in Table 5,(25R/S)-antcin H were proved stable during analysis, with RSD below 5.00% for short-term stability and below 1.27% for long-term stability.
Quantitative analysis for (25R/S)-antcin H in tumor tissues by LC-MS/MS
Using the validated LC-MS/MS method, the contents of(25R)-antcin H and (25S)-antcin H in ACE were firstly determined, and their contents were 3.04% (w/w) and 3.86% (w/w), respectively, with a ratio (rR/S) of 0.78. The tumor homogenate samples collected at 1 h and 4 h after drug administration were also analyzed by the LC-MS/MS method, and the results are shown in Table 6.For mice in the 1 h group, the average concentrations of(25R)-antcin H and (25S)-antcin H in tumors were 2.03 and 0.66 μg/g tumor tissues, respectively, with a ratio(rR/S) of 3.19. For mice in the 4 h group, the contents were 2.04 and 0.59 μg/g tumor tissues with a ratio (rR/S) of 3.33.
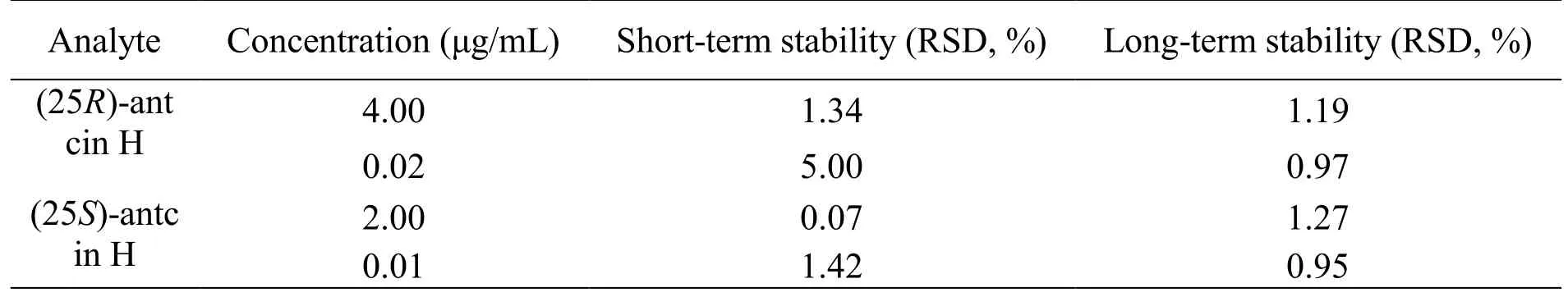
Table 5 The stability of (25R/S)-antcin H
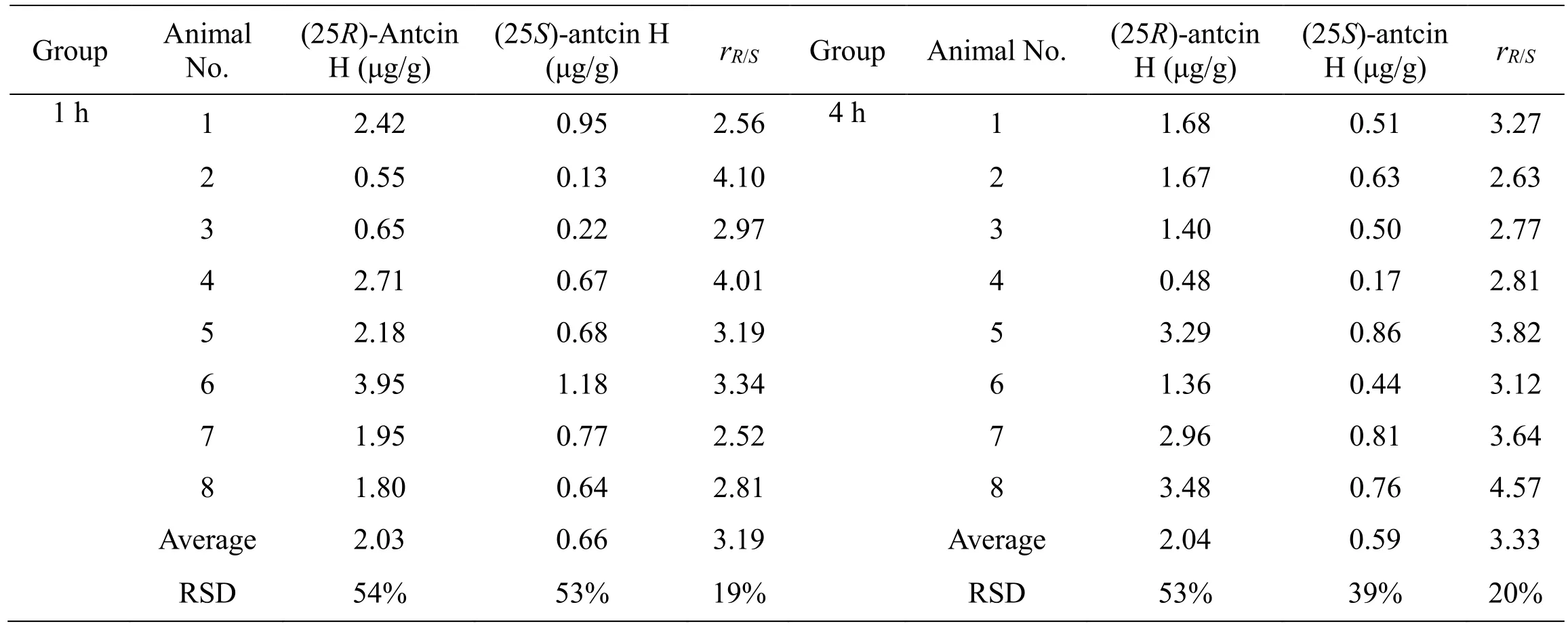
Table 6 The concentrations of (25R/S)-antcin H in mice tumor tissues
Discussion
In our previous report, we investigated the metabolites and pharmacokinetics of AC in healthy Sprague-Dawley rats, and found that ergostane and lanostane triterpenoids were the major plasma-exposed compounds [22]. The present study aims to further clarify the components which could enter tumor tissues after oral administration of AC in Sarcoma-180 tumor-bearing mice, as well as their concentrations in tumors.
After oral administration of ACE, a total of 33 compounds were detected in tumor homogenate samples using UHPLC-orbitrap/MS analysis, including 28 prototypes and 5 metabolites (Table 2 and Figure 2).Three of the five metabolites (M1–M3) were tentatively identified as the isomers of antcin K, and the other two ones (M4–M5) were identified as hydroxylated products of dehydrosulphurenic acid and sulphurenic acid,respectively. In addition, majority of the triterpenoids had low concentrations in tumor tissues except for(25R/S)-antcin H. Interestingly, (25R/S)-antcin K (E1/2),(25R/S)-antcin H (E16/17) and (25R/S)-antcin B (E21/22)were the six most abundant compounds in ACE, but only(25R/S)-antcin H had high concentrations in tumor tissues,even 4 h after drug administration. Considering the potent cytotoxicities of (25R/S)-antcin H [28–30], we deduce these two compounds may contribute remarkably to the anticancer activities of AC.
To determine the concentrations of (25R/S)-antcin H in tumor tissues, an LC-MS/MS method was developed and fully validated. Selectivity, precision, accuracy, extraction recovery, matrix effect, and stability of the method met FDA requirements. The tumor homogenate samples collected at 1 h and 4 h after oral administration of ACE were analyzed. As shown in Table 6, the concentrations of(25R)-antcin H and (25S)-antcin H in the tumor tissues were 0.55–3.95 and 0.13–1.18 μg/g for the 1 h group, and 0.48–3.29 and 0.17–0.86 μg/g for the 4 h group,respectively. The elimination of the two compounds was slow in tumors, since no significant differences were observed between 1 h and 4 h groups. In addition, the concentrations of (25R)-antcin H in tumors was much higher than (25S)-antcin H for both 1 h and 4 h groups with an average ratios (rR/S) of 3.19 and 3.33, respectively(P< 0.01). Considering that the content of (25R)-antcin H was lower than that of (25S)-antcin H (rR/S, 0.78) in ACE,we deduce that (25R)-antcin H had higher tumor affinity than (25S)-antcin H. The underlying mechanisms leading to the different tumor affinity need to be further investigated.
Conclusion
In this study, the metabolites of AC were profiled in tumor tissues of Sarcoma-180 tumor-bearing mice by UHPLC-orbitrap/MS analysis. A total of 28 prototypes of triterpenoids and 5 metabolites were detected after oral administration of ACE. Among them, (25R/S)-antcin H were the two most abundant compounds in tumor tissues,and their concentrations were determined by LC-MS/MS.The concentration of (25R)-antcin H was remarkably higher than (25S)-antcin H in the tumor tissues. These compounds may contribute to the anticancer activities of AC.
 TMR Modern Herbal Medicine2018年2期
TMR Modern Herbal Medicine2018年2期
- TMR Modern Herbal Medicine的其它文章
- Shenxian Shengmai Oral Liquid for treatment of slow arrhythmia: a randomized controlled trial
- Clinical evaluation of Xiaoyao Jieyu prescription in the treatment of persistent postural-perceptual dizziness
- Chinese medicine in the treatment of intracranial vascular stenosis in 1 case
- A meta-analysis of randomized controlled trials of Yiyiren Decoction in the treatment of rheumatoid arthritis
- Various models of atrial fibrillation induced by acetylcholine and its application in the field of traditional Chinese medicine
- Progress of Alzheimer’s disease related glucose metabolism regulating hormones and a research perspective in nootropics of herbal medicine
