The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis
Lei Wang , Huai-Cheng Chen , Xi Yang , Jian-Jian Tao , Guang Liang Jian-Zhang Wu Wen-Can Wu, Yi Wang Zong-Ming Song, , Xin Zhang
1 Chemical Biology Research Center, School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou, Zhejiang Province, China
2 The Eye Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, China
Abstract Chalcone is a plant metabolite widely found in fruits, vegetables, spices and tea, and has anti-tumor, anti-inflammation, immunomodulation,antibacterial and anti-oxidation activities, as well as many other pharmacological and biological effects. Our team has shown that its analogs have antioxidant activity, and oxidative stress is a pathological hallmark of retinal ischemia/reperfusion injury that can lead to retinal damage and visual loss. This investigation aims to identify a chalcone that protects retinal ganglion cells in vitro from the effects of oxidative stress and examine its mechanism. Rat retinal ganglion cell-5 cells were pretreated with chalcones and then exposed to tert-butyl hydroperoxide that causes oxidative damage. Controls received dimethyl sulfoxide only or tert-butyl hydroperoxide in dimethyl sulfoxide. Only (E)-3,4-dihydroxy-2′-methylether ketone (L2H17), of the five chalcone analogs, markedly increased the survival rate of oxidatively injured RGC-5 cells.Thus, subsequent experiments only analyzed the results of the L2H17 intervention. Cell viability and apoptosis were measured. Intracellular superoxide dismutase and reactive oxygen species levels were used to assess induced oxidative stress. The mechanism of action by L2H17 was explored by measuring the ER stress/UPR pathway and the expression and localization of Nrf2. All results demonstrated that L2H17 could reduce the apoptosis of oxidatively injured cells, inhibit caspase-3 activity, increase Bcl-2 expression, decrease Bad expression, increase the activity of superoxide dismutase, inhibit the production of reactive oxygen species, increase Nrf2 immunoreactivity, and reduce the activating transcription factor 4, phospho-eukaryotic initiation factor 2 and CHOP expression. L2H17 protects retinal ganglion cells induced by oxidative stress by regulating Nrf2, which indicates that it has the potential to become a drug for retinal ischemia/reperfusion.
Key Words: nerve regeneration; retinal ischemia/reperfusion injury; oxidative stress; reactive oxygen species; apoptosis; nuclear erythroid-related factor-2; endoplasmic reticulum stress; chalcone analogs; retinal ganglion cells; neural regeneration
Introduction
Increased oxidative stress is implicated in the common pathological process of many eye ailments, including glaucoma(Tanito et al., 2016), retinal vascular occlusion (Bharathi Devi et al., 2012), ischemic optic neuropathy (Gaydar et al., 2011),and diabetic retinopathy (Tarr et al., 2013). The retina is a highly metabolically active tissue, therefore is greatly sensitive to reduced oxygen tension, particularly in the retinal ganglion cells (Arjamaa and Nikinmaa, 2006). Oxidative stress exacerbates the retinal tissue injury because of the accumulation of excessive reactive oxygen species (ROS), which in turn cause severe retinal injury. For example, free radicals can cause lipid peroxidation, protein damage and DNA fragmentation (Forman, 2016). Alteration in the macromolecules could result in severe neuronal damage linked to visual impairment and irreversible neuronal destruction in many layers of the retina(Pinazo-Durán et al., 2014). Therefore, it is highly desirable to identify therapeutic antioxidant strategies for retinal injury.
The transcription factor nuclear factor erythroid-related factor 2 (Nrf2) triggers one of the prime antioxidant signaling pathways, which controls the antioxidant responses in vivo through transcriptional regulation of several antioxidant or detoxification genes. Nrf2 functions by binding to a cis-acting element known as the antioxidant response element (ARE). It regulates the expression of important antioxidant and detoxification genes under stress in response to external stimuli such as oxidants, xenobiotics, metals and ultraviolet light irradiation(Rushmore and Pickett, 1990; Kaspar et al., 2009). Evidence indicates that natural or chemical synthetic antioxidants can block the ischemic/hypoxic neuronal injury by up-regulation of the Nrf2/ARE pathway (Kumar et al., 2014; Chen et al.,2015). Recent studies reported that targeting Nrf2 expression with an effective molecule protected retinal ganglion cells from damage after optic nerve crush (Koriyama et al., 2010; Himori et al., 2013; Koriyama et al., 2013). Therefore, the Nrf2/ARE-antioxidant signaling axis may be an important therapeutic option to decrease oxidative damage in retina.
Chalcone is a plant metabolite widely found in fruits, vegetables, spices, and tea, and exhibits multiple pharmacological and biological activities, i.e., anti-tumor, anti-inflammatory,immunomodulatory, anti-bacterial, and antioxidant (Vogel et al., 2010; Fang et al., 2015; Wang et al., 2015; Xu et al., 2015). In particular, certain chalcone derivatives, such as isosalipurposide,exert a cytoprotective effect against oxidative injury in hepatocytes (Han et al., 2015), and xanthohumol confers protection against oxidative damage in PC12 cells (Yao et al., 2015). Our recent studies indicated that the chalcone derivative, L2H17[(E)-3,4-dihydroxy-2′-methylether ketone], is anti-inflammatory, conferring protection in experimental models of colon cancer (Xu et al., 2015), obesity-induced kidney injury, and diabetes (Fang et al., 2015). These findings indicate that L2H17 has potential antioxidant activity.
In the present study, we investigated the neuroprotective effect of various chalcone derivatives in tert-butyl hydroperoxide(TBHP)-induced retinal ischemia/reperfusion (I/R) injury in RGC-5 cells. The purpose of our study is to identify potential chemicals for the treatment of retinal I/R injury.
Materials and Methods
Cell line and reagents
The established rat retinal ganglion cell line RGC-5, was a gift from Professor Da-Xiao Lu (Jinan University, Guangzhou,China). The rat RGC-5 cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA)supplemented with 10% fetal bovine serum (Invitrogen) 1 g/L glucose, 1% antibiotic solution (100 U/mL penicillin and 100µg/mL streptomycin; Invitrogen) in the presence of 95% air and 5% CO2at 37°C. Cells between passages 16 and 20 were used for study.
Chalcone analogs
Chalcone analogs L2H7, L2H17, L3H6, L6H9, and L43H2were synthesized and characterized in our laboratory as described in previous publications (Ma et al., 2011; Wu et al.,2011; Xu et al., 2015). Before they were tested in biological experiments, the compounds were recrystallized from CHCl3/EtOH. High performance liquid chromatography was used to determine the purity (> 99%). The compounds of interest were dissolved in dimethyl sulfoxide (DMSO) for in vitro experiments. The chemical structures of all the compounds are shown in Table 1.
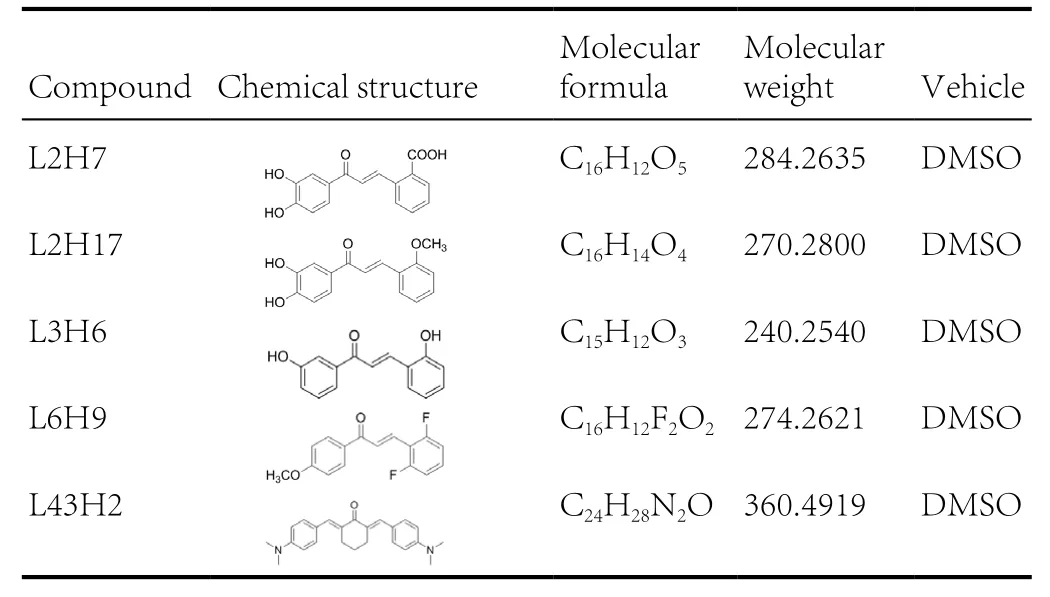
Table 1 Chemical information of the tested compounds
Assessment of cell viability using 3-(4,5-dimethylthiazol-
2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
RGC-5 cells were harvested and seeded into 96-well plates and allowed to attach for 24 hours. Then RGC-5 cells were incubated with chalcone derivatives (L2H7, L2H17, L3H6, L6H9, and L43H2) at the dose of 10 µM, or vehicle control (DMSO, 3 µL)for 2 hours, followed by incubation with TBHP (Aladdin Reagent Inc., Shanghai, China; 30 µM) for an additional 22 hours.MTT (Sigma-Aldrich, St. Louis, MO, USA; 5 mg/mL, 20 µL)in phosphate-buffered saline (PBS) was added to each well and incubated for 4 hours in the dark. The purple formazan product was dissolved by the addition of 150 µL of DMSO to each well and the plate was placed on orbital shaker for 10 minutes. The optical density (OD) values were measured at 490 nm with a 96-well plate reader (SpectraMax M2e; Molecular Devices, Sunnyvale, CA, USA). The cell viability was calculated by ODtreatmentgroup/ODDMSOgroup× 100%.
Cell apoptosis analysis
Apoptosis was assessed by Annexin V-FITC/propidium iodide staining kit (BD Biosciences Pharmingen, San Diego, CA,USA). Initially, RGC-5 cells were pretreated with L2H17 (2.5,5, or 10 µM) or vehicle control (DMSO) for 2 hours, followed by incubation with TBHP (30 µM) for 12 hours. At the end of the treatment, cells were harvested and stained with Annexin V-FITC for 10 minutes and propidium iodide for 5 minutes.The fluorescence was measured using FACS-Caliber flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA). The percentage of cells with early apoptosis (cells in Q3 area) was calculated and shown as histogram data.
Caspase-3 activity assay
Briefly, RGC-5 cells were seeded in a 6-well plate and pre-treated with L2H17 (2.5, 5, or 10 µM), curcumin (10 µM; Sigma-Aldrich), or vehicle control (DMSO) for 2 hours, followed by incubation with TBHP (30 µM) for 22 hours. After indicated time point, cells were harvested and caspase-3 activity was measured using a caspase-3 activity kit (Beyotime Institute of Biotechnology, Nantong, China) as the manufacturer’s instruction. The caspase-3 activity was expressed in enzymatic units per mg of protein.
Measurement of intracellular superoxide dismutase (SOD)activity
RGC-5 cells were pretreated with L2H17 (2.5, 5, or 10 µM) or vehicle control (DMSO) for 2 hours, followed by incubation with TBHP (30 µM) for 22 hours. The intracellular SOD activity was measured using the Total SOD Assay Kit with WST-8 Kit (Beyotime Institute of Biotechnology) according to the manufacturer’s protocol.
Detection of intracellular ROS
To investigate the intracellular ROS generation in RGC-5 cells,cells were pretreated with L2H17 (2.5, 5, or 10 µM) or vehicle control (DMSO), followed by incubation with TBHP (30 µM)for 2 hours. After the treatment, RGC-5 cells were harvested using trypsin-EDTA and ROS generation was quantified using 2,7-dichlorofluorescein diacetate and the fluorescence intensity peak was recorded using flow cytometry (BD Biosciences,Franklin Lakes, NJ, USA). The images were viewed using a fluorescence microscope (200×; Nikon, Tokyo, Japan).
Western blot assay
RGC-5 cells were pretreated with L2H17 (2.5, 5, or 10 µM),N-acetyl cysteine (5 mM; Sigma-Aldrich) or DMSO for 2 hours, followed by TBHP (30 µM) incubation for 22 hours.RGC-5 cells (5 × 105) were washed with PBS and suspended in a protein lysis buffer and debris was removed by centrifugation at 12,000 r/min for 10 minutes at 4°C. Nuclear protein was extracted using a nuclear protein extraction kit according to the manufacturer’s instructions (Beyotime Biotech, Nantong,China). The protein concentration of the samples was determined by quick start 1× Bradford protein assay kit (Bio-Rad,Hercules, CA, USA). Then 40–50 µg protein was separated on precast 12% sodium dodecyl sulfate polyacrylamide gels. After electrophoresis, the proteins were transferred to poly (vinylidene difluoride) membrane. The membranes were blocked with 5%non-fat milk in Tris-buffered saline containing 0.1% Tween 20(TBST) for 90 minutes at room temperature, then incubated with specific primary antibodies (1:1000) in TBST overnight at 4°C. After washing three times with TBST at 5-minute interval, the membranes were incubated with horseradish peroxidase conjugated secondary antibodies (1:3000) for 1 hour at room temperature. After three additional washes with TBST, the membranes were examined using an enhanced chemiluminescence detection system (GE Healthcare, Beijing, China). Antibodies against Bcl2 (mouse anti-rat IgG), Bad (rabbit anti-mouse IgG),GAPDH (mouse anti-rat IgG), Lamin B (mouse anti-rat IgG),CHOP (mouse anti-rat IgG), Nrf2 (rabbit anti-rat IgG), and goat anti-mouse and goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against ATF-4 (rabbit anti-human) and p-eIf2 (rabbit anti-human) were obtained from Cell Signaling technology (Danvers,MA, USA). Membranes were tested with GAPDH as a loading control. The optical density of the immunoreactive bands was analyzed using Image J computer software (National Institute of Health, Bethesda, MD, USA). The data were normalized to their respective controls. Three independent experiments were performed for western blot assay.
Immunofluorescence staining
To determine the intracellular level of Nrf2, treated RGC-5 cells were fixed in 4% paraformaldehyde for 5 minutes and gently washed three times with PBS. The cells were treated with 3% bovine serum albumin for 30 minutes, then incubated overnight at 4°C with anti-Nrf2 antibody (rabbit anti-rat IgG, 1:1000, Santa Cruz Technology, Santa Cruz, CA,USA), followed by incubation with fluorescein isothiocyanate(FITC)-labeled goat anti-rabbit IgG (1:3000; Santa Cruz Biotechnology) for 1 hour at room temperature. Finally, cells were stained with nuclear specific dye DAPI for 5 minutes. The images were observed under a fluorescence microscope (200×,Nikon, Tokyo, Japan).
Statistical analysis
All the experiments were repeated at least three times. Values from quantitative experiments are expressed as the mean ± SEM and were calculated using GraphPad Pro 5.0 software (GraphPad,San Diego, CA, USA). The unpaired t-test was performed to determine the differences between treatment groups. A value of P <0.05 was considered statistically significant.
Results
L2H17 pretreatment inhibited TBHP-induced cell death
Previous studies indicated that chalcone derivatives inhibit oxidative stress in different experimental models (Lounsbury et al., 2015; Martinez et al., 2015; Yao et al., 2015). We first determined whether our chalcone analogs prevented TBHP-induced RGC-5 cell viability by performing MTT assay. Briefly,RGC-5 cells were pretreated with different chalcone analogs(L2H7, L2H17, L3H6, L6H9, and L43H2 at the dose of 10µM) or DMSO for 2 hours, followed by incubation with TBHP(30 µM) for 22 hours. The viability of RGC-5 cells was determined via using MTT assay. Of the analogs evaluated, L2H17 was most effective in preventing the TBHP-induced loss of cell viability (Figure 1A). TBHP (30 µM) reduced cell viability by approximately 50%, and the pretreatment with L2H17(2.5, 5, or 10 µM) prevented the TBHP-induced reduction in cell viability in a dose-dependent manner (Figure 1B). TBHP also causes oxidative stress-induced apoptosis in cells (Dai et al., 2016). In our RGC-5 cell experimental model, TBHP increased caspase-3 activity which was prevented by L2H17 dose-dependently (Figure 1C). Flow cytometry data indicated that L2H17 pretreatment markedly reduced TBHP-induced cell apoptosis dose-dependently (Figure 1D–I). Similarly,TBHP induced a 4-fold decrease of Bcl, but a 7-fold increase of Bad expression (Figures 1J–L). With L2H17 pretreatment,the TBHP-induced changes in expression of the apoptosis-related proteins were prevented (Figure 1J–L).

Figure 1 L2H17 pretreatment decreased TBHP-induced RGC-5 cell death via reducing apoptosis.
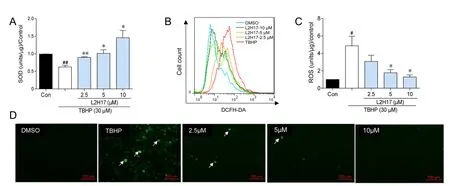
Figure 2 L2H17 pretreatment decreased TBHP-induced oxidative stress in RGC-5 cells.
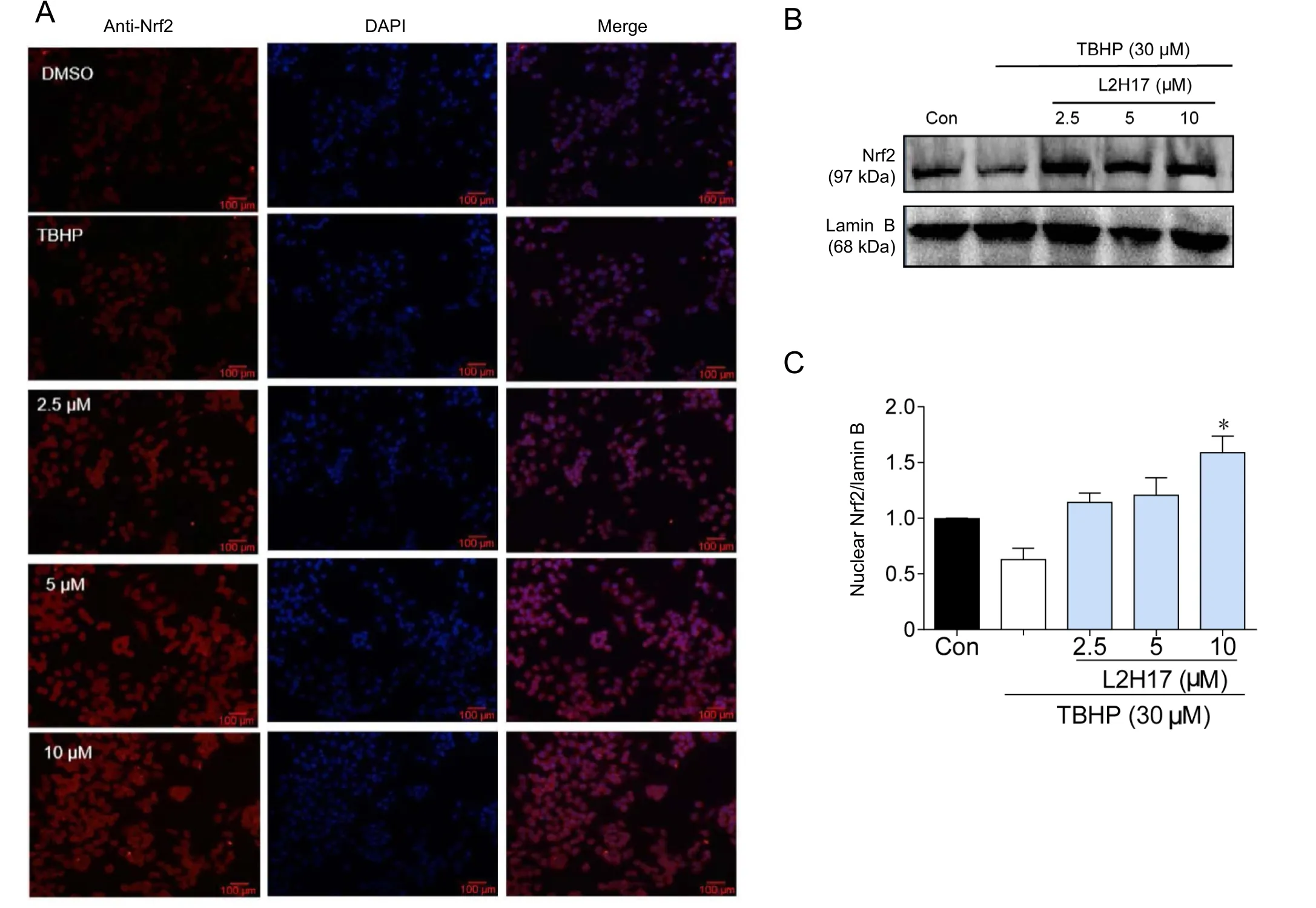
Figure 3 L2H17 pretreatment exhibited the anti-oxidative effect via increasing Nrf2 expression.
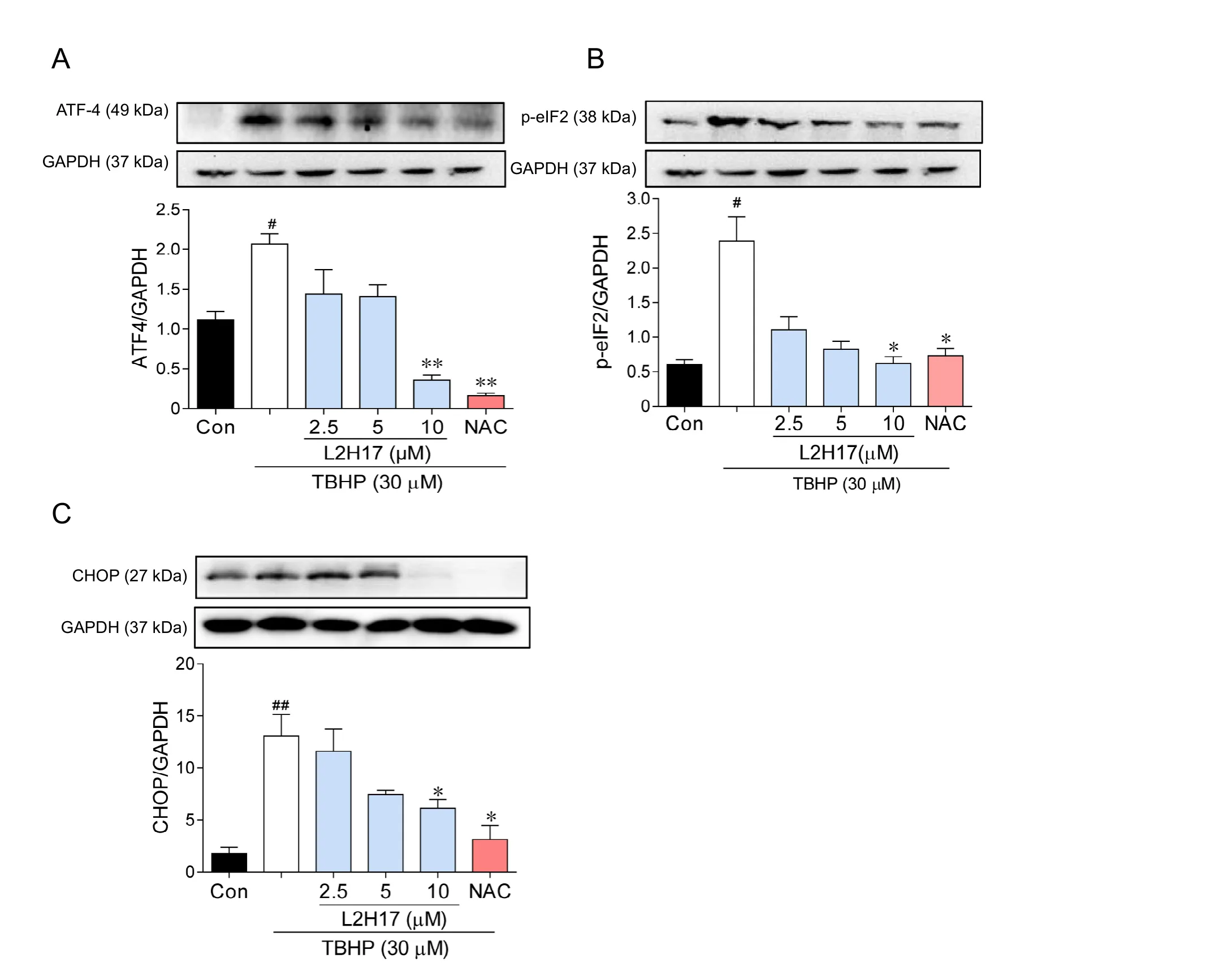
Figure 4 L2H17 pretreatment suppressed endoplasmic reticulum stress activation via its anti-oxidative effect(western blot assay).
L2H17 pretreatment prevented TBHP-induced oxidative stress by activating Nrf2
We investigated whether protective effects of L2H17 were related to its ability to inhibit TBHP-induced oxidative stress in vitro. Oxidative stress generated by TBHP was determined by measurement of intracellular content of SOD and ROS. As shown in Figure 2A, TBHP significantly decreased intracellular SOD activity, but the decrease was prevented with L2H17 pretreatment in a dose-dependent manner. TBHP also increased intracellular ROS production 5-fold over the control, which was in turn prevented by L2H17 pretreatment (Figure 2B–C). Corroborative fluorescent images of RGC-5 cells show TBHP-induced increases of intracellular ROS (green), and ROS generation was inhibited by increasing L2H17 concentrations (Figure 2D).
The Nrf2 pathway is one of the major cellular defense mechanism to control oxidative stress (Liu et al., 2017). Thus, we tested whether the antioxidative stress effects of L2H17 could be attributed to Nrf2. Results indicated that TBHP stimulation alone moderately decreased the immunofluorescent localization of cytoplasmic Nrf2 in RGC-5 cells (Figure 3A). However, pretreatment with different concentrations of L2H17 resulted in a robust increase of Nrf2 expression (Figure 3A). Similarly, western blot assay revealed that L2H17 pretreatment not only prevented the TBHP-induced decrease of Nrf2, but also increased its expression above control levels (Figure 3B–C). Our data suggest that L2H17 exhibited its antioxidative effects by activating the Nrf2 pathway.
L2H17 pretreatment reduced endoplasmic reticulum (ER)stress through an antioxidative mechanism
Oxidative stress in the retina can cause ER stress and the unfolded protein response (UPR), leading to apoptosis (Zhang et al., 2014). We determined whether the anti-apoptotic activities of L2H17 were related to inhibition of ER stress/UPR pathway in RGC-5 cells. TBHP stimulation dramatically increased the expression of marker proteins of the ER stress/UPR pathway,specifically, ATF4, p-eIF2 and CHOP; however, the pretreatment with L2H17 prevented the TBHP-induced increases(Figure 4). Findings suggest that the protective effects of L2H17 were through inhibition of ER stress and UPR. To reduce oxidative stress, we further treated RGC-5 cells with N-acetyl cysteine, a classic antioxidant to scavenge ROS in vitro. As shown in Figure 4, treatment of RGC-5 cells with N-acetyl cysteine for 2 hours significantly inhibited the activation of ER stress,providing further support that the protective mechanism of L2H17 was mediated through upstream inhibition of oxidative stress (Figure 4).
Discussion
Oxidative stress is created when there is uncontrolled generation of free radicals and imbalance in the antioxidant defense system, resulting in excessive ROS (Sun et al., 2017). Oxidative stress is also thought to be a critical factor in the pathogenesis of retinal I/R injury, triggering ischemic cell damage and leading to hypersecretion of glutamate and aspartate (Fukuda et al., 2010; Lalkovičová and Danielisová, 2016). In this study,we examined the antioxidant properties of a novel chalcone analog, L2H17, as a potential curative candidate for retinal I/R injury. The key findings from this study are that (1) L2H17 was an highly effective agent in preventing the loss of cell viability and apoptotic death in RGC-5 cells challenged with the oxidant, TBHP; (2) L2H17 prevented the oxidative stress induced by TBHP; and (3) L2H17 increased expression and nuclear localization of Nrf2, which in turn regulated the expression of several antioxidant proteins. We conclude that L2H17 protected against oxidant-mediated injury of retinal ganglionic cells most likely by triggering the cells’ antioxidant defense mechanism.
The chalcone L2H17 effectively prevented the development of free radicals in RGC-5 cells in response to TBHP. Our findings indicated that this protection was due to a combination of several factors. First, the cells’ antioxidant systems appear to be highly sensitive to L2H17. We found that L2H17 prevented the loss of SOD in response to TBHP and, surprisingly, L2H17 at high concentrations promoted the expression of SOD, suggesting preservation of the intracellular content of the antioxidant. Although we measured only SOD, it is possible that other cellular antioxidants, e.g. catalase or glutathione peroxidase,were also sensitive to L2H17 and contributed to the overall antioxidant activity of L2H17. Although precisely how L2H17 regulates SOD or other antioxidant systems remain unclear,our results indicated that L2H17 was a potent stimulator of Nrf2 expression and nuclear localization in the retinal ganglion cells.
The nuclear transcription factor Nrf2 is well known for its ability to regulate the expression of antioxidant and detoxification enzymes to maintain intracellular redox balance (Papp et al., 2012). The L2H17-induced increased nuclear localization of Nrf2 can be attributed to its release from the cytoplasmic repressor protein, Keap1 (actin-binding Kelch-like protein),through ubiquitination. Lu et al. (2016) reported that inducers of Nrf2 such as free radicals and electrophilic compounds cause Keap1 ubiquitination and Nrf2 dissociation from Keap1.Nrf2 translocates into the nucleus and binds to antioxidant response elements for gene transcription of genes encoding toxifying and antioxidant molecules. The specific molecular events by which L2H17 induces Nrf2 nuclear translocation have yet to be understood, but numerous studies using natural chalcones and their derivatives show that Nrf2 is a key target of regulation. More experiments are needed to fully investigate the mechanism of Nrf2 regulation by L2H17 and other chalcone derivatives.
A major consequence of the ability of L2H17 to upregulate and preserve the cell’s antioxidant systems is a corresponding decrease in the cellular content of ROS. Our results confirmed that L2H17 prevented the TBHP-induced cellular accumulation of ROS, thereby reducing oxidative stress in cells. However, we cannot exclude the possibility that the L2H17-mediated decrease of cellular ROS content could be also attributed to inhibition of the cellular oxidase systems that generate ROS and to direct oxygen radical scavenging.
The oxidant, TBHP, induced dramatic increases in apoptosis of the retinal ganglionic cells, which was accompanied by significant increases of marker proteins activating transcription factor 4, p-eIF2 (regulating initiator of the mRNA translation machinery), and C/EBP homologous protein of the ER stress/UPR pathway in the retinal ganglion cells. CHOP is known to decrease the expression of anti-apoptotic Bcl-2 protein. It is also involved in the execution of apoptosis program by reducing mitochondrial membrane potential, releasing cytochrome c into the cytosol and initiating apoptosome formation via caspase-3 activation (Cao and Kaufman, 2014). This was an expected finding since oxidative stress is known to activate UPR in the retina, leading to apoptosis (Zhang et al., 2011,2014; Nashine et al., 2014). Consistent with this mechanism,the TBHP-induced activation of the ER stress/UPR pathway was accompanied by a decreased expression of Bcl-2 and a corresponding increase of the pro-apoptotic protein, Bad. The results support a mechanistic link between the TBHP-induced oxidative stress and cell apoptosis in the retinal cells. Our findings that L2H17 was able to reduce the oxidative stress of TBHP-stimulated cells and prevent the TBHP-induced changes of ER stress/UPR marker proteins and Bcl-2 and Bad levels,giving protection against apoptosis, support the mechanistic link between oxidative stress and apoptosis.
In conclusion, the present study demonstrated that TBHP induced a marked oxidative stress in retinal ganglion cells, resulting in apoptosis. Our novel findings are that the chalcone derivative, L2H17, protected the cells from oxidative damage.Moreover, the protective mechanism of L2H17 was associated with the upregulation of the cell’s antioxidant systems, in particular, SOD and Nrf2. Although experiments are needed to study the full mechanism of Nrf2 regulation by L2H17, we can now conclude that L2H17 can act as an important antioxidant agent and be a potential prophylactic candidate for retinal I/R-related diseases.
Acknowledgments: The authors thank Dr. Rajamanickam Vinothkumar(Wenzhou Medical University, China) for editing of the paper, and Professor Da-Xiang Lu from Jinan University (Guangzhou, China) for providing the RGC-5 cell line.
Author contributions: LW, HCC, XY and JJT performed the experiment.XZ, GL, WCW, ZMS, YW designed the study. JZW contributed essential reagents or tools. ZMS and WY analyzed the data. LW, XZ and YW wrote the paper. All authors approved the final version of the paper.
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support: This study was supported by the National Natural Sci-ence Foundation of China, No. 81473295 (to ZMS), 81373312 (to XZ)and 81371028 (to WCW). The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of this funding organization.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- 3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
- Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease
- Role of nuclear factor κB in multiple sclerosis and experimental autoimmune encephalomyelitis
- Nanometer ultrastructural brain damage following low intensity primary blast wave exposure
- Altered leukocyte gene expression after traumatic spinal cord injury: clinical implications
- Promoting axonal regeneration following nerve surgery: a perspective on ultrasound treatment for nerve injuries

