Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease
Bridget Martinez , Philip V. Peplow
1 Department of Molecular & Cellular Biology, University of California, Merced, CA, USA
2 Department of Medicine, St. Georges University School of Medicine, Grenada
3 Department of Physics and Engineering, Los Alamos National Laboratory, Los Alamos, NM, USA
4 Department of Anatomy, University of Otago, Dunedin, New Zealand
Abstract Parkinson’s disease (PD) is an age-related neurodegenerative disease for which the characteristic motor symptoms emerge after an extensive loss of dopamine containing neurons. The cell bodies of these neurons are present in the substantia nigra, with the nerve terminals being in the striatum. Both innate and adaptive immune responses may contribute to dopaminergic neurodegeneration and disease progression is potentially linked to these. Studies in the last twenty years have indicated an important role for neuroinflammation in PD through degeneration of the nigrostriatal dopaminergic pathway. Characteristic of neuroinflammation is the activation of brain glial cells, principally microglia and astrocytes that release various soluble factors. Many of these factors are proinflammatory and neurotoxic and harmful to nigral dopaminergic neurons. Recent studies have identified several different agents with immunomodulatory properties that protected dopaminergic neurons from degeneration and death in animal models of PD. All of the agents were effective in reducing the motor deficit and alleviating dopaminergic neurotoxicity and, when measured, preventing the decrease of dopamine upon being administered therapeutically after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, 6-hydroxydopamine,rotenone-lesioning or delivery of adeno-associated virus-α-synuclein to the ventral midbrain of animals.Some of these agents were shown to exert an anti-inflammatory action, decrease oxidative stress, and reduce lipid peroxidation products. Activation of microglia and astrocytes was also decreased, as well as infiltration of T cells into the substantia nigra. Pretreatment with fingolimod, tanshinoine I, dimethyl fumarate, thalidomide,or cocaine- and amphetamine-regulated transcript peptide as a preventive strategy ameliorated motor deficits and nigral dopaminergic neurotoxicity in brain-lesioned animals. Immunomodulatory agents could be used to treat patients with early clinical signs of the disease or potentially even prior to disease onset in those identified as having pre-disposing risk, including genetic factors.
Key Words: Parkinson’s disease; immunomodulatory agents; neuroprotection; inflammation; oxidative stre ss; animal models; microgliosis; astrogliosis
Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disease, second in prevalence to Alzheimer’s disease. A range of clinical symptoms are exhibited, with the most common affecting motor function and include resting tremor, rigidity,akinesia, bradykinesia and postural instability (Winklhofer and Haass, 2010). The characteristic motor symptoms of PD appear after an extensive loss of dopamine containing neurons,the cell bodies of which are located in the substantia nigra and the nerve terminals in the striatum (Savitt et al., 2006). Pre-motor symptoms arise much earlier. A common symptom is constipation which can be experienced many years before motor dysfunction onset in PD patients (Savica et al., 2009). Computational models have been used to investigate the dopamine deficiency on PD symptoms (Daneshzand et al., 2017a, b).Characteristic of the disease is accumulation of protease-resistant α-synuclein (α-syn) in synapses and axons, formation of neuronal inclusions called Lewy bodies (LBs), and selected neuronal degeneration in the neocortex, limbic, and nigrostriatal systems, with neuroinflammation (Dickson, 2001). Recent evidence supports the view that α-syn plays a central role in the etiopathogenesis of PD (Winner et al., 2011; Lashuel et al.,2013; deSouza and Schapira, 2017). Both innate and adaptive immune responses may contribute to dopaminergic neurodegeneration and disease progression is potentially linked to these (Braak et al., 2007). While currently no proven protective treatments are available for patients with PD (Olanow et al., 2009; Athauda and Foltynie, 2015), some agents such as levodopa (L-dopa) and apomorphine can provide relief from the symptoms of PD but are less effective as the disease progresses. In addition to the loss of efficacy, these agents are associated with a range of side effects, some common such as nausea, vomiting, while others are more severe and include psychic disturbances and dyskinesia (Cotzias et al., 1970).
Studies in the last twenty years have shown an important role for neuroinflammation in PD through the degeneration of the nigrostriatal dopaminergic pathway. Characteristic of neuroinflammation is the activation of brain glial cells, principally microglia and astrocytes that release various soluble factors such as free radicals, cytokines, and lipid metabolites. Many of these factors are proinflammatory and neurotoxic and are particularly harmful to nigral dopaminergic neurons that are also vulnerable to oxidative damage (Czlonkowska et al., 2002;Liu et al., 2003).The resident immune cells in the brain are the microglia and are sensitive to even minor disturbances in central nervous system (CNS) homeostasis. They become readily activated during most neuropathological conditions (Liu and Hong, 2003). Dopaminergic neurodegeneration is alleviated in various experimental animal models of PD by reducing neuroinflammation with anti-inflammatory drugs (Choi et al.,2005; Jin et al., 2008). We have searched the PubMed database for recent studies in years 2012–2017 aimed at downregulating immune and inflammatory processes in animal models of PD using immunomodulatory agents. These could be important in slowing the progression of PD and might be exploited as treatments in patients with PD. An ambitious yet imperative goal in research, and of paramount importance in translational medicine, is the development of new therapeutic approaches that can impede or prevent the progression of PD.
Immunomodulatory Therapies for PD
Pharmaceutical therapies
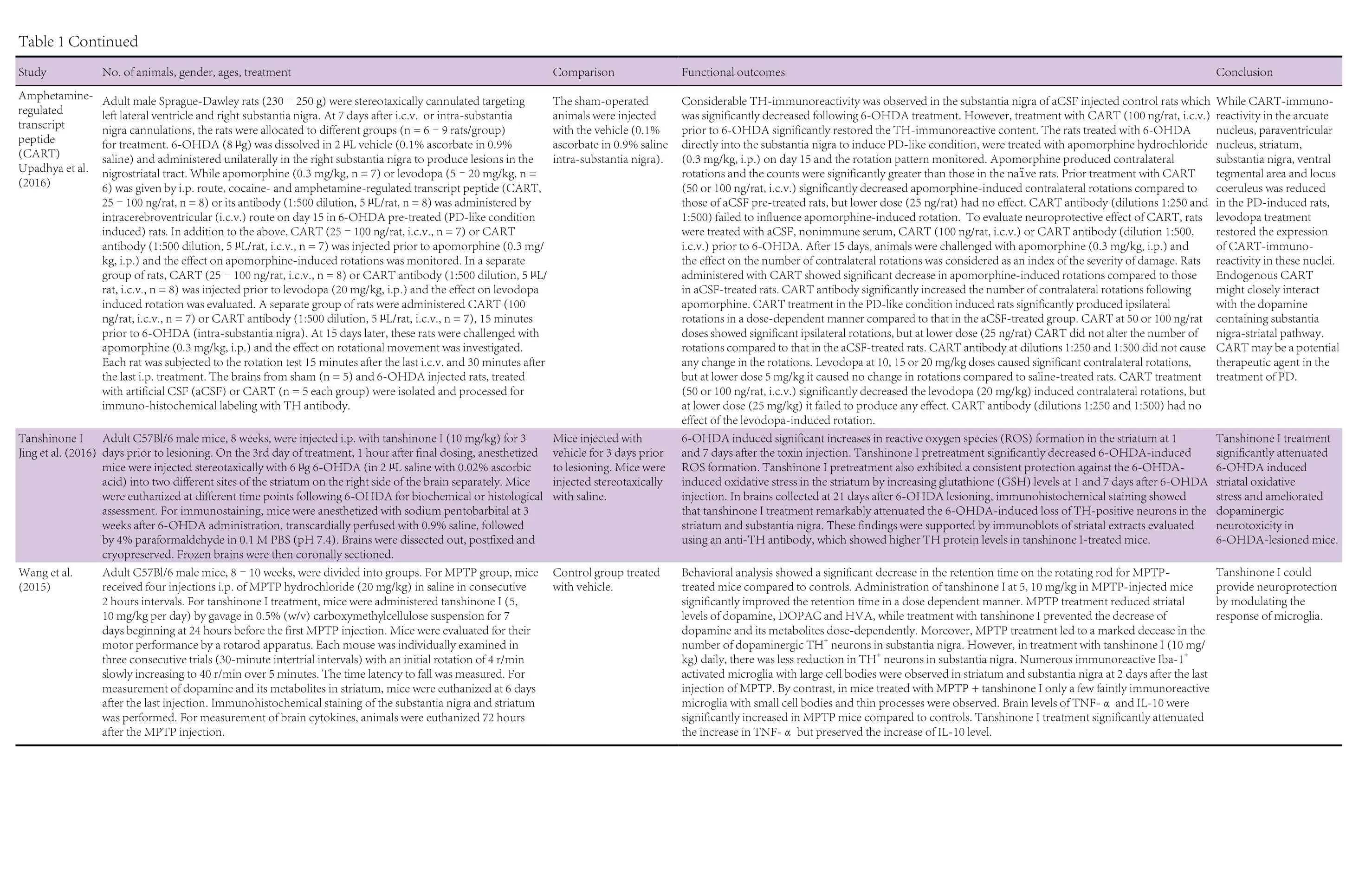
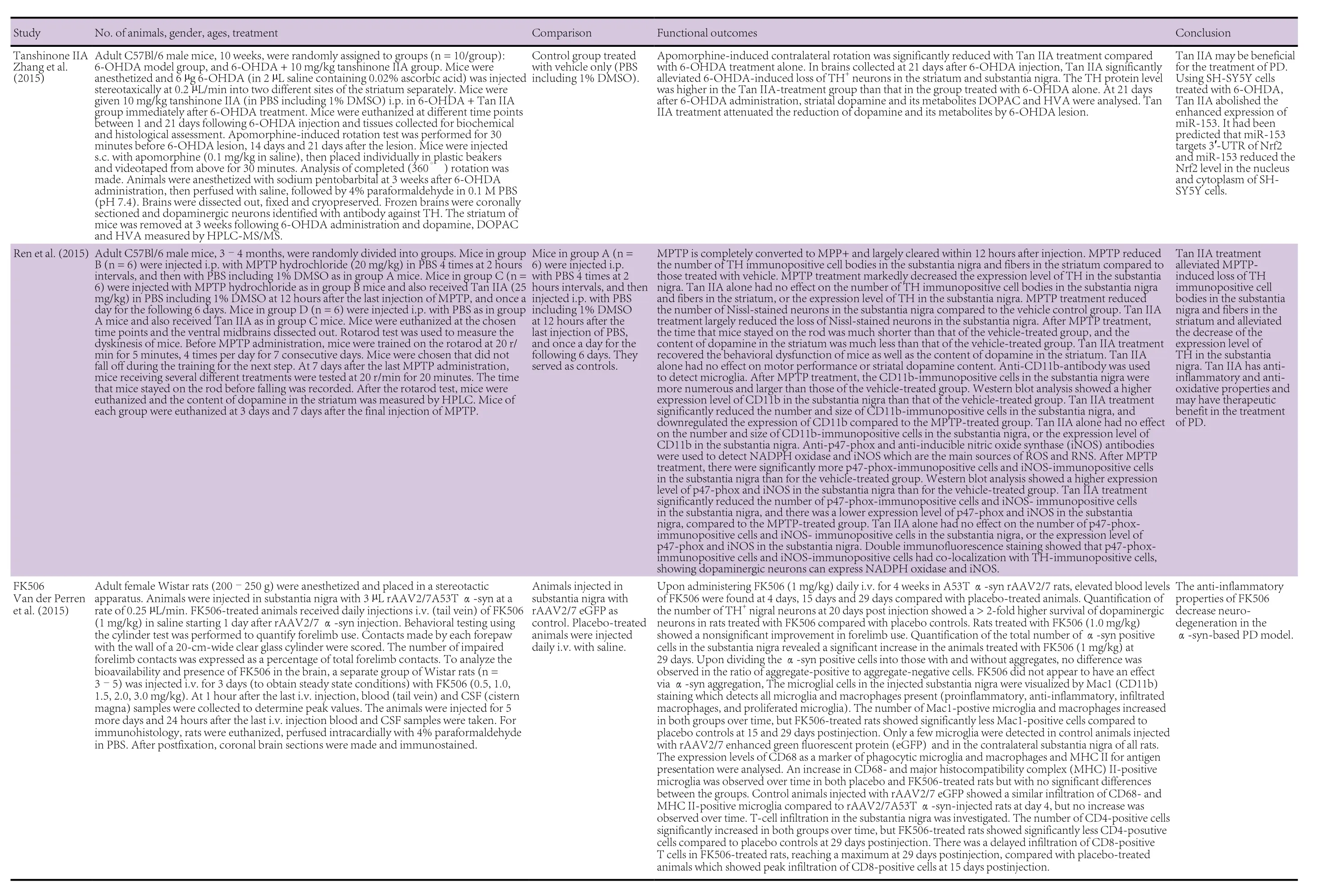
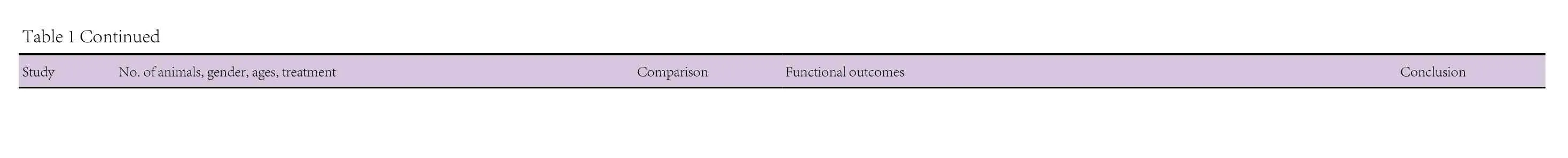
The pharmaceutical therapies were with fingolimod (FTY720),acetoside, amphetamine-regulated transcript peptide (CART),tanshinone I, tanshinone IIA, dimethyl fumarate, ginsenoside Rg1, tacrolimus (FK506), lenalidomide, thalidomide, cyclosporin, Nurr1 agonist SA00025, interferon (IFN)-β, semapimod (CNI-1493), and pycnogenol. These have all been shown to have immunomodulatory properties (FTY720: Kovarik et al., 2004; Lakshmikanth et al., 2016; acetoside: He et al.,2011; cocaine- and amphetamine-regulated transcript peptide(CART): Bik et al., 2008; tanshinone I: Lee et al., 2013; tanshinone IIA: Qin et al., 2010; dimethyl fumarate: Albrecht et al.,2012; Strassburger-Krogias et al., 2014; ginsenoside Rg1: Kenarova et al., 1990; FK506: Kaminska et al., 2004; lenalidomide:Kotla et al., 2009; thalidomide: Bodera and Stankiewicz, 2011;cyclosporin: Tajima et al., 2003; Nurr1 agonist SA00025: Maijenburg et al., 2010; IFN-β: Kasper and Reder, 2014; CNI-1493: Martiney et al., 1998; pycnegenol: Cheshier et al., 1995).The twenty animal studies utilizing these pharmaceutical agents are summarized in Table 1. Fourteen of these studies had used mouse models, four had employed rat models, and two had used both mouse and rat models. In the mouse studies,the ages of the animals ranged from 7 weeks to 12 months and where gender was specified had used males. The rat studies had used animals the ages of which, by reference to body weight/age growth charts, would have ranged from 6 to 13 weeks and where gender was specified had used females.
Mouse PD studies FTY720
FTY720 treatment of 6-hydroxydopamine (6-OHDA)- or rotenone-induced PD mice reduced the deficit of motor function and the loss of TH+neurons in the substantia nigra, and attenuated the decrease of striatal dopamine and its metabolite levels (Zhao et al., 2017). FTY720 pretreatment of 6-OHDA-lesioned mice also reduced motor deficits and loss of nigral dopaminergic neurons, while also decreasing 6-OHDA-induced inflammation. Activation of AKT and ERK1/2 pathways and an increase in brain-derived neurotrophic factor (BDNF) expression were associated with the protective effects of FTY720 (Ren et al., 2017). Interestingly,long-term oral FTY720 reduced enteric nervous system α-syn aggregation and constipation, enhanced gut motility, and increased levels of BDNF in transgenic mice overexpressing mutant human α-syn (Vidal-Martinez et al., 2016).
Tanshinone I
Tanshinone I pretreatment of 6-OHDA-lesioned mice ameliorated dopaminergic neurotoxicity in the substantia nigra and striatum. It also protected against 6-OHDA-induced oxidative stress in the striatum by increasing glutathione (GSH) levels after 6-OHDA injection (Jing et al., 2016). In another study,tanshinone I pretreatment of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-injected mice increased retention time on the rotating rod and prevented the decrease in dopamine and its metabolites. It also alleviated the reduction in dopaminergic TH+neurons in the substantia nigra associated with MPTP treatment. Tanshinone I pretreatment inhibited the MPTP-induced microglial activation in the substantia nigra and striatum, attenuated the increase in the brain level of tumor necrosis factor-α (TNF-α), and preserved the increase of interleukin (IL)-10 level (Wang et al., 2015).
Tanshinione IIA
Tanshinione IIA given immediately after 6-OHDA treatment reduced apomorphine-induced contraleral rotations and alleviated 6-OHDA-induced loss of TH+neurons in the substantia nigra and striatum. Tanshinione IIA also attenuated the reduction of dopamine and its metabolites associated with 6-OHDA lesioning (Zhang et al., 2015). Similar findings with tanshinione IIA were reported earlier and it was also shown to decrease the number and size of CD11b-immunopositive cells in the striatum and downregulate the expression of CD11b in the striatum which was increased by MPTP treatment. Tanshinione IIA inhibited NADPH oxidase and inducible nitric oxide synthase (iNOS) in the substantia nigra which are the main sources of ROS and RNS (Ren et al., 2015).
Dimethyl fumarate
Dimethyl fumarate reduced the motor deficit, protected dopaminegic neurons in the substantia nigra against α-syn toxicity,and decreased microgliosis and astrogliosis after delivery of adeno-associated viral vector expressing human α-syn to the ventral midbrain. The protective effect was not found to occur in Nrf2-knockout animals (Lestes-Becker et al., 2016). An earlier study using dimethyl fumarate pretreatment showed it protected against 6-OHDA-induced oxidative stress by reducing ROS and increasing glutathione in the striatum. It protected against 6-OHDA-induced loss of TH+neurons in the substantia nigra and striatum, decreased microgliosis and astrogliosis, and attenuated the reduction in striatal dopamine and its metabolites in 6-OHDA lesioned animals. Dimethyl fumarate also decreased apomorphine-induced asymmetrical rotations contralateral to the 6-OHDA intrastriatal injection site (Jing et al., 2015).

?
?

?

?
?
?

?

?

?

?

?
Lenalidomide
Lenalidomide reduced motor deficits and ameliorated dopamine fiber loss in the striatum, together with a decrease in microgliosis in the striatum and hippocampus, in mThy1-αsyn transgenic animals. Lenalidomide reduced the expression of the proinflammatory cytokines TNF-α, IL-6, IL-1β, and IFN-γ and increased the expression of the anti-inflammatory cytokines IL-10 and IL-13, as well as inhibiting NF-κB signaling in mThy1-α-syn transgenic animals. CX3CL1 (Fractalkine) level in transgenic animals was increased by lenalidomide treatment (Valera et al., 2015).
Thalidomide
Thalidomide, like lenalidomide, restored dopamine fiber loss in the striatum, and reduced TNF-α, IL-6, IL-1β, and IFN-γ expression in mThy1-α-syn transgenic animals.However, it did not affect microgliosis in the striatum and hippocampus, or the expression of IL-10 in transgenic animals(Valera et al., 2015). Thalidomide treatment before or after MPTP exposure increased dopamine content in the striatum and decreased monoamine oxidase B in the substantia nigra and striatum. In addition, thalidomide given before or after MPTP exposure lowered lipid peroxidation products in the substantia nigra and striatum (Palencia et al., 2015).
Ginsenoside Rg1
Ginsenoside Rg1 decreased MPTP-induced dopaminergic neuronal loss in the substantia nigra. The ratio of CD3+CD4+to CD3+CD8+T cells and CD4+CD25+Foxp3+regulatory T cells in the blood were increased in MPTP-induced animals following Rg1 treatment. The serum levels of TNF-α, IFN-γ,IL-1β and IL-6 in MPTP-induced animals were reduced by Rg1. Microgliosis was inhibited and infiltration of CD3+T cells into the substantia nigra of MPTP-lesioned animals was reduced by Rg1 treatment (Zhou et al., 2015).
IFN-β
Age-associated motor learning defects, neuromuscular deficiencies, and cognitive impairment were caused by deletion of Ifnb gene, which encodes IFN-β. Ifnb–/–pathology was associated with LBs resulting from defective neuronal autophagy(Ejerskov et al., 2015).
CNI-1493
CNI-1493 attenuated dopaminergic cell loss in the substantia nigra and alleviated striatal loss of dopamine content in MPTP-injected animals. CNI-1493 reduced microgliosis in the substantia nigra of MPTP-treated animals (Noelker et al.,2013).
Pycnogenol
Pycnogenol improved behavioral motor deficits of MPTP-injected animals. Lipid peroxidation products were reduced by pycnogenol and the activities of antioxidant enzymes and glutathione were increased by pycnogenol in MPTP-lesioned animals. Also pycnogenol attenuated dopamine depletion in the striata and reduced the nigrostriatal dopaminergic neuron loss following MPTP injection. Pycnogenol treatment reduced microgliosis and astrogliosis in MPTP-injected animals. In addition, pycnogenol pretreatment attenuated the activation of NF-κB in nuclear extracts. The MPTP-induced expression of cyclooxygenase-2 (COX-2) and iNOS protein and the secretion of TNF-α and IL-1β in the striatum were inhibited by pycnogenol (Khan et al., 2013).
Cyclosporin
Cyclosporin enhanced motor and cognitive function in Thy1-α-syn transgenic animals, decreased the striatal level of human α-syn and partially restored the level of TH protein. Cyclosporin enhanced motor function, exhibited an anti-inflammatory effect by lowering the expression level of NFATc3 and alleviated mitochondrial stress in the midbrain of MPTP-lesioned animals. The expression levels of GFAP and GLT-1 in the striatum of MPTP-treated animals were also reduced by cyclosporine, suggesting that it reduced astrogliosis and glutamate levels, the latter being associated with a lowering of excitotoxicity (Tamburino et al., 2015).
Rat PD studies IFN-β
Lentiviral IFN-β overexpression arrested dopaminergic neuron loss in a familial PD model induced by injecting human α-syn (hSCNA) in the substantia nigra of animals (Ejerskov et al., 2015).
Acetoside
Parkinsonism symptoms were attenuated by administration of acetoside in rotenone-injected animals. Acetoside suppressed rotenone-induced α-syn, caspase-3 upregulation and microtubule-associated protein 2 (MAP2) downregulation (Yuan et al., 2016).
CART
Pretreatment with CART restored TH+content in the substantia nigra and decreased apomorphine-induced contralateral rotations in 6-OHDA lesioned animals (Upadhya et al., 2016).
FK506
FK506 increased the survival of dopaminergic neurons in a rAAV2/7 α-syn overexpression model. α-Syn aggregation was not decreased, but the infiltration of both T helper and cytotoxic T cells and the number and subtype of microglia and macrophages were lowered by FK506. At 15 days the percentage of ‘isolated activated microglia’ in substantia nigra increased but was less prominent in FK506- than placebo-treated animals. At 29 days microglial cells with ‘tendency to form clusters’ were mainly present in placebo group and more abundant than in FK506-treated animals (Van der Perren et al., 2015).
Cyclosporin
Cyclosporin treatment following AAV α-syn vector injection into substantia nigra and receiving mesencephalic neural cell graft resulted in larger-sized grafts with an increased number of dopamine neurons formed from the graft than in the vehicle-treated group (Tamburino et al., 2015).
Nurr1 agonist SA00025
SA00025 was partially neuroprotective of dopaminergic neurons and fibers in animals receiving a priming injection of polyinosinic-polycytidylic acid (to exacerbate inflammation)and subsequent injection of 6-OHDA. SA00025 brought about changes in microglial morphology indicative of a resting state and a decrease in reactive microglia, together with a decrease in microglial Iba-1 staining intensity in the substantia nigra.SA00025 also decreased astrocyte GFAP staining intensity in the substantia nigra and IL-6 levels (Smith et al., 2015).
Neuroprotective Effects of Immunomodulatory Agents in PD
Neuroprotective therapy for PD aims to protect the at-risk dopaminergic neurons in the substantia nigra from degeneration that results in premature cell death and depletion of dopamine.It is envisaged that neuroprotective drugs could be used to treat patients with early clinical signs of the disease or potentially even prior to disease onset in those identified as having pre-disposing risk, including genetic factors (Tarsy, 2017).
Pharmaceutical therapies that target inflammation and the immune response are promising approaches for the treatment of PD. The pharmaceutical studies described in this review have identified several agents with immunomodulatory properties that protected dopaminergic neurons from degeneration and death in animal models of PD. All of the agents were effective in reducing the motor deficit and alleviating dopaminergic neurotoxicity and, when measured, prevented the decrease of dopamine upon being administered therapeutically after MPTP-, 6-OHDA-, rotenone-lesioning or delivery of AAV-α-syn to the ventral midbrain of animals. Interestingly, pretreatment with FTY720 (Zhao et al., 2017), tanshinoine I (Jing et al., 2016), dimethyl fumarate (Jing et al., 2015), thalidomide(Palencia et al., 2015), or CART (Upadhya et al., 2016) as a preventive strategy ameliorated motor deficits and nigral dopaminergic neurotoxicity in brain-lesioned animals. When tested for in animal models of PD, agents such as tanshinone I (Jing et al., 2016), tanshinone IIA (Ren et al., 2015), dimethyl fumarate (Jing et al., 2015), and pycnogenol (Khan et al., 2013) decreased oxidative stress. Also lipid peroxidation products were lowered by thalidomide (Palencia et al., 2015) and pycnogenol(Khan et al., 2013). Tanshinone I (Wang et al., 2015), lenalidomide (Valera et al., 2015), thalidomide (Valera et al., 2015),Rg1 (Zhou et al., 2015), pycnogenol (Khan et al., 2013) and SA00025 (Smith et al., 2015) decreased the levels of proinflammatory cytokines TNF-α, IL-6, IL-1β, and IFN-γ, while the levels of anti-inflammatory cytokines IL-10 and IL-13 were maintained or increased by tanshinone I (Wang et al., 2015)and lenalidomide (Valera et al., 2015). Cyclosporin exhibited an anti-inflammatory effect by lowering the expression level of NFATc3 in the midbrain of MPTP-lesioned animals (Tamburino et al., 2015). Microgliosis was decreased by dimethyl fumarate (Jing et al., 2015; Lestes-Becker et al., 2016), lenalidomide (Valera et al., 2015), Rg1 (Zhou et al., 2015), CNI-1493(Noelker et al., 2013), pycnogenol (Khan et al., 2013), and SA00025 (Smith et al., 2015), while astrogliosis was reduced by dimethyl fumarate (Jing et al., 2015; Lestes-Becker et al., 2016),pycnogenol (Khan et al., 2013), cyclosporine (Tamburino et al., 2015), and SA00025 (Smith et al., 2015) in animal PD models. FK506 inhibited the infiltration of both T helper and cytotoxic T cells and decreased the number and subtype of microglia and macrophages (Van der Perren et al., 2015), whereas tanshinone IIA reduced the number and size of CD11b+cells in the striatum (Ren et al., 2015). Rg1 inhibited the infiltration of CD3+T cells into the substantia nigra and increased the ratio of CD3+CD4+to CD3+CD8+T cells and CD4+CD25+Foxp3+regulatory T cells in the blood (Zhou et al., 2015). The actions of the immunomodulatory agents in inhibiting microgliosis and astrogliosis and lowering the levels of pro-inflammatory cytokines and NFATc3, as well as modifying the infiltration of immune cells, are consistent with decreasing the neuroinflammation associated with aggregation of α-syn and thereby reducing neuronal degeneration and death (Rai et al., 2017; von Euler Chelpin and Vorup-Jensen, 2017).
Future Perspectives
Persistent inflammatory responses, involving T cell infiltration and microglial cell activation, are common characteristics of human patients with PD and involved in the degeneration of dopaminergic neurons. There is a need to develop therapeutic strategies that can impede or halt the disease through the modulation of the peripheral immune system by controlling the existing neuroinflammation (von Euler Chelpin and Vorup-Jensen, 2017). Several potential neuroprotective agents for PD had shown some promise in animals and/or humans, including selegiline and rasagiline (both monoamine oxidase inhibitors),and the natural substance coenzyme Q10. However, no treatment had proven to be effective for neuroprotection in human PD patients (Tarsy, 2017).
6-OHDA administration induces an intense IgG deposition in the substantia nigra as well as increased infiltration of both T-and B- lymphocytes into the injected side of the midbrain. The adaptive immune response was associated with extensive degeneration of dopamine neurons and microglial activation (Theodore and Maragos, 2015). Classically activated neuroinflammatory microglia by secreting IL-1α, TNF-α and C1q induce a subtype of reactive astrocytes termed A1, and are strongly induced by CNS injury and disease. A1 astrocytes lose the abili-ty to promote neuronal survival, outgrowth, synaptogenesis and phagocytosis, and induce neuron and oligodendrocyte death(Liddelow and Barres, 2017; Liddelow et al., 2017). Normal aging was shown to induce neuroinflammatory A1-like astrocyte reactivity (Clarke et al., 2018) and would suggest that it is involved in the onset of PD. Some of the pharmaceutical agents reviewed herein could have potential by reducing the number and functional state of activated microglia and astrocytes (Figure 1) and inhibiting T cell infiltration into the PD brain.
Most of the mouse and rat studies reviewed had been performed with relatively young adult animals. Future studies need to be conducted with aged animals. This is particularly relevant with regard to the role of neuromelanin in neuroinflammation. Neuromelanin is formed by the oxidation of dopamine(Segura-Aguilar et al. 2014) and is present in the neurons of the substantia nigra with increasing amounts in cats, dogs, primates and humans (DeMattei et al., 1986). It was previously concluded that rodents did not possess neuromelanin (Marsden, 1983) but this seems to be an artifact of the young age of the animals studied as it has now been established that they can and do accumulate neuromelanin with its concentration being dependent on age (Zecca et al., 2001). In very old rats (23 months), but not in younger animals, neuromelanin granules were detected by electron microscopy (DeMattei et al., 1986).Accumulated neuromelanin is known to trap and bind PD-inducing toxins, making neurons containing these granules more susceptible to toxic insult. The presence of extraneuronal neuromelanin has been investigated in human subjects with idiopathic PD and MPTP exposure (McGeer et al., 1988; Langston et al., 1999). Most of the extraneuronal neuromelanin is phagocytosed by microglia resulting in microglia and astrocyte activation. It suggests that neuromelanin could be the effector of the chronic inflammation in the substantia nigra and degeneration of dopaminergic neurons in PD.
It is likely that many of the human PD patients are taking medication, and an observational study concluded that diabetes prevalence was closely similar between patients with PD and subjects without the disease (Becker et al., 2008). Animal models of PD should also incorporate possible medications that could be used by human PD patients such as L-dopa, antidiabetic, antihypertensive, and antihyperlipidemic drugs. Also both male and female animals should be used. Where gender was specified, the mouse studies had used males, whereas the rat studies had used females. It was surprising that no in vivo studies were found in the PubMed search of the effects of immunomodulatory agents in human PD patients. It would seem that some of the pharmaceutical therapies described in the recent animal PD studies and reviewed here would warrant being trialed in human patients. In addition, cell-based therapies with immunomodulatory properties such as mesenchymal stem cells (MSCs), human umbilical cord blood cells, and endothelial progenitor cells could also be investigated for possible benefit in PD patients. MSCs were reported to stabilize axonal transports for autophagic clearance of α-syn in Parkinsonian models (Oh et al, 2017). MSC therapy was found to improve clinical outcome in patients with stable chronic stroke (Steinberg et al., 2016).
A future translational task will be to exploit endogenous mechanisms of neuroprotection for therapeutic purposes by combining behavioral and pharmacological interventions. This type of approach is likely to benefit many PD patients, despite the clinical, etiological, and genetic heterogeneity of the disease(Francardo et al., 2017).
Author contributions: Both authors contributed to the study equally.
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
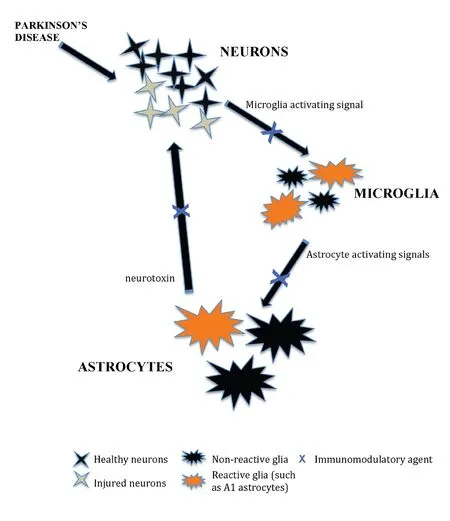
Figure 1 A schematic to illustrate the effect of immunomodulatory agents in enhancing the survival of dopaminergic neurons in animal models of Parkinson’s disease by reducing microgliosis and astrogliosis (modified from Liddelow and Barres, 2017).
- 中国神经再生研究(英文版)的其它文章
- Seeing the wood for the trees: towards improved quantification of glial cells in central nervous system tissue
- 3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
- The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis
- Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats
- Saikosaponin a increases interleukin-10 expression and inhibits scar formation after sciatic nerve injury
- Various changes in cryopreserved acellular nerve allografts at −80° C

