Saikosaponin a increases interleukin-10 expression and inhibits scar formation after sciatic nerve injury
Meng-Qiang Huang , Xiao-Yu Cao , Xu-Yi Chen , Ying-Fu Liu, Shuang-Long Zhu Zhong-Lei Sun, Xian-Bin Kong, Jing-Rui Huo, Sai Zhang , Yun-Qiang Xu
1 Graduate School, Tianjin Medical University, Tianjin, China
2 Department of Orthopedics, Tianjin Medical University General Hospital, Tinajin, China
3 Department of Rehabilitation Medicine, First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province, China
4 Tianjin Key Laboratory of Neurotrauma Repair, Institute of Traumatic Brain Injury and Neuroscience, Center for Neurology and Neurosurgery of Affiliated Hospital, Logistics University of Chinese People’s Armed Police Force, Tianjin, China
5 Science and Technology Experiment Center, Cangzhou Medical College, Cangzhou, Hebei Province, China
6 Graduate School, Jinzhou Medical University, Jinzhou, Liaoning Province, China
7 Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Abstract Nerve scarring after peripheral nerve injury can severely hamper nerve regeneration and functional recovery. Further, the anti-inflammatory cytokine, interleukin-10, can inhibit nerve scar formation. Saikosaponin a (SSa) is a monomer molecule extracted from the Chinese medicine,Bupleurum. SSa can exert anti-inflammatory effects in spinal cord injury and traumatic brain injury. However, it has not been shown whether SSa can play a role in peripheral nerve injury. In this study, rats were randomly assigned to three groups. In the sham group, the left sciatic nerve was directly sutured after exposure. In the sciatic nerve injury (SNI) + SSa and SNI groups, the left sciatic nerve was sutured and continuously injected daily with SSa (10 mg/kg) or an equivalent volume of saline for 7 days. Enzyme linked immunosorbent assay results demonstrated that at 7 days after injury,interleukin-10 level was considerably higher in the SNI + SSa group than in the SNI group. Masson staining and western blot assay demonstrated that at 8 weeks after injury, type I and III collagen content was lower and nerve scar formation was visibly less in the SNI + SSa group compared with the SNI group. Simultaneously, sciatic functional index and nerve conduction velocity were improved in the SNI + SSa group compared with the SNI group. These results confirm that SSa can increase the expression of the anti-inflammatory factor, interleukin-10, and reduce nerve scar formation to promote functional recovery of injured sciatic nerve.
Key Words: nerve regeneration; saikosaponin a; anti-inflammatory factor; inflammation; interleukin-10; nerve scar; peripheral nerve injury; sciatic nerve injury; sciatic functional index; nerve conduction velocity; neuroelectrophysiological function; neural regeneration
Introduction
Peripheral nerve injury is a common clinical injury (Li et al.,2014). Currently, there are many available methods to treat peripheral nerve injury such as autologous nerve graft (the ‘gold standard’) (Masgutov et al. 2018), micro-suture (Leuzzi et al., 2014), and tissue-engineered nerve catheter bridges (Rbia et al., 2017). However, these methods have limitations or side effects, and it is necessary to identify auxiliary methods to promote peripheral nerve repair and regeneration (Rbia et al.,2017). A series of pathophysiological changes occur after pe-ripheral nerve injury including Wallerian degeneration (Gaudet et al., 2011; Tricaud et al., 2017), apoptosis (Zhao et al., 2017),dedifferentiation of Schwann cells (Wang et al., 2015), scarring(Lemke et al., 2018), inflammation (Hartlehnert et al., 2017),and edema (Chen et al., 2015). Inflammation and scarring can hamper regeneration of injured nerve, and may even lead to nerve necrosis and other severe consequences (Han et al.,2016; Li et al., 2017). Moreover, there is evidence showing that the formation of scarring after peripheral nerve injury is strongly associated with the occurrence of inflammation (Atkins et al., 2007; Yao et al., 2018). Therefore, it may be possible to inhibit scar formation to promote regeneration and repair of nerve by inhibiting inflammation (Atkins et al., 2006).
Interleukin-10 (IL-10) is the most important anti-inflammatory factor, and plays a key role in inflammation after peripheral nerve injury (Khan et al., 2015). Once a peripheral nerve is injured, Schwann cells and macrophages rapidly produce proinflammatory cytokines, leading to inflammatory reactions(Mietto et al., 2015). The role of IL-10 is to reduce and gradually stop inflammation, and provide a good microenvironment for regeneration of peripheral nerve (Taskinen et al., 2000;Siqueira et al., 2015). Atkins et al., (2007) reported that low doses of IL-10 reduces scar formation after sciatic nerve injury(SNI) and promotes axonal regeneration. This suggests that drugs that increase IL-10 expression may inhibit nerve scar formation and promote repair of peripheral nerve injury.
Radix Bupleuri is an important herbal medicine in China,and used as a traditional medicine in Asia for a number of diseases (Yang et al., 2017). Saikosaponin a (SSa) is an important active saikosaponin of Radix Bupleuri, and plays a crucial role in anti-inflammatory (Lu et al., 2012; Zhu et al., 2013; Fu et al., 2015), antitumor (Kang et al., 2017), antiviral (Chen et al., 2015), neuromodulatory, and immunoregulatory activities (Yuan et al., 2017). Studies have shown that SSa plays an active role in alleviating inflammation (Chen et al., 2018),reducing cerebral edema in traumatic brain injury rats (Mao et al., 2016), and inhibiting the nuclear factor kappa B inflammatory pathway to relieve neuropathic pain in rats (Zhou et al., 2014). However, it has not yet been reported whether SSa can promote repair of peripheral nerve injury. In this study, we focused on clarifying the role of SSa in peripheral nerve injury,and investigated the effect of SSa on the inflammatory response and nerve scar formation after peripheral nerve injury. Our findings provide a theoretical basis for auxiliary repair of SSa for peripheral nerve injury.
Materials and Methods
Drugs
SSa (purity > 98%; Nanjing Pu Yi Biological Technology Co.,Ltd., Nanjing, China) was dissolved in 0.9% saline at a ratio of 1:1, stored at −20° C, and shaken before each experiment.
Animals
Sixty male Sprague–Dawley rats (aged 6 weeks, weighing 250–300 g) were purchased from the Academy of Military Medical Sciences Laboratory Animal Center, China (license No. SCXK [Army] 2012-0004). The rats were housed in cages and maintained at 24°C on a standard 12-hour light/dark cycle (lights on at 7:00 a.m.). Rats had free access to food and water until 24 hours before SNI. This study was approved by the Animal Care and Use Committee of Tianjin Medical University, China.
Group assignment and surgical procedures
All rats were randomly assigned to three groups (n = 20), and treated with SSa or physiological saline: (1) sham group; (2)SNI group: SNI + physiological saline; (3) SNI + SSa group:SNI + 5 mg/kg SSa.
Rats were intraperitoneally anesthetized with 4% chloral hydrate (1 mL/100 g; Qingdao Yulong Alga Co., Ltd., Qingdao,China). SNI models were generated according to a previous method (Horasanli et al., 2017). For each rat, all four limbs were fixed in the prone position. After shaving, a longitudinal incision was made on the dorsal side of the left lower limb to expose the biceps femoris. The biceps femoris was bluntly dissociated to expose the sciatic nerve. The superior segment of the sciatic nerve was transected and an epineural neurorrhaphy was performed with a No.10-0 Prolene suture (Shanghai Medical Suture Needle Factory Co., Ltd., Shanghai, China). The biceps femoris was sutured using an absorbable suture and the skin sutured with silk sutures (Figure 1). After disinfecting, the wound was dressed. In the sham group, the muscles and skin were sutured directly after sciatic nerve exposure.
Rats in the SNI and SNI + SSa groups were injected with SSa or saline (at a dose of 10 mg/kg), respectively, at the injury site within 15 minutes after operation. Rats were injected once daily for 7 days. The dose was decided based on drug solubility and a previous study (Zhou et al., 2014). Twenty-four hours after surgery, the left lower limb of rats in the SNI and SNI +SSa groups had contracted and would not move. In contrast,the left lower limb was basically normal in rats of the sham group. This indicated successful establishment of our models.
Sciatic functional index (SFI)
Rat SFI score was evaluated as previously described (Yao et al., 2016). At different time points after injury (1, 7, 14, 28, and 56 days), hind feet of the rats were dipped in ink, and the rats allowed to walk across a plastic tunnel. The footprints were recorded on paper loaded onto the bottom of the tunnel. Distance between the top of the third toe and the most posterior part of the foot in contact with the ground (print length, PL),distance between the first and fifth toes (toe spread, TS), and distance between the second and fourth toes (intermediary toe spread, ITS) were measured on the experimental side (EPL,ETS, and EITS, respectively) and on the contralateral normal side (NPL, NTS, and NITS, respectively). SFI was calculated as follows (Bain et al., 1989): SFI = 109.5 × (ETS − NTS)/NTS −38.3 × (EPL − NPL)/NPL + 13.3 × (EITS − NITS)/NITS −8.8.
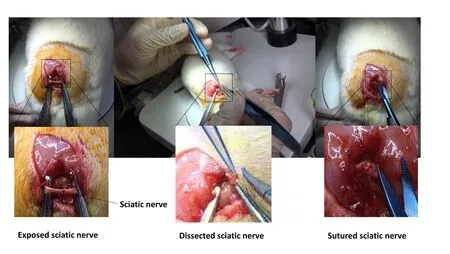
Figure 1 Modeling schematic.

Figure 2 SSa increased IL-10 level at 7 days after sciatic nerve injury in rats(enzyme linked immunosorbent assay).
In general, SFI values of approximately 0 indicated normal nerve function and −100 indicated total dysfunction.Enzyme linked immunosorbent assay (ELISA)Seven days after surgery, six rats in each group were sacrificed and the left sciatic nerve removed. The lesion was taken as the center, with an overall length of approximately 2 cm. Tissue samples were weighed and homogenized in a 1:9 ratio with phosphate-buffered saline, and then centrifuged to collect supernatant liquid. Optical density values of sample liquid were measured using a rat IL-10 ELISA kit (Lanpai Biological Technology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. A concentration histogram was plotted by calculating the concentration of each factor in the sample based on a standard curve.
Nerve conduction velocity
Electrophysiological evaluation was performed on day 56 after injury. Five rats were selected from each group and the left nerve exposed following anesthesia. Briefly, electrical stimuli were applied to the injured sciatic nerve site using a biological function experimental system (Viasys Solar Secure, Inc., San Diego, CA, USA). The stimulating electrode was then moved distally by a fixed 10-mm distance. Compound muscle action potentials were compared with the contralateral control nerve,and expressed as percentages. Motor nerve conduction velocity was calculated from the latency and distance between the two stimulating positions (i.e., 10 mm) (Lin et al., 2017).
Masson staining
Eight weeks after surgery, five rats in each group were sacrificed and the left sciatic nerve removed. The lesion was taken as the center, with an overall length of approximately 2 cm.Slices were dewaxed and stained with 10% potassium dichromate and 10% trichloroacetic acid for 30 minutes. Nuclei were stained with hematoxylin for 20 minutes. Cells were differentiated with hydrochloric acid and ethanol for 15 seconds,returned to blue with weak ammonia for 15 seconds, stained with Masson solution (Cell Signaling Technology, Irvine, CA,USA) for 1 minute, rinsed with 1% acetic acid, and dehydrated with an increasing ethanol series. Sections were permeabilized with xylene I and II for 10 minutes and finally mounted in resin. Collagen fiber proliferation in longitudinal sections was observed by light microscopy (Olympus, Tokyo, Japan). Cell nuclei were stained red and collagen fibers blue.
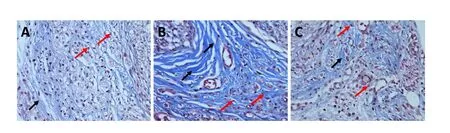
Figure 3 SSa reduced nerve scar formation at 8 weeks after sciatic nerve injury in rats (Masson staining, optical microscope).
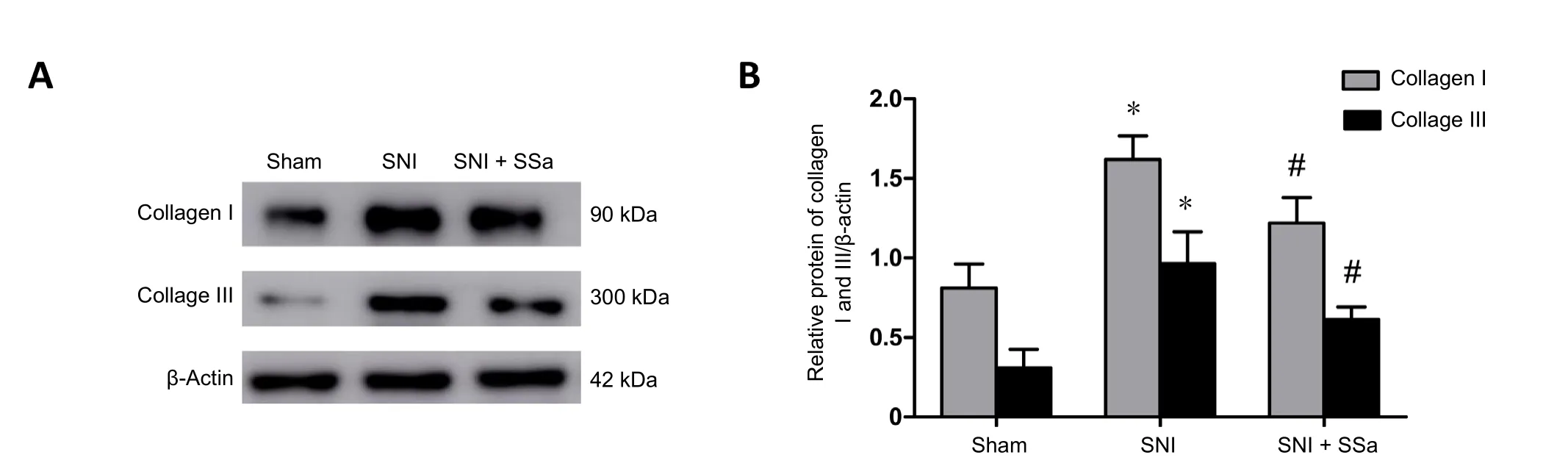
Figure 4 SSa reduces expression of type I and III collagen at 8 weeks after sciatic nerve injury in rats.
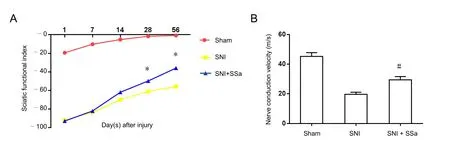
Figure 5 SSa promoted recovery of motor function and nerve conduction function at 8 weeks after sciatic nerve injury in rats.
Western blot assay
At 8 weeks after surgery, four rats in each group were sacrificed as described above. Near the injury site, approximately 1 cm sciatic nerve was collected for further western blot assay.Samples were homogenized on ice. Protein samples (30 μg)were mixed with sample buffer, supplemented with 0.0625 M Tris-HCl (pH 6.8), 2% (w/v) sodium dodecyl sulfate, 5% (w/v) β-mercaptoethanol, 10% (v/v) glycerin, and 0.002% (w/v)bromophenol blue for 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Zanotto et al., 2013). After separation, protein samples were transferred onto polyvinylidene difluoride membrane for incubation at room temperature for 1 hour with rabbit anti-rat type I collagen polyclonal antibody(Abcam, Shanghai, China or rabbit anti-rat type III collagen polyclonal antibody (Abcam) (both diluted to 1:500). After three washes with saline, protein samples were incubated with anti-rabbit or anti-mouse peroxidase-conjugated immunoglobulin (IgG, diluted at 1:10,000) at room temperature for 1 hour (Desoubeaux et al., 2017). Chemiluminescence signal was detected using an enhanced chemiluminescence Kit(Beyotime Institute of Biotechnology, Nanjing, China) and analyzed using ScionImage software (ImageJ, Bethesda, MD,USA). Rabbit anti-rat β-tubulin polyclonal antibody (1:1000;Abcam) was used as an internal reference.
Statistical analysis
Data are expressed as the mean ± SD and were analyzed using SPSS 22.0 software (IBM, Armonk, IL, USA). Statistical analysis was performed using one-way analysis of variance followed by the least significant difference post-hoc test. P-values less than 0.05 were considered statistically significant.
Results
SSa increased level of anti-inflammatory factor IL-10 after SNI Chen et al. (2015) reported that IL-10 level peaked at 7 days after SNI in rats. Level of IL-10 in the sciatic nerve of three groups of rats was examined by ELISA at 7 days after surgery. Our results showed normal IL-10 level in the sciatic nerve of rats in the sham group, while expression was slightly higher in the SNI group compared with the sham group (P < 0.05), which may reflect protective immunity in rats. Meanwhile, IL-10 level was significantly higher in the SNI + SSa group compared with the SNI group (Figure 2; P < 0.01), indicating that SSa increases IL-10 expression.
SSa inhibited nerve scar formation after SNI
Nerve scar is the glial scar formed by accumulation of type I and III collagen after nerve injury, and which forms a physical barrier between injured axons and severely hampers axonal regeneration (Benga et al., 2017). Masson staining detected nerve scar formation in all three groups of rats at 8 weeks after surgery. Further, the amount of type I and III collagen was determined by western blot assay. The Masson staining results showed almost no blue collagen fibers in the sham group,suggesting there was no neural scarring. However, many blue collagen fibers were detected in the SNI group (Figure 3).Compared with the SNI group, the number of blue collagen fibers was considerably reduced in the sciatic nerve of the SNI+ SSa group, but also markedly increased compared with the sham group. These results indicate that SSa reduced nerve scar formation after SNI in rats. Moreover, western blot assay results showed that expression of type I and III collagen was significantly increased in the SNI group compared with the sham group, while expression was significantly decreased in the SNI+ SSa group compared with the SNI group (Figure 4; P < 0.05).Altogether, Masson staining and western blot assay results indicate that treatment with SSa reduces nerve scar formation after SNI in rats.
SSa promoted recovery of lower extremity motor function and neurophysiological function
SFI was used to assess recovery of lower extremity motor function at various time points after SNI. Additionally, nerve conduction velocity was used to assess recovery of neurophysiological function at 8 weeks after surgery. Rats in the sham group had a slightly lower SFI value at only 1 week after surgery because of trauma caused by sciatic nerve exposure and its slightly worsened motor function. After this, the SFI value began to rise and was almost close to 0, indicating that lower extremity motor function returned to normal in rats of the sham group. In the first two weeks after surgery, the SFI value of rats in both the SNI group and SNI + SSa group was low and close to −100, indicating almost complete loss of motor function in the lower extremities. Thus, the SNI model was successfully established in both groups. However, at 4 and 8 weeks after surgery, higher than before SFI values were found in the SNI+ SSa group and SNI group, indicating gradual recovery of motor function. Meanwhile, SFI value was higher in the SNI+ SSa group than SNI group (Figure 5A; P < 0.05). Thus, SSa promotes recovery of lower extremity motor function. Neurophysiological function of rats in all three groups was also evaluated at 8 weeks after surgery. These results found normal nerve conduction velocity of rats in the sham group, indicating normal sciatic nerve function. Furthermore, nerve conduction velocity was lower in rats from the SNI group and SNI + SSa group than the sham group. While nerve conduction velocity was higher in rats of the SNI + SSa group than the SNI group,indicating improved recovery of sciatic nerve function in the SNI + SSa group compared with the SNI group (Figure 5B; P< 0.05). Altogether, this suggests that SSa promotes recovery of lower extremity motor function and neurophysiological function.
Discussion
Inflammation and scarring after peripheral nerve injury play the dual role of inhibiting and promoting nerve regeneration(Barton et al., 2017). In the early stage of injury, the release of inflammatory cytokines and scar formation are a self-protective response (Ngeow, 2010; Lang et al., 2014). However, once the inflammatory factors accumulate in large quantities and scar tissue further forms, nerve regeneration is inhibited (Benga et al., 2017).
Inflammation includes recruitment of inflammatory cells and release of inflammatory cytokines (Dubový et al., 2013).Schwann cells express proinflammatory factors and chemokines immediately after peripheral nerve injury. These factors trigger recruitment and activation of macrophages, which are the major cells that express the anti-inflammatory cytokine, IL-10. IL-10 can inhibit pro-inflammatory cytokines to exert an anti-inflammatory effect (Potas et al., 2015; Wang et al., 2015;Chen and Jin, 2016; Hartlehnert et al., 2017). Simultaneously,IL-10 facilitates nerve regeneration and functional recovery after peripheral nerve injury (Fregnan et al., 2012). Siqueira et al. (2015) investigated the role of IL-10 in the inflammatory response and functional recovery after peripheral nerve injury.They found that IL-10-deficient mice failed to rapidly reduce expression of pro-inflammatory cytokines, with recovery function and axonal regeneration affected. Our ELISA results showed increased IL-10 expression in the SNI + SSa group at one week after SNI in rats, whereas IL-10 expression was only slightly increased in the SNI group compared with the sham group. We believe that SSa increases IL-10 expression, while a slight increase of IL-10 in the SNI group might reflect the rat immune response to injury. Simultaneously, our SFI and nerve conduction velocity results showed that SSa promoted recovery of lower extremity motor function and neurophysiological function. Therefore, we conclude that SSa increases IL-10 expression and promotes recovery of neurological function.
Inflammatory responses occur after peripheral nerve injury.Fibroblasts in the resting state largely activate, proliferate, and produce many type I and III collagens (da Silva et al., 2017;Li et al., 2018). The extracellular matrix formed by collagen accumulates in tissue and is not easily absorbed by the body,resulting in scar formation and inhibition of axonal regeneration (Hara et al., 2017). Nerve scar formation not only prevents passage of regenerative axons, but also causes the nerve and surrounding tissue to become adhesive, resulting in ischemic nerve changes and further damage (Ko et al., 2018).Consequently, inhibiting scar formation can promote repair and regeneration of peripheral nerve (Li et al., 2018). Xue et al. (2016) reported that calcium channel blockers inhibit nerve scar formation, and promote functional recovery and nerve regeneration in sciatic nerve injured rats. Li et al. (2016) produced a methylprednisolone microsphere sustained-release membrane to repair the injured sciatic nerve of rats, and found that it effectively inhibited scar formation and promoted nerve regeneration. Our western blot results showed type I and III collagen content was considerably higher in the SNI group than the sham group, while it was lower in the SNI + SSa group than the SNI group. Therefore, treatment with SSa inhibits scar formation. Further, our Masson staining results are consistent with the western blot assay.
A study has shown a direct relationship between scar formation and release of inflammatory cytokines (Atkins et al.,2007). Among them, pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-6 promote nerve scar formation, while anti-inflammatory factors such as IL-10 inhibit nerve scar formation (Khan et al., 2017). Atkins et al.,(2007) injected IL-10 locally into SNI mice, and found that IL-10 reduced nerve scar formation and promoted axonal regeneration and functional recovery. Sakalidou et al., (2011)injected IL-10 into end-to-side peroneal/tibial nerve lesion model rats, and found that IL-10 reduced scar formation and enhanced nerve regeneration. Ngeow et al. (2011) injected IL-10 peptide fragment locally into the SNI site of C57/B6 mice,and again found that IL-10 reduced nerve scar formation and enhanced nerve regeneration. Therefore, we speculate that after SNI in rats, SSa inhibits formation of neural scarring and promotes recovery of neurological function by increasing IL-10 expression.
In summary, SSa can promote recovery of motor and nerve conduction function in SNI rats by increasing expression of the anti-inflammatory cytokine, IL-10, and inhibiting nerve scar formation. In this study, we enriched the methods to repair peripheral nerve injury. However, we did not determine whether SSa inhibits nerve scar formation by increasing IL-10 expression, which still needs to be confirmed by further investigation.
Author contributions: MQH, XYC (Cao), and XYC (Chen) designed the study and wrote the paper. MQH, XYC, SLZ, ZLS, and XBK collected the data. MQH, YFL, JRH, SZ and YQX analyzed the data. All authors approved the final version of the paper.
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the National Natural Science Foundation of China, No. 11672332, 11102235,8167050417; the National Key Research and Development Plan of China, No. 2016YFC1101500; the Key Science and Technology Support Foundation of Tianjin City of China, No. 17YFZCSY00620; the Natural Science Foundation of Tianjin City of China, No. 15JCYBJC28600,17JCZDJC35400. The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Tianjin Medical University of China. All experimental procedures described here were in accordance with the National Institutes of Health(NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.Open peer reviewer: Alonzo D Cook, Brigham Young University, USA.Additional file: Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Seeing the wood for the trees: towards improved quantification of glial cells in central nervous system tissue
- 3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
- The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis
- Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats
- Various changes in cryopreserved acellular nerve allografts at −80° C
- Patterns of cortical reorganization in facial synkinesis: a task functional magnetic resonance imaging study

