3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
Xiao Li , Rui-Zhen Liu , Qi Zeng, Zhi-Hua Huang Jian-Dong Zhang Zong-Liang Liu Jing Zeng , Hai Xiao
1 Gannan Medical University, Ganzhou, Jiangxi Province, China
2 Department of Ultrasound, First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi Province, China
3 Department of Pathology, First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi Province, China
Abstract 3′-Daidzein sulfonate sodium (DSS) is a new synthetic water-soluble compound derived from daidzein, a soya isoflavone that plays regulatory roles in neurobiology. In this study, we hypothesized that the regulatory role of DSS in neurobiology exhibits therapeutic effects on hippocampal damage and memory impairment. To validate this hypothesis, we established rat models of chronic cerebral hypoperfusion (CCH) by the permanent occlusion of the common carotid arteries using the two-vessel occlusion method. Three weeks after modeling, rat models were intragastrically administered 0.1, 0.2, and 0.4 mg/kg DSS, once a day, for 5 successive weeks. The Morris water maze test was performed to investigate CCH-induced learning and memory deficits. TUNEL assay was used to analyze apoptosis in the hippocampal CA1, CA3 regions and dentate gyrus. Hematoxylin-eosin staining was performed to observe the morphology of neurons in the hippocampal CA1, CA3 regions and dentate gyrus. Western blot analysis was performed to investigate the phosphorylation of PKA, ERK1/2 and CREB in the hippocampal PKA/ERK1/2/CREB signaling pathway. Results showed that DSS treatment greatly improved the learning and memory deficits of rats with CCH, reduced apoptosis of neurons in the hippocampal CA1, CA3 regions and dentate gyrus, and increased the phosphorylation of PKA, ERK1/2, and CREB in the hippocampus. These findings suggest that DSS protects against CCH-induced memory impairment and hippocampal damage possibly th rough activating the PKA/ERK1/2/CREB signaling pathway.
Key Words: nerve regeneration, learning and memory deficits; chronic cerebral hypoperfusion; 3′-daidzein sulfonate sodium; PKA/ERK1/2/CREB signaling pathway; neuroprotection; hippocampus; neural regeneration
Introduction
Cerebrovascular disease refers to a group of conditions,such as acute ischemic and chronic cerebral hypoperfusion(CCH), that affect the blood supply to the brain. CCH refers to a reduction in cerebral blood flow of between 25% and 50% that persists over a period of 6 months (Sekhon et al.,1994, 1997). CCH impairs neuronal and capillary structure and function, leading to memory loss and cognitive deficits (Sekhon et al., 1994, 1998). The learning and memory impairments caused by CCH are permanent and seriously affect the daily life of both patients and their families (Ma et al., 2016). Finding effective medicines that can improve the learning and memory impairments associated with CCH is one of the urgent missions in neuroscience research.
3′-Daidzein sulfonate sodium (DSS) is a new synthetic water-soluble compound derived from daidzein, an active ingre-dient of the kudzu vine root. Previous work has indicated that DSS has diverse pharmacological actions such as anti-arrhythmia (Zeng et al., 2006a), anti-oxidation (Zeng et al., 2009),and anti-hypoxia (Zeng et al., 2006b). Furthermore, we found that DSS can protect against myocardial and cerebral ischemia/reperfusion (I/R) injury (Zeng et al., 2009; Zhong et al., 2011).Moreover, DSS was reported to reduce mitochondrial swelling, elevate mitochondrial membrane potential, increase mitochondrial superoxide dismutase and glutathione peroxidase activity, and decrease mitochondrial malondialdehyde levels,indicating that DSS can improve mitochondrial function after a cerebral I/R injury (Yuan et al., 2017). Based on the previous work, we hypothesized that DSS can reduce the learning and memory impairments caused by CCH.
CCH can be simulated in animals by the permanent occlusion of the common carotid arteries using the two-vessel occlusion method (2VO) (Farkas et al., 2007). The 2VO model has been used as a common experimental model to investigate cognitive impairment and neuronal damage (Nanri and Watanabe, 1999; Jia et al., 2012; Lana et al., 2014). In the present study, a rat 2VO model was used to investigate whether 5-week DSS gavage can prevent against CCH-induced learning and memory deficits, and reduce neuronal damage and apoptosis in the hippocampus.
Materials and Methods
Animals
Fifty specific pathogen-free male Sprague-Dawley (SD) rats,aged 8 weeks, weighing 200–240 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., China (licence No. SCXK (Jing) 2016-006). All animals included were housed in a specific pathogen-free animal house and provided with free access to food and water. They were allowed to adapt to laboratory conditions for 7 days before the experiment. All animal experimental procedures were approved by the Institute of Animal Care and Use Committee of Gannan Medical University. All experimental procedures complied with the Principles of Laboratory Animal Care, formulated by the National Institutes of Health, and applicable regulations and laws regarding the use and care of laboratory animals. All SD rats were randomly divided into five groups with 10 rats in each group: sham-operated (sham), CCH model (CCH), 0.1 mg/kg DSS-treated CCH model (CCH + 0.1 mg/kg DSS), 0.2 mg/kg DSS-treated CCH model (CCH + 0.2 mg/kg DSS), or 0.4 mg/kg DSS-treated CCH model (CCH + 0.4 mg/kg DSS).
CCH model establishment
The CCH model used in this study was established according to the methods reported by Ni et al. (1995). Briefly, the left common carotid artery was separated under 10% chloral hydrate (3.5 mL/kg) anesthesia. The left carotid arteries were simultaneously doubly ligated with 4-0 suture (ETHICON, Norderstedt, Germany). The same method was used to ligate and cut the right carotid arteries. The sham-operated control rats received same surgical cut but without the carotid artery ligation.
DSS treatment
DSS was synthesized and identified by Laboratory of Natural Medicinal Chemistry, Shenyang Pharmaceutical University, China and was given to us for use. DSS was dissolved in a physiological saline solution. Three weeks after modeling, the CCH + 0.1, 0.2, 0.4 mg/kg DSS groups received a 0.1, 0.2, 0.4 mg/kg DSS gavage once daily for 5 successive weeks. The rats in the sham and CCH groups received a physiological saline solution gavage of the same volume (3 mL) and at the same frequency as the DSS groups.
Morris Water Maze (MWM) test
The MWM test was carried out in a black circular tank(150-cm-diameter) (The Institute of Materia Medica at Chinese Academy of Medical Sciences, Beijing, China) after DSS treatment for 5 weeks. The tank was divided into four quadrants(namely I, II, III and IV) and the tank contained 40 cm3of water(23–25° C). A circular platform (9 cm diameter) was placed 2 cm beneath the water level. The spatial learning test was conducted for 4 consecutive days with four trials per day (30-minute intertrial intervals). In each trial, rats of each group were randomly placed in a different quadrant except for the quadrant where platform was located. The maximum trial length was 60 seconds. In the probe trial, the platform was removed and all methods were the same as the spatial learning test. The swimming paths of the rats were tracked using EthoVision (Noldus,Wageningen, the Netherlands).
Isolation of brain tissues
At 5 weeks after DSS, all rats were anesthetized under 10%chloral hydrate (3.5 mL/kg) anesthesia and were perfused through the left cardiac ventricle with 20 mL of saline solution,followed by 20 mL of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed. The right brain tissues were frozen in liquid nitrogen and post-fixed in the same fixative solution at 4 °C for 2 hours. Then, the left brain tissues were cryoprotected by 30% sucrose in phosphate buffer at 4 °C. The frozen right brain tissues were used for western blot analysis. The fixed left brain tissues were used for terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labelling (TUNEL) assay and hematoxylin and eosin (HE) staining.
TUNEL assay
The TUNEL assay was carried out using the In Situ Cell Death Detection Kit (Roche Molecular Biochemicals, Mannheim,Germany). To perform deparaffinization, the sections were kept at 65°C for 2 hours and heated at 57°C for 1 hour.Subsequently, the sections were washed in xylene and rehydrated through a graded series of alcohol (100%, 95%, 90%,80% and 70%). After washes in distilled water, the sections were incubated with proteinase K solution (20 µg/mL) at 37°C for 25 minutes for antigen retrieval. After blocking endogenous peroxidase activity, the sections were incubated with TUNEL solution for 60 minutes at 37°C in a moist and dark environment. Subsequently, sections were washed and incubated with converter-POD solution in a humidified environment at 37°C for 30 minutes. After PBS washes, sections were stained with diaminobenzidine tetrahydrochloride and counterstained with Mayer’s hematoxylin. Finally, they were washed, dehydrated, soaked, and covered. Cells with apoptotic nuclei (TUNEL-positive) that stained yellow brown in the hippocampal CA1, CA3, and dentate gyrus (DG) regions were counted using light microscopy (400×) (Carl Zeiss, Jena,Germany).
HE staining
The slides were stained with a hematoxylin staining solution.Then the sections were washed and differentiated with 0.3%acid alcohol. After wahses, the sections were stained with 0.5%eosin for 2 minutes. Finally, the sections were washed, dehydrated, mounted, and scanned using the same method as the TUNEL assay. The number of neurons was counted using Image Pro-Plus 6.0 software (Media Cybernetics, Silver Spring,MD, USA) at 400× magnification in the same field of CA1,CA3, and DG regions in each rat. Neuronal cell morphology was estimated by 2 senior pathologists who were blinded to experimental procedures of the study.
Western blot assay
The total protein of the hippocampus was isolated using a radioimmunoprecipitation assay buffer. The protein concentration was determined with a BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). 30 μg total protein was separated on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA,USA). The PVDF membranes were blocked with 5% non-fat powdered milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 hour at 37°C and subsequently incubated overnight at 4°C with the following primary antibodies:rabbit monoclonal anti-PKA (phospho T197) (p-PKA) (dilution, 1:3000), rabbit monoclonal anti-ERK1 (pT202/pY204)+ ERK2 (pT185/pY187) (p-ERK1/2) (dilution, 1:6000), and rabbit monoclonal anti-CREB (phospho S133) (p-CREB) (dilution, 1:5000; Abcam, Cambridge, MA, USA). After washes in TBST, all PVDF membranes were incubated with HRP-conjugated goat anti-rabbit antibody (1:8000, Southern biotech,Birmingham, AL, USA) for 1 hour at room temperature. Protein bands were visualized using the Immun-Star™ HRP Chemiluminescence Kit (Bio-Rad). The internal loading control was glyceraldehyde-3-phosphate dehydrogenase (GAPDH).The film was scanned and the integrated optical density was calculated using Image Pro-Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). The protein was expressed as a relative integrated optical density ratio of the target protein to reference protein.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 (IBM SPSS,Armonk, NY, USA). Descriptive data were expressed as the mean ± standard deviation (SD). Intergroup comparisons were conducted using one-way analysis of variance (ANOVA)followed by post hoc tests of least significant difference (LSD)for multiple pairwise comparisons. A P-value less than 0.05 was considered statistically significant.
Results
DSS prevented CCH-induced learning and memory deficits
To investigate the role of DSS on CCH-induced learning and memory deficits, the MWM test was conducted after 5 weeks of DSS gavage. Representative swimming paths of rats in each group on day 4 of the place navigation test are shown in Figure 1A. In the spatial learning test, from day 2 to day 4, rats in the CCH group had longer escape latencies (Figure 1B) and a greater travelled distance (Figure 1C) than rats in the sham group (P < 0.05). On day 1 of spatial learning test, obvious changes were not found in escape latencies and travelled distance between rats in the CCH group and DSS-treated groups(Figure 1B, C). We found that on day 2 to day 4, rats in the 0.2 and 0.4 mg/kg DSS groups had significantly shorter escape latencies and travelled distance than rats in the CCH group(P < 0.05). On day 4 of the place navigation test, the escape latencies (Figure 1B) and travelled distance in the DSS-treated groups decreased in a dose-dependent manner (Figure 1C). In the probe trial, the number of rats in the DSS-treated groups crossing the platform area was greater than that in the CCH group, but it was still lower than that in the sham group (P <0.05) (Figure 1D). The time spent by rats in the DSS-treated groups in quadrant IV was longer than that in the CCH group,but it was shorter than that in the sham group (P < 0.05) (Figure 1E). Results from the DSS-treated groups showed that the number of times the rats crossed the platform location and the time spent in quadrant IV were dose-dependent.
DSS alleviated neuronal damage caused by CCH
The neuroprotective effect of DSS was evaluated based on the number and morphology of neurons in the hippocampus after 5 weeks of DSS treatment. In the sham group, neurons in the hippocampal CA1, CA3, and DG regions were normal in quantity and morphology (Figure 2A). The number of neurons in the hippocampal CA1, CA3, and DG regions in the CCH group was markedly decreased than that in the sham group (P < 0.05)(Figure 2). In addition, great changes in neuronal morphology were observed, such as coagulative necrosis, vacuolation of neuron cell bodies, pyknosis of the nuclei, and a disordered arrangement (Figure 2). Abnormalities in neuronal morphology and reduction in the number of neurons were ameliorated in the CCH + 0.1 mg/kg DSS, CCH + 0.2 mg/kg DSS, and CCH + 0.4 mg/kg DSS groups (P < 0.05; Figure 2).
DSS reduced apoptosis caused by CCH in the hippocampus
The TUNEL assay was carried out to determine the role of DSS in CCH-induced apoptosis in the hippocampus. The number of TUNEL-positive cells in the hippocampal CA1, CA3, and DG regions in the CCH group was significantly greater than that in the sham group (P < 0.05) (Figure 3A and B). In the CCH + 0.1 mg/kg DSS, CCH + 0.2 mg/kg DSS, and CCH +0.4 mg/kg DSS groups, the number of TUNEL-positive cells decreased by 50%, 67%, and 77% respectively in hippocampal CA1 region, by 43%, 60%, and 86% respectively in hippo-campal CA3 region, and by 51%, 73%, and 81% respectively in hippocampal DG region. These results show that DSS can reduce apoptosis caused by CCH in the hippocampus.
DSS reversed the decrease in the phosphorylation of PKA,ERK1/2, and CREB by CCH
To explore the possible regulatory mechanisms underlying the function of DSS on memory deficit and hippocampal cell death, we examined the alterations of p-PKA, p-ERK1/2,and p-CREB. Western blot assay (Figure 4) showed that the expression levels of p-PKA, p-ERK1/2, and p-CREB in the hippocampus of rats in the CCH group significantly decreased compared to those in the sham group (P < 0.05). After 0.1, 0.2,and 0.4 mg/kg DSS treatments, p-PKA in the hippocampus increased by 52%, 88%, and 182% respectively, p-ERK1/2 increased by 17%, 56%, and 61% respectively, and p-CREB increased by 44%, 104%, and 190% respectively. These results suggest that DSS can reverse the decrease in phosphorylation of PKA, ERK1/2, and CREB by CCH.
Discussion
Long-term treatment is required to improve learning and memory deficits caused by CCH. Therefore, drug treatments must be safe for the patients. It is necessary to extract effective monomer components from food material to improve learning and memory deficits caused by CCH. Consumption of soy is associated with numerous health benefits including protection against obesity, cardiovascular disease, osteoporosis, cancer, and immune deficiency (Ørgaard and Jensen, 2008; Wenzel et al.,2008; Zaheer and Humayoun Akhtar, 2017). Daidzein, a soya isoflavone, played roles in various neurobiological regulatory mechanisms (Ahmed et al., 2017). Other studies have demonstrated that in an animal model, daidzein can improve memory impairment induced by many factors (Kim et al., 2010; Zeng et al., 2010). Soya isoflavone supplementation, including isoflavone equivalents such as daidzein, genistein, and glycitein, has been shown to enhance spatial working memory in men (Thorp et al., 2009). However, because of its poor water and lipid solubility, daidzein is diffuclt to use clinically. DSS is a new synthetic water-soluble compound derived from daidzein. In our present study, we investigated whether DSS protects against the rat’s learning and memory deficits induced by CCH. Using the 2VO model is an accepted method of investigating neuronal damage and cognitive impairment (Ni et al., 1995; Ohta et al., 1997;Farkas et al., 2004). Our results support the use of DSS as the effective treatment for the learning and memory deficits caused by CCH.
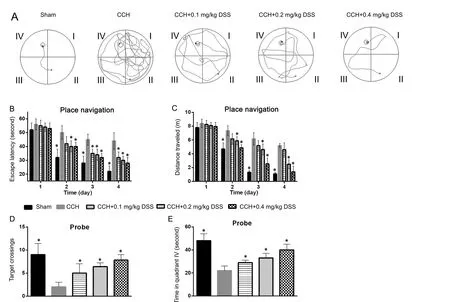
Figure 1 Effects of DSS gavage on water maze performance deficits induced by chronic cerebral hypoperfusion in rat models.
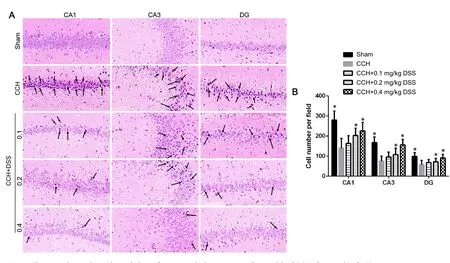
Figure 2 Changes in the number and morphology of neurons in the hippocampus of rat models of CCH after 5 weeks of DSS treatment.

Figure 3 DSS reduced apoptosis caused by CCH in the hippocampus.

Figure 4 DSS reversed the decrease in the phosphorylation of PKA,ERK1/2 and CREB in the hippocampus by CCH.
The Morris water maze is a common instrument used to measure hippocampus-dependent spatial learning and memory function. On day 1 of the spatial learning test, no significant changes were found in escape latencies and travelled distance between CCH group and the DSS-treated groups. We found that the escape latencies and travelled distance were significantly shortened in the DSS-treated groups compared with the CCH group in a dose-dependent manner on days 2 to 4.Furthermore, in the probe trial, the number of times the rats crossed the platform location and the time in quadrant IV were increased in DSS-treated groups than in the CCH group,but they were smaller or shorter than those in the sham group.These results indicate that DSS treatment can reduce spatial learning and memory deficits. Results showed that 5 weeks of DSS treatment can reduce CCH-induced neuronal damage and apoptosis in the hippocampus. The protective effects of DSS on the hippocampus are similar to those of daidzein(Yamada et al., 2016). These findings suggest that DSS might have similar pharmacological activity to that of daidzein, but with better water solubility, making it easier to use clinically in the treatment of learning and memory impairments caused by CCH.
To determine the regulating mechanisms responsible for the benefits of DSS, we investigated the effects of DSS on the PKA/ERK1/2/CREB signaling pathway. CREB can be activated by many physiological signals, such as synaptic activity, depolarization, neurotransmitters, and mitogenic signals (Ginty et al., 1994; Deisseroth et al., 1996). Activated CREB can bind to CREB binding-protein and subsequently activates target genes,including the genes that control development, function, and plasticity of the nervous system (Mayr and Montminy, 2001).CREB could be phosphorylated at Ser-133 by the ribosomal protein SG kinase of 90 kDa, a substrate of ERK1/2 (Xing et al.,1996; Yamada et al., 2016). The activation of ERK1/2 could be mediated by PKA activation (Spirli et al., 2012). Western blot results showed that DSS can increase the phosphorylation levels of PKA, ERK1/2, and CREB in the hippocampus, which were decreased by CCH. These results indicate that DSS can activate the PKA/ERK1/2/CREB signaling pathway that is otherwise inactivated by CCH. Based on the important role the PKA/ERK1/2/CREB signaling pathway plays in the nervous system,we suggest that DSS protected against memory impairment and hippocampal damage caused by CCH by activating this pathway.
However, our study has some limitations. Firstly, the effect of DSS on learning and memory impairments caused by CCH needs to be further demonstrated in more animal models.Secondly, further studies are needed to confirm the regulatory mechanism of PKA/ERK1/2/CREB signaling pathway. For instance, the direct target of CREB needs to be identified to confirm that DSS uses this pathway to alleviate the learning and memory impairments caused by CCH.
In conclusion, we identified the effects of DSS on the learning and memory deficits and neuronal damage and apoptosis in the hippocampus caused by CCH. Morris water maze test results revealed that DSS prevented against CCH-induced learning and memory deficits. Hematoxylin-eosin staining and TUNEL assay results showed that DSS alleviated neuronal damage and reduced neuronal apoptosis in the hippocampal CA1, CA3, and DG regions. Western blot assay results revealed that DSS increased the levels of p-PKA, p-ERK1/2, and p-CREB in the hippocampus. Our results suggest that DSS protects against learning and memory deficits and hippocampal damage caused by CCH by activating the PKA/ERK1/2/CREB signaling pathway. However, further studies are needed to provide confirmation.
Acknowledgments: We thank the members of our laboratories for their insight and technical support. We thank Natural Pharmaceutical Chemistry Laboratory of Shenyang Pharmaceutical University for donating 3′-daidzein sulfonate sodium.
Author contributions: XL, RZL and ZLL were responsible for experimental operation in establishing animal models, terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling assay, western blot analysis and hematoxylin-eosin staining. XL and HX wrote the paper. JDZ was responsible for statistical analysis and data collection. JDZ and ZHH were responsible for establishing animal models and Morris water maze test. RZL and QZ were responsible for western blot analysis. ZHH was responsible for statistical analysis. JZ and HX were responsible for the design of the experiments. All authors approved the final version of the paper.
Conflicts of interest: The authors declare that they have no conflict of interest.
Financial support: This work was supported by the National Natural Science Foundation of China (No. 81560583) and the Natural Science Foundation of Jiangxi Province of China (No. 20142BAB205021). The funding bodies played no role in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement: The experiments were approved by the Institute of Animal Care and Use Committee of Gannan Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review reports:
Reviewer 1: Evgeniya Pushchina, A.V. Zhirmunsky Institute of Marine Biology Far Eastern Branch of Russian Academy of Sciences, Russia.
Comments to authors: The article presents new and interesting data about the neuroprotective effect of DSS, in particular on the prevention of memory deficits and damage to neurons. The work was performed at a modern methodological level and contains new interesting data. Appropriate methodological approaches were used in this work, in particular, modern methods for identifying apoptotic neurons, methods for quantitative evaluation of protein, and behavioural tests. All these approaches provide an opportunity to solve the problems posed in the study. The article uses adequate statistical methods of results. Thus, the article presents the results of a study on the current direction related to the neuroprotective properties of DSS.
Reviewer 2: Victor S. Alemany, Columbia University, USA.
- 中国神经再生研究(英文版)的其它文章
- Seeing the wood for the trees: towards improved quantification of glial cells in central nervous system tissue
- The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis
- Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats
- Saikosaponin a increases interleukin-10 expression and inhibits scar formation after sciatic nerve injury
- Various changes in cryopreserved acellular nerve allografts at −80° C
- Patterns of cortical reorganization in facial synkinesis: a task functional magnetic resonance imaging study

