Proanthocyanidin B2 attenuates high-glucose-induced neurotoxicity of dorsal root ganglion neurons through the PI3K/Akt signaling pathway
Yuan-Pin Zhang , Si-Yan Liu Qian-Yu Sun Jing Ren Hua-Xiang Liu Hao Li
1 Department of Rheumatology, Shandong University Qilu Hospital, Jinan, Shandong Province, China
2 Department of Endocrinology and Metabolism, Fudan University Huashan Hospital, Shanghai, China
3 Department of Orthopedics, Shandong University Qilu Hospital, Jinan, Shandong Province, China
Abstract High glucose affects primary afferent neurons in dorsal root ganglia by inhibiting neurite elongation, causing oxidative stress, and inducing neuronal apoptosis and mitochondrial dysfunction, which finally result in neuronal damage. Proanthocyanidin, a potent antioxidant, has been shown to have neuroprotective effects. Proanthocyanidin B2 is a common dimer of oligomeric proanthocyanidins. To date, no studies have reported the neuroprotective effects of proanthocyanidin B2 against high-glucose-related neurotoxicity in dorsal root ganglion neurons. In this study, 10 µg/mL proanthocyanidin B2 was used to investigate its effect on 45 mM high-glucose-cultured dorsal root ganglion neurons. We observed that challenge with high levels of glucose increased neuronal reactive oxygen species and promoted apoptosis, decreased cell viability,inhibited outgrowth of neurites, and decreased growth-associated protein 43 protein and mRNA levels. Proanthocyanidin B2 administration reversed the neurotoxic effects caused by glucose challenge. Blockage of the phosphatidylinositol 3 kinase/Akt signaling pathway with 10 µM LY294002 eliminated the protective effects of proanthocyanidin B2. Therefore, proanthocyanidin B2 might be a potential novel agent for the treatment of peripheral diabetic neuropathy.
Key Words: nerve regeneration; high glucose; diabetes; neurons; neuropathy; reactive oxygen species; apoptosis; growth-associated protein 43;cell viability; neural regeneration
Introduction
Hyperglycemia influences primary afferent neurons in the dorsal root ganglion (DRG) by inhibiting the elongation of neuronal processes, causing oxidative stress, inducing neuronal apoptotic cell death, which result in mitochondrial dysfunction and neuronal damage in DRG, the target tissue in diabetic somatosensory neuropathy (Shimoshige et al., 2009; Abdel Nazeer et al., 2010; Chowdhury et al., 2010; Akude et al., 2011).However, how to prevent neurotoxicity of DRG caused by high glucose concentrations is still a challenge in this research field.
Proanthocyanidin is widely exists in natural product, such as seeds, fruits, vegetables and leaves of various plant species (Miura et al., 2008; Zunino, 2009; Ferraro et al., 2014). Oligomeric proanthocyanidins are important secondary metabolites that are beneficial to human health (An et al., 2015). Oligomeric proanthocyanidins have an important role in antioxidation(Houde et al., 2006; Asha Devi et al., 2011; Zhang et al., 2015;Nazima et al., 2016; Niu et al., 2016; Pinent et al., 2016) and radical scavenging (Ferreira and Slade, 2002; Bagchi et al.,2014), especially against diabetic oxidative stress. In diabetic model rats and mice, proanthocyanidin or oligomeric proanthocyanidins decreased serum glucose, glycosylated protein(Lee et al., 2008), and serum urea nitrogen levels. Proanthocyanidin also inhibited oxidative stress by suppressing the generation of reactive oxygen species (ROS) (Yokozawa et al.,2012). Oligomeric proanthocyanidins regulate inflammatory reactions caused by oxidative stress in diabetes (Yokozawa et al., 2012). Proanthocyanidin B2 (ProB2), a common dimer of oligomeric proanthocyanidins, is widely used to investigate the characteristics of proanthocyanidin. It was reported that ProB2 administration protected rat bladder and retina against hyperglycemic damage by ameliorating oxidative stress status via nuclear erythroid 2-related factor 2 pathway activation (Chen et al., 2015; Sun et al., 2016). Furthermore, it was suggested that the antioxidative and anti-inflammatory properties of ProB2 might protect against atherosclerosis (Yu et al., 2012) and ameliorate kidney damage in rats with type 2 diabetes (Bao et al.,2015). Phosphatidylinositol 3 kinase (PI3K)/Akt signaling activation is associated with the protective effects of proanthocyanidin on rat myocardial cell anoxia/reoxygenation injury (Hu et al., 2014). Proanthocyanidin was also reported to ameliorate biochemical abnormalities and antioxidant system status in diabetic rats probably because of its potent antioxidant effects(Mansouri et al., 2015a). To date, no studies have investigated whether ProB2 has neuroprotective effects against high-glucose-related neurotoxicity of primary sensory neurons. We hypothesized that ProB2 is neuroprotective for cultured DRG neurons with high-glucose-induced neurotoxicity. This is the first study to explore the promoting effects of ProB2 on neuroregeneration in peripheral diabetic neuropathy by using primary cultured DRG neurons with high-glucose-induced neurotoxicity. These findings may provide novel evidence for improving diabetic neuropathy by using ProB2.
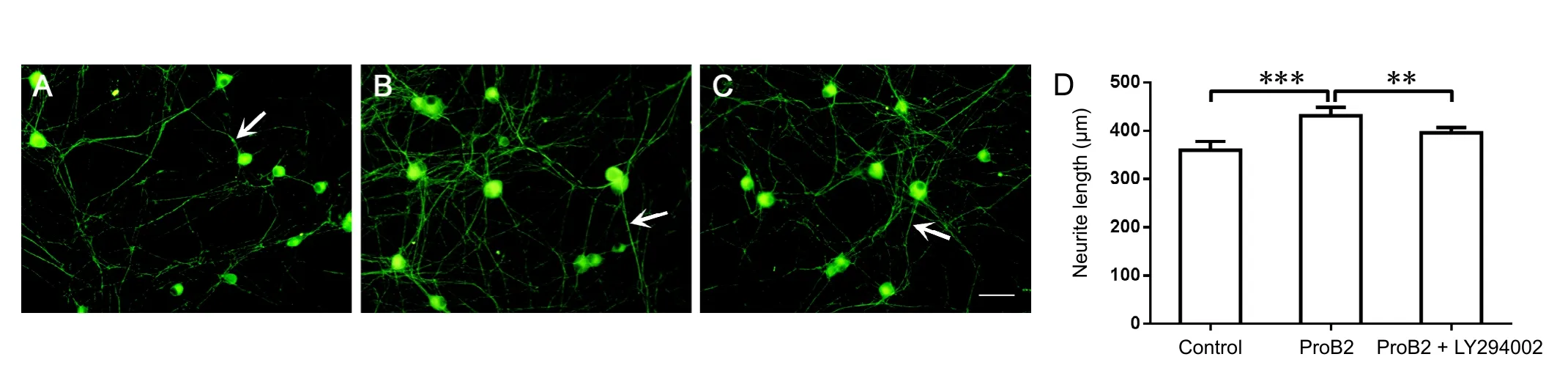
Figure 1 Effect of proanthocyanidin B2 (ProB2) on the neurite length of single neurons under normal culture conditions.
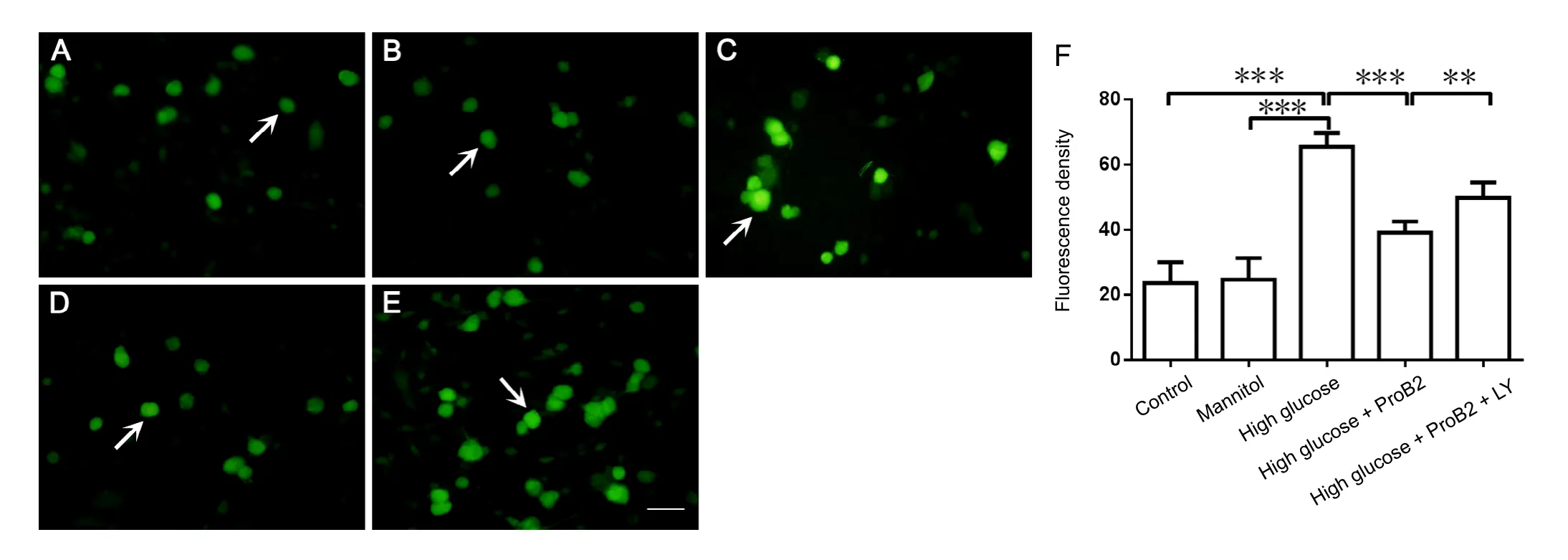
Figure 2 Effect of proanthocyanidin B2 (ProB2) on the intracellular reactive oxygen species (ROS) in dorsal root ganglion neurons with high glucose toxicity.
Materials and Methods
Neuronal culture from DRG
Seventy-two specific-pathogen-freeNewborn Wistar rats (<1 day after birth) were provided by the Experimental Animal Center at Shandong University of China (animal license No.SCXK20130009). The animal protocols were approved by the Animal Experimental Ethics Committee of Shandong University of China (approval No. 201402260001). Rats were subjected to anesthesia with 1.5% pentobarbital sodium (2 mL/kg, intraperitoneally) prior to and throughout the protocol to minimize suffering. DRGs were removed, digested with trypsin (0.25%)(Sigma, St. Louis, MO, USA), centrifuged at 1 × 103r/min for 5 minutes and triturated. Then cells were added to 24-well plates(2 × 105cells/well) (Costar, Corning, NY, USA) with each well containing a coverslip precoated with 0.1 mg/mL poly-L-lysine(Sigma). The culture medium was DMEM/F12 (Gibco, Grand Island, NY, USA) with additional fetal bovine serum (5%), B-27(2%) (Gibco), and L-glutamine (0.1 mg/mL) (Sigma). The culture conditions were 37°C in a 5% CO2incubator for 24 hours. Then, neurons were primed with cytosine arabinoside(5 μg/mL) to exclude non-neuronal cells by inhibiting their growth for an additional 24 hours. Cells were allowed to acclimatize under different settings for an additional 24 hours before observation.
Drug administration
DRG neurons were cultured in DMEM/F12 for 48 hours and the medium was replaced with neurobasal medium for the following experiments.
To examine the effect of ProB2 on neurite outgrowth in normal culture conditions (non-high concentration glucose challenge), DRG neuronal cultures were allowed to acclimatize under the following settings for an additional 24 hours: (1) Control group: neurobasal medium; (2) ProB2 group: 10 µg/mL ProB2(Sigma) (Kopustinskiene et al., 2015) in neurobasal medium; (3)ProB2 + LY294002 group: 10 µM LY294002 (Invitrogen, Camarillo, CA, USA) (Bai et al., 2017; Zhang et al., 2017), and 10µg/mL ProB2 in neurobasal medium.
To examine the protective effect of ProB2 on DRG neurons with high concentration glucose toxicity, the DRG neuronal cultures were allowed to acclimatize under different settings for an additional 24 hours: (1) Control group: neurobasal medium; (2) mannitol group: 20 mM mannitol (mimics high glucose osmotic conditions (Solarbio Science Technology Co., Ltd., Beijing, China) (Xu et al., 2012)); (3) high glucose group: 45 mM glucose (additional 20 mM glucose) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China); (4)high glucose + ProB2 group: 45 mM high glucose with 10 µg/mL ProB2; (5) high glucose + LY294002 + ProB2 group: 45 mM glucose with 10 µM PI3K inhibitor LY294002 and 10 µg/mL ProB2.
All aforementioned cultures under each condition were cultured at 37°C with 5% CO2for an additional 24 hours.
Measurement of ROS production in DRG neurons
Neuronal ROS concentrations were monitored by a fluorescence probe (2′,7′-dichlorofluorescein diacetate, DCFH-DA)(Sigma) sensitive to oxidation and cell permeability. After the treatment of DRG neurons, 10 μM DCFH-DA was applied to the culture medium at 37°C for 20 minutes. Then, D-Hanks solution was used to remove additional culture medium and clear the background for fluorescent microscopy (Olympus,Tokyo, Japan) (485 nm excitation and 530 nm). Twenty neurons in each sample were randomly selected for fluorescence intensity determination using Image-Pro Plus 5.1 software (Media Cybernetics, Silver Spring, MD, USA). The neuronal fluorescence density was measured automatically and the mean density was obtained for each sample.
Neuronal apoptosis in DRG determined by Hoechst 33342 staining
After the treatment of DRG neurons, the apoptosis of neurons was observed by Hoechst 33342 staining, which directly shows morphological alterations of the nucleus in each neuron. A fixing solution of 4% paraformaldehyde was used for 10 minutes to fix DRG cells. Hoechst 33342 (5 μg/mL) dye was applied to DRG neurons for 5 minutes in a darkroom. After staining,phosphate-buffered saline (0.1 M, pH 7.4) was used as a washing solution to remove the remaining dye. In the fluorescent micrographs, apoptotic neurons are characterized by shrinking nuclei and condensed or fragmented chromatin. Five separate visual fields at 200× magnification (just adjacent but not overlapping) in the central part of each sample were selected for cell counting. Both the total number of cells and apoptotic cells were counted separately in the same visual field. The apoptotic rate of DRG neurons was obtained by counting both cell types.Apoptotic rate = apoptotic neurons/total number of neurons× 100%.
Evaluation of DRG neuron viability by Cell Counting Kit-8 (CCK-8)
After treatment of DRG neurons, the viability of neuronal cells was estimated with CCK-8. The amount of formazan dye generated by dehydrogenases in cells is directly proportional to the number of living cells. The protocol of neuronal viability assay with CCK-8 was described previously (Bai et al., 2017; Zhang et al., 2017). Briefly, CCK-8 (10 μL) was added to each well in a 96-well plate for 2 hours at 37°C. Then, the cell absorbance was read at 450 nm with a microplate reader. The viability of neuronal cells in the control group represented the standard level of viability and this was compared with other experimental groups. The relative value of cell viability in each group was obtained.
Measurement of neurite length of DRG neurons by immunofluorescent staining
After incubation under different experimental conditions for 24 hours, βIII-tubulin was fluorescently labeled as a marker to monitor the elongation or retraction of growing neurites.The primary antibody in this fluorescence labeling protocol was mouse monoclonal anti-βIII-tubulin antibody (1:1000;Abcam, Cambridge, MA, USA; overnight at 4°C). The secondary antibody was goat anti-mouse antibody conjugated with Cy2 (1:200; Abcam; 60 minutes at 25°C). Images were observed and captured using a fluorescent microscope (BX63;Olympus, Tokyo, Japan) and a digital photo processing system. Twenty individual neurons in each sample were randomly selected for neurite length measurement. All neurites in each distinct neuron were analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA) to measure the elongation or retraction of processes extending from the neuronal cell bodies.
Real time-polymerase chain reaction (PCR) for mRNA levels of growth-associated protein 43 (GAP-43)
The mRNA levels of GAP-43 and GAPDH (internal control)were assayed by PCR. Synthesis of cDNA was guided by the manufacturer’s instructions of cDNA synthesis kit (Thermo Scientific Molecular Biology, Vilnius, Lithuania). Total RNA from DRG cells from each sample was isolated with TRIzol(TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China).Real-time PCR was conducted with SYBR Green dye (Thermo Scientific Molecular Biology, Lithuania, EU, USA). The PCR was run at 50° C for 2 minutes, 94° C for 15 minutes,followed by 40 cycles at 94° C for 15 seconds, 58° C for 30 seconds, and 72°C for 30 seconds. Relative quantification of the target gene was calculated using the 2-ΔΔCtmethod. The primer sequences are shown in Table 1.
Western blot assay for GAP-43 protein
The primary antibody was rabbit anti-GAP-43 monoclonal IgG (1:5000; Abcam) or mouse anti-GAPDH monoclonal IgG(1:1000; Hangzhou Xianzhi Biological Technology Co., Ltd.,Hangzhou, China), which were incubated overnight at 4°C.The secondary antibody was goat anti-rabbit IgG-horseradish peroxidase (1:3000; Santa Cruz Biotechnology, Santa Cruz,CA, USA) or goat anti-mouse IgG-horseradish peroxidase(1:3000; Santa Cruz Biotechnology), which were incubated for 60 minutes at 25°C. In each group, the relative gray value of the target protein GAP-43 was compared to the internal control protein GADPH. The relative value of GAP-43/GADPH in the control group was standardized to 1. The relative quantification of the target protein GAP-43 in other experimental groups was determined by comparisons with the standardized value in the control group.
Double fluorescence staining of GAP-43 and microtubule associated protein 2 (MAP2)
We evaluated GAP-43 in situ in DRG neurons and the proportion of DRG neurons that synthesized GAP-43 protein by the double fluorescence staining of GAP-43 and MAP2.GAP-43-immunoreactive (IR) DRG neurons represented neurons that synthesized GAP-43 protein. MAP2-positve staining of DRG neurons indicated the total number of neurons present. Double fluorescence staining of GAP-43 and MAP2 was performed using rabbit polyclonal anti-GAP-43 (1:500; Abcam) or mouse monoclonal anti-MAP2 (1:400; Abcam) as the primary antibodies, which were incubated overnight at 4°C.The secondary antibodies were goat anti-rabbit antibody conjugated with Cy3 (1:200; Abcam) or goat anti-mouse antibody conjugated with Cy2 (1:200; Abcam), which were incubated for 60 minutes at 25°C.
GAP-43-IR neurons were counted at 200× magnification by fluorescence microscopy. Five adjacent, but not overlapping, visual fields in the center of the coverslip were selected for GAP-43-IR neuronal counting. MAP2-IR neurons in the same area were taken as the summation of neurons under detection.The number of GAP-43-IR neurons was divided by the number of MAP2-IR neurons (total number of DRG neurons),and then the proportion of GAP-43-IR neurons was calculated based on these numbers.
Statistical analysis
Quantitative data were expressed as the mean ± standard deviation (SD), and analyzor by one-way analysis of variance in SPSS 19.0 (IBM, Armork, NY, USA). The Student-Newman-Keuls test was used for data with a homogeneity of variance. A value of P <0.05 was considered statistically significant.
Results
Neurite outgrowth under normal conditions
After ProB2 incubation, the elongation of neurites from the neuronal cell body to the peripheral area was significantly longer than in the control group (P < 0.001). After preincubation with the PI3K inhibitor LY294002 in ProB2 incubated cultures,the elongation of neurites was significantly restricted (P < 0.01;Figure 1).
ProB2 inhibited ROS levels in DRG neurons incubated with high glucose
Exposure to high glucose (45 μM) significantly elevated neuronal ROS levels (P < 0.001). Incubation with ProB2 (10µg/mL) decreased the ROS levels induced by high glucose challenge (P < 0.001). LY294002 preincubation blocked the effect of ProB2 on the inhibition of ROS generation (P < 0.01).Mannitol did not affect the ROS levels (P > 0.05; Figure 2).
ProB2 inhibited DRG neuron apoptosis induced by high glucose
After ProB2 treatment, the apoptotic rate of neuronal cells was determined by Hoechst 33342 staining. ProB2 (10 µg/mL) incubation decreased apoptosis induced by high glucose challenge(P < 0.01). LY294002 preincubation blocked the effect of ProB2 on the inhibition of apoptosis (P < 0.01). Mannitol did not affect apoptosis (P > 0.05; Figure 3).
ProB2 improved DRG neuron viability after injury induced by high glucose
After ProB2 treatment, the alteration of neuronal cell viability was monitored by a CCK-8 kit. ProB2 treatment increased cell viability, which was inhibited under high glucose conditions (P< 0.001). LY294002 preincubation blocked the ProB2 effect on the promotion of cell viability (P < 0.001). Mannitol did not affect cell viability (P > 0.05; Figure 4).

Figure 3 Effect of proanthocyanidin B2 (ProB2) on apoptosis of dorsal root ganglion neurons with high glucose toxicity.
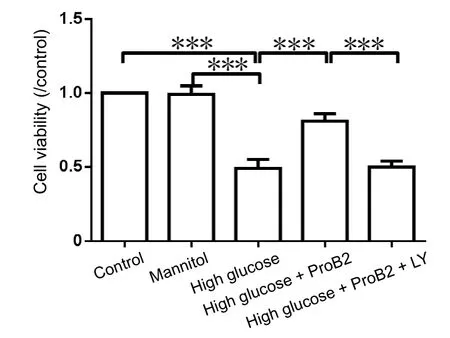
Figure 4 Effect of proanthocyanidin B2 (ProB2) on the cell viability of dorsal root ganglion neurons incubated in high glucose.

Figure 5 Effect of proanthocyanidin B2 (ProB2) on the neurite length of a single neuron in dorsal root ganglion neurons with high glucose toxicity.
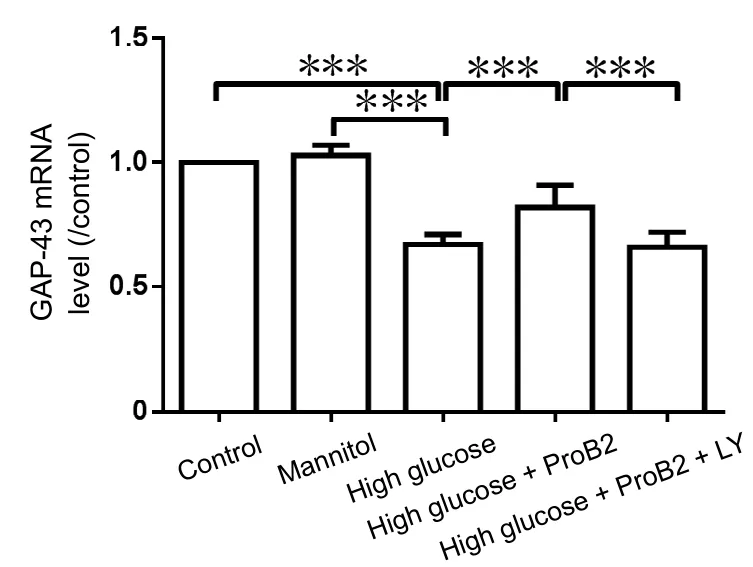
Figure 6 Effect of proanthocyanidin B2 (ProB2) on growth-associated protein 43 (GAP-43) mRNA levels in dorsal root ganglion neurons detected by real time-polymerase chain reaction.
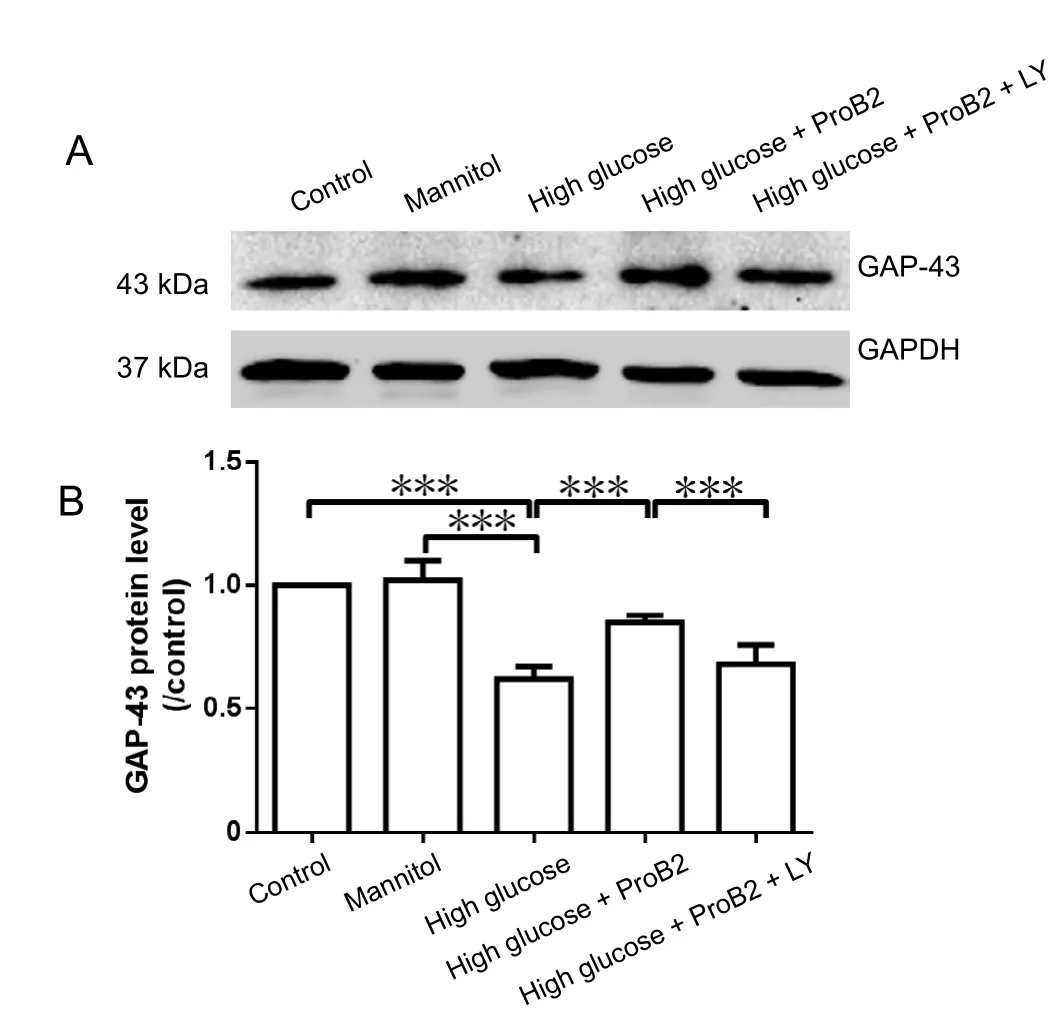
Figure 7 Effect of proanthocyanidin B2 (ProB2) on growth-associated protein 43 (GAP-43) protein expression levels in the dorsal root ganglion neurons incubated in high glucose detected by western blot assay.
ProB2 improved neurite extension in DRG neurons incubated with high glucose
High glucose exposure significantly inhibited neurite elongation (P < 0.001). After ProB2 incubation, the elongation of neurites from the neuronal cell body to the peripheral area was significantly longer than in the high glucose group (P < 0.001).Preincubation with LY294002 in ProB2 incubated cultures with high glucose challenge, significantly restricted the elongation of neurites (P < 0.001). Mannitol did not affect neurite elongation (P > 0.05; Figure 5).
ProB2 increased GAP-43 mRNA expression in DRG neurons incubated with high glucose
Results of real time-PCR showed that ProB2 (10 µg/mL) incubation increased GAP-43 mRNA levels that were inhibited by high glucose challenge (P < 0.001). LY294002 preincubation blocked the effect of ProB2 on the promotion of GAP-43 mRNA expression (P < 0.001). Mannitol did not affect GAP-43 mRNA expression (P > 0.05; Figure 6).
ProB2 improved GAP-43 protein expression in DRG neurons incubated with high glucose
GAP-43 is a marker for axonal regeneration (Murata et al.,2006). High glucose incubation inhibited GAP-43 protein expression (P < 0.001). ProB2 (10 µg/mL) incubation increased GAP-43 protein levels that were inhibited by high glucose challenge (P < 0.001). PI3K inhibitor LY294002 preincubation blocked the effect of ProB2 on the promotion of GAP-43 protein expression (P < 0.001). Mannitol did not affect GAP-43 protein expression (P > 0.05; Figure 7).

Figure 8 Effect of ProB2 on growth-associated protein 43(GAP-43)-immunoreactive (IR) neurons in the dorsal root ganglion neurons incubated with high glucose detected by double fluorescence staining.

Table 1 Primer sequences for the targeting genes
ProB2 increased the percentage of GAP-43-IR neurons in DRG neurons incubated with high glucose
Double fluorescence staining results showed that ProB2 (10 µg/mL) incubation increased the proportion of GAP-43-IR neurons that were inhibited by high glucose challenge (P < 0.01).The effects of ProB2 on the GAP-43-IR neuronal proportion were blocked by LY294002 (P < 0.01; Figure 8).
Discussion
This is the first study to report that ProB2 rescues DRG neurons from high glucose induced neurotoxicity, which enhances our understanding of anti-diabetic peripheral neuropathy. Peripheral neuropathy is a severe diabetic complication reported worldwide (Hur et al., 2011; Farmer et al., 2012; Shi et al.,2013) and is often accompanied by chronic neuropathic pain(Krishnan et al., 2008; Gibbons and Freeman, 2010; Nones et al., 2013), which severely degrades the quality of life of diabetic patients (Ramos et al., 2007; Mousa et al., 2013; Wang et al.,2017). Current therapies only maintain normal blood glucose levels, which are usually difficult to control (Harati, 2007;Comelli et al., 2010). No other treatments have been shown to retard the progression of diabetic peripheral neuropathy(Tesfaye and Selvarajah, 2009; Serafín et al., 2010; Zilliox and Russell, 2011). The development of novel agents to relieve diabetic neuropathy is urgently required. A recent study reported that the oral intake of proanthocyanidin was safe and well tolerated in human subjects (Sano, 2016). However, the anti-neurotoxic effects or neuroprotective effects of proanthocyanidin on primary sensory neurons under high glucose challenge are still unclear. The results of the present study demonstrate the neuroprotective effects of ProB2 for DRG neurons incubated with high glucose levels. The major significance of these findings is the novel concept of the efficacy of anti-high glucose neurotoxicity, and the novel therapeutic agent, ProB2, which can be used to advance our knowledge in the field of neural regeneration for primary sensory DRG neurons.
Results from this study demonstrated that treatment with LY294002 had a lower impact on ROS and apoptosis compared with cell viability, neurite length, and GAP-43 expression, indicating LY294002 might have a different impact on different parameters. Furthermore, LY294002 administration in the presence of ProB2 did not return outcome measures to control levels, indicating other mechanisms are associated with the protective effects of ProB2 in relieving high-glucose-induced neurotoxicity. ROS production is increased in diabetes,because glucose challenge induced oxidative stress. The inhibition of ROS production by oligomeric proanthocyanidin administration in rat and mouse diabetic models might be by directly controlling blood glucose levels. In a high glucose challenged culture model, it is difficult to determine whether oligomeric proanthocyanidins directly affect glucose levels. In diabetic neuropathy, neurodegeneration may inhibit GAP-43 expression. As a neuroregeneration marker, increased GAP-43 expression levels after the administration of ProB2 observed in this culture model may reflect the initiation of the regeneration of injured DRG neurons.
Cultured DRG neurons are commonly used in in vitro models to study primary sensory neurotoxicity (Lu et al.,2014). The benefits of this in vitro model are the convenience of testing high-glucose-induced neurotoxicity, which excludes other factors; therefore, this model does not completely mimic the in vivo characteristics of primary sensory neurotoxicity. Patients with fasting plasma glucose ≥ 7.0 mM or 2-hour venous plasma glucose ≥ 11.1 mM with oral glucose tolerance test or optional venous plasma glucose ≥ 11.1 mM with diabetic symptoms were diagnosed as diabetes. In cell culture, normal neurobasal medium contains basic 25 mM glucose concentration for normal neurite outgrowth in vitro.High glucose in vitro was 45 mM (additional 20 mM glucose),which was higher than diabetic patients (Xu et al., 2012). High concentration glucose is accompanied by increased mitochondrial ROS and subsequent apoptotic DRG neuronal death(Berent-Spillson and Russell, 2007; Vincent et al., 2010). However, there is some controversy regarding whether apoptotic DRG neuronal death is associated with the progression of the pathology of afferent neuronal diabetic neuropathy under diabetic conditions. Furthermore, whether high concentration glucose produces ROS toxicity and subsequent axonal injury,loss of myelination, induction of nerve fiber degeneration, and loss of regenerative ability is unclear (Srinivasan et al., 2000;Zochodne et al., 2001; Cheng and Zochodne, 2003; Kamiya et al., 2005). Stress oxidation was reported to be a potential mechanism to induce neuronal cell death glucose (Páramo et al., 2010). Consistent with a previous study reporting increased intracellular ROS and apoptotic rate, DRG neurons exposed to high concentration glucose showed decreased cell viability (Xu et al., 2012). In this study, high glucose caused increased intracellular ROS and apoptotic rate, decreased cell viability, inhibition of neurite outgrowth, and decreased GAP-43 protein and mRNA in DRG neurons. ProB2 administration reversed the neurotoxic effects caused by glucose challenge. Foods rich in ProB2 protected neurons in a rat acute ischemic stroke model by preventing or reducing oxidative stress and inflammation(Yunoki et al., 2014). ProB2 also prevented cardiac disorders caused by diabetes in rats (Mansouri et al., 2015b). Interestingly, a recent study reported ProB2 had significant anti-hyperalgesic and anti-nociceptive effects in rats with neuropathic pain induced by chronic constructive sciatic nerve injury (Kaur et al., 2016). These recent studies support the notion that ProB2 has multi-protective effects after tissue injury, including neuroprotective effects after nerve injury under different experimental conditions.
A limitation of this study was that the in vitro model does not completely mimic in vivo diabetic peripheral neuropathic conditions. Therefore, future studies should use a diabetic peripheral neuropathic animal model to test the efficacy of ProB2 on neuron status and neural regeneration of nerve endings.
In conclusion, ProB2 was beneficial at improving oxidative stress, inhibiting neuronal apoptosis, promoting neuronal regeneration, and elevating the cell viability of DRG neurons under high-glucose-induced neurotoxicity. The theoretical significance of the present study raises the novel concept of using ProB2 to retard high-glucose-induced neurotoxicity.Furthermore, it provides novel experimental evidence for the further practical application of ProB2 for the treatment of intractable diabetic neuropathy.
Author contributions: YPZ performed the experiments, participated in study design, and wrote the manuscript. SYL, QYS, and JR performed the experiments. HXL participated in study design and data analysis. HL designed this study and revised the manuscript. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81501935; the Shandong Provincial Natural Science Foundation of China, No. ZR2014HQ065. Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the papaer for publication.
Institutional review board statement: The study protocol was approved by the Animal Experimental Ethics Committee of Shandong University of China (approval number: 201402260001).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.Open peer review report:
Reviewer: Aldo Calliari, Universidad de la República, Uruguay.
Comments to authors: The manuscript explores the effect of pro anthocyanidin B2 on cultured dorsal root ganglion neurons exposed to high glucose concentrations as a model to study therapeutic approaches for diabetic neuropathy. In my opinion, the work has two basic drawbacks: a) it provides few novel information on the field. b) The experimental work is not properly conducted; some conclusions are invalid and the discussion is very shallow.
- 中国神经再生研究(英文版)的其它文章
- Seeing the wood for the trees: towards improved quantification of glial cells in central nervous system tissue
- 3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
- The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis
- Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats
- Saikosaponin a increases interleukin-10 expression and inhibits scar formation after sciatic nerve injury
- Various changes in cryopreserved acellular nerve allografts at −80° C

