Electroacupuncture modulates the activity of the hippocampus-nucleus tractus solitarius-vagus nerve pathway to reduce myocardial ischemic injury
Shuai Cui , Kun Wang Sheng-Bing Wu, Guo-Qi Zhu, Jian Cao, Yi-Ping Zhou Mei-Qi Zhou ,
1 Research Institute of Acupuncture and Meridian, Anhui University of Chinese Medicine, Hefei, Anhui Province, China
2 Clinical Medical College of Acupuncture, Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong Province, China
3 Key Laboratory of Xin’ An Medicine, Ministry of Education, Anhui University of Chinese Medicine, Hefei, Anhui Province, China
4 Department of Science and Technology, Anhui University of Chinese Medicine, Hefei, Anhui Province, China
Abstract The hippocampus is involved in the regulation of the autonomic nervous system, together with the hypothalamus and brainstem nuclei, such as the paraventricular nucleus and nucleus tractus solitarius. The vagus nerve-nucleus tractus solitarius pathway has an important role in cardiovascular reflex regulation. Myocardial ischemia has been shown to cause changes in the autonomic nervous system, affecting the dynamic equilibrium of the sympathetic and vagal nerves. However, it remains poorly understood how the hippocampus communicates with brainstem nuclei to regulate the autonomic nervous system and alleviate myocardial ischemic tissue damage. A rat model of acute myocardial ischemia (AMI)was made by ligating the left anterior descending branch of the coronary artery. Three days before ischemia, the hippocampal CA1 region was damaged. Then, 3 days after ischemia, electroacupuncture (EA) at Shenmen (HT7)-Tongli (HT5) was performed (continuous wave, 1 mA, 2 Hz,duration of 30 minutes). Cluster analysis of firing patterns showed that one type of neuron was found in rats in the sham and AMI groups. Three types of neurons were observed in the AMI + EA group. Six types of neurons were found in the AMI + EA + Lesion group. Correlation analysis showed that the frequency of vagus nerve discharge in each group was negatively correlated with heart rate (HR) (P < 0.05, r = −0.424), and positively correlated with mean arterial pressure (MAP) (P < 0.05, r = 0.40987) and the rate-pressure product (RPP) (P < 0.05, r = 0.4252). The total frequency of the nucleus tractus solitarius discharge in each group was positively correlated with vagus nerve discharge (P < 0.01, r = 0.7021), but not with hemodynamic index (HR: P > 0.05, r = −0.03263; MAP: P > 0.05, r = −0.08993; RPP: P > 0.05, r = −0.03263). Some neurons (Neuron C)were negatively correlated with vagus nerve discharge, HR, MAP and RPP in the AMI + EA group (vagus nerve discharge: P < 0.05, r = −0.87749; HR: P < 0.01, r = −0.91902; MAP: P < 0.05, r = −0.85691; RPP: P < 0.01, r = −0.91902). Some neurons (Neurons C, D and E) were positively correlated with vagus nerve discharge, HR, MAP and RPP in the AMI + EA + Lesion group (vagus nerve discharge: P < 0.01, r = 0.8905, P < 0.01,r = 0.9725, P < 0.01, r = 0.9054; HR: P < 0.01, r = 0.9347, P < 0.01, r = 0.9089, P < 0.05, r = 0.8247; MAP: P < 0.05, r = 0.8474, P < 0.01, r = 0.9691,P < 0.01, r = 0.9027; RPP: P < 0.05, r = 0.8637, P < 0.01, r = 0.9407, P < 0.01, r = 0.9027). These findings show that the hippocampus-nucleus tractus solitarius-vagus nerve pathway is involved in the cardioprotective effect of EA at the heart meridian. Some interneurons in the nucleus tractus solitarius may play a particularly important role in the cardiomodulatory process.
Key Words: nerve regeneration; acute myocardial ischemia; hippocampus; nucleus tractus solitarius; vagus nerve discharge; electroacupuncture;Shenmen (HT7); Tongli (HT5); autonomic nerve; neural regeneration
Introduction
Acute myocardial ischemia (AMI) can be caused by a reduction in blood supply or blockage of the coronary artery. This leads to severe acute ischemia in myocardial tissue, resulting in myocardial ischemic necrosis (Lu and Zhong, 2009; Chen et al., 2016). The World Health Organization in 2004 reported on the global burden of 136 diseases, and showed that ischemic heart disease is the leading cause of death, accounting for 12.2% of all deaths. That number is expected to rise to 14.2%by 2030. Myocardial ischemia is associated with sudden cardiac death (Zaman and Kovoor, 2014) and high disability and mortality, and is a major focus of cardiovascular disease research.
The hippocampus is involved in regulating the autonomic nervous system by the endocrine system, together with the hypothalamus and brainstem nuclei, including the paraventricular nucleus and nucleus tractus solitarius (NTS) (Liu et al., 2007). A previous study showed that myocardial ischemia increases expression of the N-methyl-D-aspartic acid receptor NR1 and NR2B subunits in the rat hippocampus, thereby activating this brain region (Zhu et al., 2012). Myocardial ischemia can also cause cognitive dysfunction in the hippocampus. For example, transient changes in cognitive function after acute myocardial ischemia/reperfusion injury is associated with an increase in tumor necrosis factor alpha and interleukin-1 beta mRNA and protein expression in the hippocampus (Zhu et al., 2008). The NTS is the primary reflex center of the viscera,and relays afferent and efferent vagal transmission (Randich and Aicher, 1988). The dorsal medial subnucleus neurons of the NTS filter signals from peripheral cardiovascular receptors, and relay afferent information from the vagus and glossopharyngeal nerves (Zanutto et al., 2010). In addition, the vagus-NTS pathway plays a major role in the regulation of the cardiovascular reflex, and the vast majority of visceral vagal fibers are relayed by the NTS to multiple brain regions (Fu et al.,2012).
We hypothesize that the cardioprotective effect of acupuncture in myocardial ischemia may involve regulation of the autonomic nervous system via the hippocampus, through both inhibition of sympathetic activity and excitation of the vagus nerve. Previous studies have shown that myocardial ischemia induces changes in the autonomic nervous system that affect the dynamic balance between the sympathetic system and the vagus nerve (Cui et al., 2016).
Electroacupuncture (EA) improves the symptoms of myocardial ischemia, modulates autonomic nervous system activity, and reverses the effect of myocardial ischemia on the hippocampal CA1 region (Wu et al., 2015). However, the pathways involved in alleviating myocardial ischemic damage are unclear.
In the present study, we used multi-channel electrophysiological recordings in vivo to examine NTS neuronal activity,and at the same time, we observed the vagus nerve and the hemodynamic index, in an effort to clarify how the hippocampus-NTS-vagus nerve pathway provides cardioprotection.
Materials and Methods
Animals
Fifty male Sprague-Dawley rats, 8 weeks of age and weighing 250–300 g, were supplied by the Feeding Center at Anhui Medical University of China (license No. SCXK (Wan) 2011-002). Rats were housed in separate cages (Kangwei IR60) with an independent air supply system for 1 week at 24 ± 2° C and 65% relative humidity under natural lighting. All rats were allowed free access to food and water. All animal procedures were conducted in accordance with the Animal Use Guidelines of Anhui University of Chinese Medicine and Anhui Laboratory Animal Center. The study was approved by the Animal Ethics Committee of Anhui University of Chinese Medicine of China (approval No. 201604-001).
Production of the AMI animal model
Six rats were randomly allocated to the sham group. In the sham group, only punctures were made, and the left anterior descending branch of the coronary artery was not ligated. The remaining 44 rats were used for the AMI model by ligating the left anterior descending branch of the coronary artery (Klocke et al., 2007). High T-wave and J-point elevation (≥ 0.1 mV)on electrocardiogram (ECG) indicated successful generation of the AMI model. Of these 44 animals, 30 met the criteria for AMI, while the remaining 14 died or did not satisfy the criteria.The 30 AMI model rats were randomly divided into the AMI group, AMI + EA group, and the AMI + EA + Lesion group (n= 10 per group).
Lesioning of the hippocampal CA1 region
Three days before artery ligation, the hippocampal CA1 region was lesioned using a previously described method (Wu et al.,2015). Briefly, kainic acid, 1 mg/L, (Sigma-Aldrich, St. Louis,MO, USA) was injected into the hippocampal CA1 region bilaterally at bregma −4.16 mm, lateral 2.8 mm, depth 2.8–3.0 mm, according to the rat brain atlas (Paxinos and Watson,2009) (Additional Figure 1A, B). Three days after surgery, neuronal death was evident in the hippocampal CA1 region (Additional Figure 2A–D).
The electrode array (diameter: 35 μm; Plexon Inc., Hong Kong Special Administrative Region, China) was implanted in the NTS and fixed using dental cement. The coordinates according to the rat brain atlas are: bregma −12.72 mm, lateral 0.8–1.6 mm, depth 7.8–8.2 mm (Additional Figure 1C, D).
The rat neck surgery mainly involved separation of the arteries and the vagus nerves. Hemodynamic index was recorded during carotid artery cannulation. The discharge of the vagus nerve was recorded using a needle electrode.
All of the experimental indexes (NTS neuronal discharge,ECG, vagus nerve discharge and hemodynamics) were recorded simultaneously (Additional Figure 2E).
EA treatment
The Shenmen (HT7)-Tongli (HT5) segment in the Shaoyin heart meridian of the hand was selected with reference to the human meridian line. The acupuncture positioning criteria of rats were in accordance with Chinese Veterinary Acupuncture and previous results (Cui et al., 2016). The Shenmen (HT7)acupoint is located on the wrist, at the ulnar end of the crease of the wrist. The Tongli (HT5) acupoint is in the radial margin of the flexor carpi ulnaris, 1 inch above the transverse striation of the wrist. For the rats in the AMI + EA group and the AMI+ EA + Lesion group, three needles (Φ0.30 × 25 mm) were inserted at the Shenmen (HT7)-Tongli (HT5) segment with a spacing of 1 mm. The device (Huatuo brand, SDZ-IV type)was purchased from Suzhou Medical Products Co., Ltd., Suzhou, China. The EA parameters were as follows: continuous wave; current of 1 mA; frequency of 2 Hz; duration of 30 minutes, once a day. The EA therapy was started 1 day after AMI,and was performed for three consecutive days in the AMI +EA group and AMI + EA + Lesion group. Rats in the AMI and sham groups received sham stimulation with the instrument switched off.
2,3,5-Triphenyltetrazolium chloride (TTC) staining
The rats were killed by intraperitoneal overdose injection of chloral hydrate. The hearts were taken out and quick-frozen at −20° C for 20 minutes. The hearts were then sliced into six sections and soaked in 2% TTC solution (Servicebio, Wuhan,China) in the dark in a 37° C incubator for 15–30 minutes.The sections were then fixed with paraformaldehyde, and photographed. The area of myocardial infarction was calculated using Image Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA) (Figure 1A).
Peripheral vagus nerve discharge recording
All rats were anesthetized with 10% chloral hydrate (3.5 mL/kg; Sinopharm Chemical Reagent Co., Ltd., St. Louis, MO,USA) by intraperitoneal injection. The room temperature was controlled at 26 ± 2° C, and the rat was placed on a heating pad (Chengdu Taimeng Software Co., Ltd., Chengdu, China) maintained at 36 ± 1 ° C. The cervical vagus nerve was dissociated, and hooked with a bipolar platinum recording electrode. The reference electrode was inserted into the subcutaneous tissue. The ground electrode was connected to the lower limb. Recording parameters were set, and the discharge frequency of the vagus nerve was recorded by the BIOPAC multi-channel physiological recorder (BIOPAC Systems Inc.,St. Louis, MO, USA). Afterwards, fast Fourier transformation filtering was performed on the recorded signals offline. The neural signals were recorded to determine the discharge frequency 5 minutes after EA.
Recording of neuronal discharge in the central nucleus
All rats were anesthetized with 10% chloral hydrate (3.5 mL/kg) by intraperitoneal injection and then fixed on the brain stereotactic apparatus (Stoelting Co., Ltd., St. Louis, MO, USA).The NTS coordinates were according to the rat brain atlas(Paxinos and Watson, 2009). Craniotomy was performed, and an 8-channel microelectrode array was electronically moved to the target nucleus at 5 μm/s. When satisfactory discharge activity was observed, the stable neuronal discharge was recorded for 5 minutes. After the signals were filtered and processed, the selected neuronal discharge signals were subjected to cluster analysis with the Offline Sorter (version 3.3.5, Plexon Inc., Dallas, TX, USA). NeuroExplorer (version 4.13, Nex Technologies, Lexington, MA, USA) was used to analyze the waveform,frequency, characteristics of neuronal discharge, and correlation with the field potential.
Hemodynamics and ECG recording
All rats were anesthetized with 10% chloral hydrate (3.5 mL/kg) by intraperitoneal injection and secured on a heating pad (maintained at 36–37° C) in the supine position. Using conventional standard II lead ECG, the electrodes were inserted into the subcutaneous tissue of the right upper limb and left lower limb. The BIOPAC multi-channel physiological recorder(BIOPAC Systems Inc.) was used to continuously observe respiratory activity, arterial blood pressure, and ECG waveform.After the rats achieved a stable status, the heart rate (HR),mean arterial pressure (MAP) and rate-pressure product (RPP)values were recorded and analyzed with Acknowledge 4.1 (BIOPAC Systems Inc.).
Statistical analysis
Data are expressed as the mean ± SD. Statistical analysis was performed using SPSS 19.0 software (IBM SPSS, Inc., St. Louis, MO, USA). The difference between groups was analyzed by one-way analysis of variance. Test for homogeneity of variance was performed before the comparisons were made between groups. The least significant difference test was used for homogeneity of variance and Tamhane’s T2 test was used for heterogeneity of variance.
Cluster analysis, interspike-interval analysis and autocorrelation analysis were used to distinguish and analyze the pattern of NTS neuronal discharge. The pyramidal neurons and interneurons were distinguished based on their characteristic discharge activity. The characteristics of pyramidal neurons were as follows: (1) low mean discharge frequency (0.5–10 Hz) and irregular discharge pattern; (2) histogram showing that a short interspike interval (3–10 ms) is dominant, with exponential attenuation; (3) wide waveform (> 300 μs). The characteristics of interneurons were as follows: (1) high mean discharge frequency (> 5 Hz); (2) interspike interval histogram showing delayed spikes and slow attenuation; (3) narrow waveform (< 250 μs)(Pang and Zhang, 2014).
Real-time spectrum analysis was used to observe local field potential changes. HR, MAP, RPP, vagus nerve discharge, and NTS and interneuronal spike counts were analyzed by linear correlation analysis. The correlation coefficient (R) was calculated. Linear regression was performed using the following model: y = a + bx.
Results
Hippocampal regulation by EA at the heart meridian
effectively reduced myocardial infarction and ischemic damage Three days after the EA treatment, the left ventricular infarction areas were calculated in the AMI, AMI + EA and AMI +EA + Lesion groups. Compared with the AMI group, the area of myocardial infarction was significantly reduced in the AMI+ EA and AMI + EA + Lesion groups (P < 0.01). Compared with the AMI + EA group, the area of myocardial infarction was significantly increased in the AMI + EA + Lesion group (P< 0.05; Figure 1B).
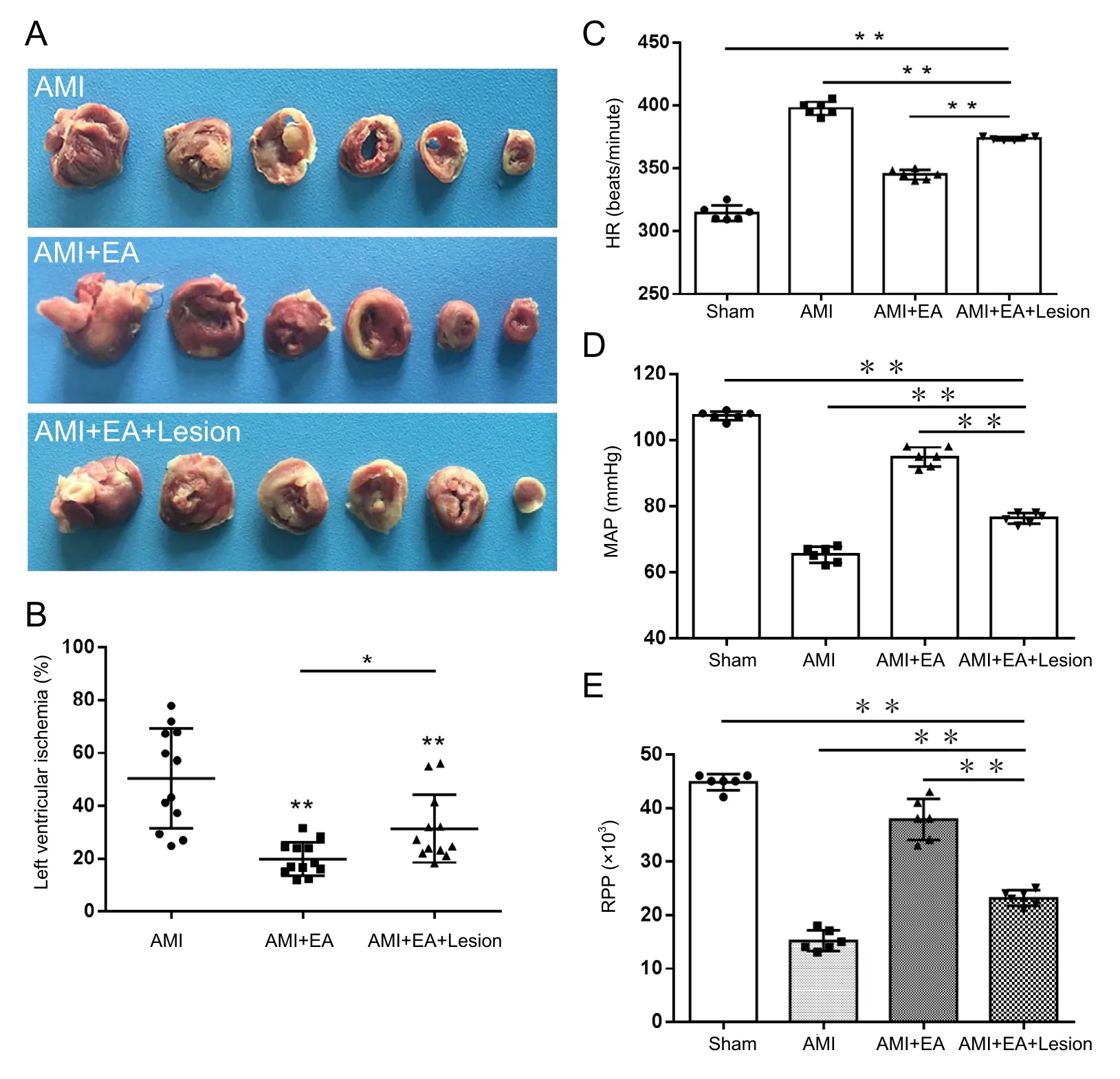
Figure 1 Effect of EA and CA1 lesioning on myocardial infarction area, hemodynamic parameters and vagus nerve discharge activity in AMI rats.
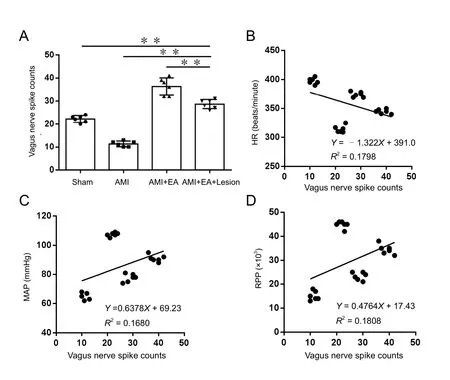
Figure 2 Effect of EA and CA1 lesioning on the discharge frequency of the vagus nerve in AMI rats.(A) Stable discharge signal of the vagus nerve measured for 5 minutes was recorded immediately (0 minute) after the last EA session for all experimental groups. The data are expressed as the mean± SD (n = 6; one-way analysis of variance followed by the least significant difference test). **P <0.01. (B–D) Discharge frequency of the vagus nerve was negatively correlated with the HR (P < 0.05,r = −0.424), but positively correlated with the MAP and RPP (P< 0.05, r = 0.40987; P < 0.05, r =0.4252). EA: Electroacupuncture;AMI: acute myocardial ischemia;HR: heart rate; MAP: mean arterial pressure; RPP: rate-pressure product.

Figure 3 Cluster analysis of the discharge frequency of NTS neurons in AMI rats undergoing EA and CA1 lesioning.
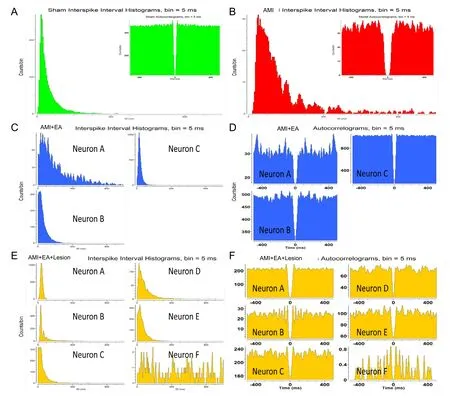
Figure 4 Distinguishing the activity patterns of NTS neurons by autocorrelation analysis and interspike-interval analysis in AMI rats undergoing EA and CA1 lesion.
HR, MAP and RPP were recorded after 3 days of EA. Compared with the sham group, HR was significantly higher (P <0.01), but the MAP and RPP were significantly lower (P < 0.01),in the AMI group. Compared with the AMI group, HR was significantly lower (P < 0.01), but the MAP and RPP were significantly higher (P < 0.01), in the AMI + EA group. Compared with the AMI + EA group, HR was significantly higher (P <0.01), but the MAP and RPP were significantly lower (P < 0.01),in the AMI + EA + Lesion group (Figure 1C–E).
EA of the heart meridian affected hippocampal regulation of vagus nerve discharge
The stable discharge activity of the vagus nerve for a period of 5 minutes was recorded immediately (0 minutes) after the last EA session. Discharge frequency of the vagus nerve was significantly lower in the AMI group compared with the sham group (P <0.01). Compared with the AMI group, discharge frequency of the vagus nerve was significantly higher in the AMI + EA group(P < 0.01). Discharge frequency of the vagus nerve was significantly lower in the AMI + EA + Lesion group compared with the AMI + EA group (P < 0.01; Figure 2A).
Correlation analysis was performed for discharge frequency of the vagus nerve and hemodynamic indexes. The discharge frequency of the vagus nerve was negatively correlated with the HR (P < 0.05, r = −0.424), but positively correlated with the MAP and RPP (P < 0.05, r = 0.40987; P < 0.05, r = 0.4252)(Figures 2B−D). These results suggest that EA at the heart meridian modulates the hippocampal regulation of vagal discharge activity to reduce myocardial ischemic injury.
EA of the heart meridian impacted the hippocampal regulation of NTS neurons
Multi-channel in vivo recording electrodes were used to record stable discharge by the NTS for 5 minutes immediately (0 minute) after the last EA session. Data processing was used to obtain the discharge time sequence of single neurons and clusters of neurons in each group (Figure 3 and Additional Figure 3). We identified one pattern of neuronal activity in the sham and AMI groups, three patterns in the AMI + EA group, and six patterns in the AMI + EA + Lesion group. Interspike interval analysis and autocorrelation analysis were performed to distinguish the firing types. In the AMI group, one interneuron unit discharge was distributed over a period of 1000 ms, and the maximum discharge was within 400 ms. In the AMI + EA group, three interneuron unit discharges were distributed over 1000 ms, and the maximum discharge was within 800 ms in Neuron A, within 300 ms in Neuron B, and within 200 ms in Neuron C. In the AMI+ EA + Lesion group, five interneuron unit discharges and one pyramidal cell unit discharge were recorded. The discharge of Neuron A was distributed over a period of 100 ms; the discharge of Neuron B was distributed over 300 ms, with a maximum within 200 ms; the discharge of Neuron C was distributed over 200 ms; the discharge of Neuron D was distributed over 400 ms;the discharge of Neuron E was distributed over 300 ms; and the discharge of Neuron F was distributed over 1000 ms. The discharge patterns differed (Figure 4).
The discharge time sequence (time = 300 seconds) of each group of neurons was converted to a histogram of discharge frequency. In the sham group, the total discharge frequency of the NTS neurons was 5010 ± 58.89 Hz for the interneurons. In the AMI group, the total discharge frequency of the NTS neurons was 931.67 ± 14.38 Hz, all of which were interneurons. In the AMI + EA group, the total discharge frequency was 14,195 ±240.51 Hz in the NTS interneurons, 1322 ± 23.37 Hz in Neuron A, 5175 ± 18.34 Hz in Neuron B, and 7716 ± 29.53 Hz in Neuron C. In the AMI + EA + Lesion group, the total discharge frequency of the NTS neurons was 23,105 ± 174.59 Hz. The values were 6984 ± 43.26 Hz, 3778 ± 53.74 Hz, 4751 ± 18.26 Hz, 2372 ± 32.13 Hz and 5014 ± 25.67 Hz for the interneurons, and 259 ± 3.28 Hz for the pyramidal cells. The total discharge frequency of NTS neurons and the discharge frequency of the interneurons were significantly different between groups(P < 0.01; Figure 5A, B).
Real-time spectrum analysis was used to investigate the changes in spectral characteristics over time. The intensities of the spectral energy of the local field potential for the four groups were in the following order: AMI + EA + Lesion group > sham group >AMI + EA group > AMI group. These findings suggest that the hippocampus is involved in mediating the cardioprotective effect of EA at the heart meridian by regulating the spectral energy of the NTS (Additional Figure 4).
NTS neuronal activity correlated with vagal discharge and hemodynamics following EA of the heart meridian
A correlation analysis was performed of the total discharge frequency of NTS neurons, the discharge frequency of the vagus nerve and hemodynamic parameters. The total discharge frequency of NTS neurons was positively correlated with the discharge frequency of the vagus nerve (P < 0.01, r = 0.7021),but not with HR (P > 0.05, r = 0.01439), MAP (P > 0.05, r =−0.08993) or RPP (P > 0.05, r = −0.03263) (Figure 6).
Correlation analysis was conducted for the AMI + EA and AMI + EA + Lesion groups. This analysis showed that of the three types of neurons in the AMI + EA group, only one neuron (neuron C) was negatively correlated with the discharge frequency of the vagus nerve (P < 0.05, r = −0.87749), the HR(P < 0.01, r = −0.91902), the MAP (P < 0.05, r = −0.85691),and the RPP (P < 0.01, r = −0.91902) (Figure 7). Among the six types of neurons in the AMI + EA + Lesion group, three correlated with vagal discharge and hemodynamic indexes.Neuron C was positively correlated with vagal discharge frequency (P < 0.01, r = 0.8905), HR (P < 0.01, r = 0.9347), MAP(P < 0.05, r = 0.8474) and RPP (P < 0.05, r = 0.8637). Neuron D was positively correlated with the discharge frequency of the vagus nerve (P < 0.01, r = 0.9725), HR (P < 0.01, r = 0.9089),MAP (P < 0.01, r = 0.9691) and RPP (P < 0.01, r = 0.9407).Neuron E was positively correlated with the discharge frequency of the vagus nerve (P < 0.01, r = 0.9054), HR (P < 0.05, r =0.8247), MAP (P < 0.01, r = 0.9027) and RPP (P < 0.01, r =0.9027) (Figure 8).

Figure 5 Discharge frequency of the NTS and classification of the neurons in AMI rats undergoing EA and CA1 lesioning.
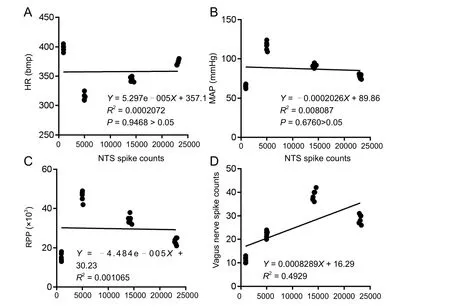
Figure 6 Correlation analysis of the total discharge frequency of NTS neurons and the total discharge frequency of the vagus nerve and hemodynamic parameters.

Figure 7 Correlation analysis of the discharge frequency of neuron C, the total discharge frequency of the vagus nerve, and hemodynamic parameters in the AMI + EA group.

Figure 8 Correlation analysis of the discharge frequency of neuron C, neuron D and neuron E, the total discharge frequency of the vagus nerve, and hemodynamic parameters in the AMI + EA + Lesion group.
Discussion
The association between the meridian-viscera and the brain was the theoretical basis of acupuncture treatment for AMI
Research on the links between the meridians, viscera and the brain is a hot topic in systems biomedicine. Systems theory and Chinese medicine are combined, emphasizing that biological phenomena should be studied and grasped from the systemic and holistic levels. The study of the association between the meridians and viscera are combined with modern neuroscience to investigate the connection between the meridian-viscera and the brain, especially the limbic-hypothalamic-autonomic axis (Zhou et al., 2008).
Acupuncture alleviates myocardial ischemic tissue damage by regulating the central nervous system, cardiovascular activity, myocardial tissues and the antioxidant system (He et al.,2014). EA at heart acupoints has been shown to regulate hypoxia-inducible factor-1α protein, downregulate ASIC2 and ASIC3 gene expression in ischemic myocardial tissue, effectively alleviate damage to myocardial cells, and reduce the area of myocardial infarction (Wang et al., 2015; Ding et al., 2017).Furthermore, it upregulates heat shock protein 27 and heat shock protein 70 expression in heart tissue to protect against myocardial ischemic damage (Sakamoto et al., 2000; Tan et al.,2017). A large number of studies have shown that EA inhibits expression of the pro-apoptotic gene Bax and upregulates the anti-apoptotic gene Bcl-2 to reduce apoptosis in myocardial tissue (Zhang et al., 2009). Furthermore, EA downregulates CLCa and inhibits PKC activation to inhibit AQP 1 protein expression, thereby exerting cardioprotection (Bai et al., 2015;Cheng et al., 2016; Cai et al., 2017). EA at the heart meridian has been shown to modulate gene expression in the hypothalamus, including that of the Trh and Crh genes. This may be involved in the protective effects of EA at the heart meridian and small intestine meridian (Zhou et al., 2007).
Our current findings suggest that the total discharge frequency of the NTS is correlated with the discharge of the vagus nerve. Furthermore, some, but not all, NTS neurons were correlated with hemodynamic indexes. Therefore, we speculate that some interneurons in the NTS mediate the cardioprotective effects of EA at the heart meridian.
The limbic-hypothalamic-autonomic axis was the neural substrate targeted by EA to alleviate heart disease
The hippocampus is a limbic forebrain structure. Not only is the hippocampus an important center for regulating memory and cognition, but also an important center for regulating cardiovascular function. The hippocampus has complex connections with the paraventricular nucleus, NTS, amygdala, locus coeruleus and medial prefrontal cortex (Chiba, 2000; Castle et al., 2005; Vertes et al., 2007; Mello-Carpes and Izquierdo, 2013; Zhang and Hernandez, 2013). A recent study showed that the cognitive dysfunction induced by myocardial ischemia in adult mice is associated with reactive gliosis in the hippocampus and a decrease in neurogenesis (Evonuk et al., 2017).
The NTS is a relay station for visceral primary afferent fibers,and has connections with many nuclei and regions in the brain.Xiao et al. (2000) found that NTS fibers project to the central amygdala via the lateral parabrachial nucleus. This pathway might play a major role in regulating cardiovascular activity.The NTS projects onto catecholaminergic cells in the bed nucleus of the stria terminalis, preoptic area, central amygdala,locus coeruleus and spinal cord, which may also be involved in regulating the cardiovascular and sympathetic nervous systems(Sim and Joseph, 1994).
Our results show that the hippocampus modulates NTS neuronal activity in rats with myocardial ischemia. Damage to the hippocampal CA1 region increased neuronal activity and the types of neuronal firing in the NTS. The NTS receives sensory signals from the heart after ischemia as well as instructions from higher centers to regulate the cardiovascular system. This may explain why different neuronal discharge patterns were recorded in the NTS.
Multi-channel recording in vivo allowed study of central neuronal activity
Multi-channel recording in vivo is an extracellular recording technique that allows measurement of the extracellular field potential at the electrode tip (Buzsaki et al., 2012). Filtering technology can help distinguish neural activity from different sources. One type of signal is the multiple-unit activity recorded around the electrode with the 300–400 Hz high-pass filter. The other type of signal is the local field potential activity obtained with the 300 Hz low-pass filter (Jansen and Ter Maat,1992; Gray et al., 1995; Csicsvari et al., 1999). This method can synchronously record the electrical activity of a large number of neurons in multiple brain regions. This is useful for the study of individuals receiving a specific stimulus (e.g., EA) or performing a specific behavioral task. The method can help clarify the spatiotemporal relationship between neuronal discharges in different brain regions. Brain coding mechanisms for external events can be studied by analyzing neuronal firing patterns(Wang et al., 2003).
Summary
The NTS is a complex mixed sensory/motor nerve relay nucleus. The NTS receives afferent sensory input from the inferior vagal nerve and provides efferent signals to higher brain centers(e.g., the hippocampus) to regulate cardiovascular activity.Therefore, EA at the heart meridian likely alleviates myocardial ischemic injury via the hippocampus-NTS-vagus nerve pathway. During EA stimulation at Shenmen (HT7)-Tongli (HT5),the acupuncture signals are conveyed to the central nervous system via the peripheral nerves. Signal processing occurs in the hippocampus, and the output from this structure then regulates the excitability of specific neurons in the NTS. Subsequently, signals from the NTS are transmitted to the vagus nerve to regulate cardiac activities and provide cardioprotection.
Conclusion
Although we identified the types of neurons involved in the cardioprotective action of EA based on discharge patterns, we did not analyze the relationship between the neurons. In addition, we did not assess relevant neurotransmitter indicators.Neurons should be associated with neurotransmitters to better identify the neuronal types involved in the protective effect of EA against myocardial ischemia. These unresolved issues will be the focus of our future studies.
Author contributions: MQZ and YPZ conceived and supervised the study.MQZ, YPZ and SC designed the study. SC, SBW and KW performed experiments. SC designed new electrode and tools, and analyzed data. SC and MQZ wrote the paper. SC, GQZ and MQZ made paper revisions. All authors reviewed the results and approved the final version of the paper.
Conflicts of interest: All authors declare no personal or financial conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81273858; a grant from the Anhui University Research and Innovation Platform Team Construction Project in China, No. 2015TD033.The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: The study protocol was approved by the Animal Ethics Committee of Anhui University of Chinese Medicine in China(approval No. 201604-001). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-Share-Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1: Central nucleus coordinate location.
Additional Figure 2: Hippocampus CA1 HE staining and experimental flow graph.
Additional Figure 3: The rate histograms of neurons in each group.
Additional Figure 4: Real-time spectrum analysis of the NTS’s local field potential discharge.
- 中国神经再生研究(英文版)的其它文章
- 3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
- Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease
- Role of nuclear factor κB in multiple sclerosis and experimental autoimmune encephalomyelitis
- Nanometer ultrastructural brain damage following low intensity primary blast wave exposure
- Altered leukocyte gene expression after traumatic spinal cord injury: clinical implications
- Promoting axonal regeneration following nerve surgery: a perspective on ultrasound treatment for nerve injuries

