Baihui (DU20)-penetrating-Qubin (GB7) acupuncture inhibits apoptosis in the perihemorrhagic penumbra
Beng Zhang , Xiao-Hong Dai, Xue-Ping Yu, Wei Zou, , Wei Teng, Xiao-Wei Sun, Wei-Wei Yu, Hao Liu, Hui WangMeng-Juan Sun Meng Li
1 Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang Province, China
2 First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang Province, China
3 Clinical Key Laboratory of Integrated Traditional Chinese and Western Medicine, Heilongjiang University of Chinese Medicine, Harbin,Heilongjiang Province, China
4 Department of Acupuncture and Moxibustion, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang Province, China
Abstract Baihui (DU20)-penetrating-Qubin (GB7) acupuncture can inhibit inflammatory reactions and activate signaling pathways related to proliferation after intracerebral hemorrhage. However, there is no research showing the relationship between this treatment and cell apoptosis. Rat models of intracerebral hemorrhage were established by injecting 60 μL of autologous blood into the right side of the caudate-putamen. Six hours later, the needle traveled subcutaneously from the Baihui acupoint to Qubin acupoint. The needle was alternately rotated (180 ± 10 turns/min) manually along clockwise and counter-clockwise directions. Stimulation lasted for 7 days, and was performed three times each for 6 minutes with 6-minute intervals between stimulations. Rats intraperitoneally receiving Sonic hedgehog pathway activator, purmorphamine(1 mg/kg per day), served as positive controls. Motor and sensory function were assessed using the Ludmila Belayev test. Extent of pathological changes were measured in the perihemorrhagic penumbra using hematoxylin-eosin staining. Apoptosis was examined by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling assay. Expression of smoothened (Smo) and glioma-associated homolog 1 (Gli1)was determined by western blot assay. Our results showed that Baihui-penetrating-Qubin acupuncture promoted recovery of motor and sensory function, reduced the apoptotic cell percentage in the perihemorrhagic penumbra, and up-regulated Smo and Gli1 expression. We conclude that Baihui-penetrating-Qubin acupuncture can mitigate hemorrhage and promote functional recovery of the brain in a rat model of intracerebral hemorrhage, possibly by activating the Sonic hedgehog pathway.
Key Words: nerve regeneration; acupuncture treatment; intracerebral hemorrhage; neurological function; Ludmila Belayev test; TUNEL assay;purmorphamine; Smo; Ptch1; Glil; neural regeneration
Introduction
Intracerebral hemorrhage (ICH) has one of the highest frequencies of mortality and morbidity in humans (Li et al., 2017;Ostrowski et al., 2017). In addition to primary injury, secondary injury (including ischemia and edema around the hematoma, toxic effects of hemoglobin metabolites, inflammatory responses, and neural cell apoptosis) contributes to the overall functional loss and determines the long-term outcome (Lu et al., 2014; Xu et al., 2017). Severity of apoptosis is strongly associated with functional deficits after ischemic stroke as well as ICH (Wu et al., 2015; Wang and Zhang, 2017).
The hedgehog (Hh) gene was first identified in drosophila in 1980 (Nüsslein-Volhard and Wieschaus, 1980). Homologous Hh genes include Sonic hedgehog (Shh), Indian hedgehog, and desert hedgehog, and were identified in mammalian species in the 1990s. Signal transduction in the Shh pathway mainly occurs in the primary cilium of cells (Wilson and Chuang, 2010).Members of the Shh pathway include Shh, patched homolog 1 (Ptch1), smoothened (Smo), and glioma-associated homolog 1 (Gli1). Without participation of Shh protein, Ptch1 (a 12 transmembrane-helix receptor protein and target of Shh) inhibits the Shh pathway by assembling in cilia and excluding Smo protein (Marigo et al., 1996). In the activated state, Shh interacts with Ptch1 and becomes vulnerable to degradation (van den Heuvel and Ingham, 1996). Subsequently, Smo (a 7 transmembrane-helix receptor protein and target protein of Ptch1) can no longer be inhibited by Ptch1 and activate Gli1, the downstream target of the Shh pathway (van den Heuvel and Ingham,1996). The Shh signaling pathway participates in normal pattern formation of the central nervous system (Rowitch et al., 1999),regulation of neural stem cell proliferation (Cheng et al., 2015),and regulation of neuronal apoptosis (Meng et al., 2016). Activating the Shh pathway can attenuate motor and sensory deficits after stroke by increasing microvascular density and neuronal survival (Chen et al., 2017) and inhibiting neuronal apoptosis(Meng et al., 2016). As important proteins in the Shh pathway,activation of the upstream protein, Smo, and downstream protein, Gli1, indicate activation of the Shh pathway.
Acupuncture has been widely used to promote functional recovery after stroke in clinical practice. Effectiveness of acupuncture is documented after stroke in limb disorders (Zhao et al., 2015; Lou et al., 2016; Shi et al., 2017), dysphagia (Zhang et al., 2016), shoulder pain (Chau et al., 2018), and shoulder-hand syndrome (Zheng et al., 2018), as well as recovery of learning and memory deficits (Liu et al., 2017b). Pathological injuries after stroke (such as edema, inflammation, and apoptosis) affect neural functions (Liu et al., 2017a; Wang et al.,2017; Chen et al., 2018; Qiu et al., 2018), and acupuncture can alleviate these functional deficits by inhibiting the underlying pathological changes (Jung et al., 2016; Liu et al., 2016; Tian et al., 2016).
Previous studies have suggested that acupuncture at the Baihui (DU20) acupoint can inhibit neural cell apoptosis under diseased conditions (Wang et al., 2002; Guo et al., 2015).Further, a previous study showed a similar pattern of glucose metabolism activation in the motor cortex after acupuncture at Baihui versus Qubin (GB7) acupoints (Fang et al., 2012).Moreover, studies have seldom analyzed the mechanism of acupuncture treatment, including Baihui-penetrating-Qubin treatment. Thus, we examined the effect of Baihui-penetrating-Qubin acupuncture on functional recovery and neuronal apoptosis after ICH.
Materials and Methods
Animals
Rats were obtained from Heilongjiang University of Chinese Medicine, Heilongjiang, China (Certificate No. SCXK (Heilongjiang) 2013-004). Briefly, specific-pathogen-free adult male Sprague-Dawley rats weighing 250 ± 20 g and aged 6–7 weeks were housed under a 12-hour light/dark cycle at 20°C and 50% relative humidity. Food and water were removed 12 hours before surgery (autologous blood injection) to prevent aspiration during anesthesia. The study was conducted in accordance with Guide for the Care and Use of Laboratory Animals (Eighth Edition, 2011) (National Research Council of the National Academies, 2011) and approved by the Animal Ethics Committee of Heilongjiang University of Chinese Medicine in China (approval IACUC: 2017-10-10-01).
Group assignment
Six groups were involved with 10 rats randomly divided into each group: control group, sham group, ICH group, ICH +acupuncture group (ICH + Ac group), ICH + sham acupuncture group (ICH + SAc group), and ICH + agonist group (ICH+ Ag group). Starting 6 hours after ICH on the same day, rats were randomized to receive daily treatments for 7 consecutive days of Baihui-penetrating-Qubin acupuncture (30-minute session per day; ICH + Ac group), sham acupuncture (ICH +SAc group), purmorphamine (a Shh pathway activator) (Abcam, Cambridge, MA, USA; 1 mg/kg per day, intraperitoneally; ICH + Ag group), or no intervention (ICH group) (n =10/group). Purmorphamine was dissolved to a concentration of 1 mg/mL using a solution containing 2% dimethyl sulfoxide and 30% polyethylene glycol 300 (Hu et al., 2016). A group of rats receiving intracerebral saline injection (sham group) and a group of healthy rats receiving no treatment at all (control group) were included as controls (n = 10/group).
ICH model establishment
ICH was modeled using autologous blood injection as described previously (Sinar et al., 1988; MacLellan et al., 2008). Rats were anesthetized with 10% chloral hydrate (350 mg/kg, intraperitoneally) and fixed on a stereotaxic frame (68001; RWD Life Science, Shenzhen, Guangdong Province, China). A midline incision was made to expose the skull. A burr hole was made 3.5 mm right of the midline and 0.2 mm posterior to bregma (Figure 1) (Paxinos and Watson, 2005). Autologous blood (60 μL) was collected from the tail vein, and injected using a 26-gauge needle to a depth of 6 mm from the brain surface (Figure 1) (Paxinos and Watson, 2005) at a speed of 20 μL/min. The needle was removed slowly 5 minutes after injection. Body temperature was maintained at 37°C during surgery.
Assessment of neurological function
Global central nervous system function (including motor and sensory) was assessed using the Ludmila Belayev test, as described previously (Bederson et al., 1986; De Ryck et al., 1989;Belayev et al., 1996). Testing was performed using a 12-point rating scale (summarized in Additional Table 1) by two experienced investigators blinded to treatment allocation. Average score was used as the final score.
Acupuncture treatment
Acupuncture was performed by experienced physicians with at least 20 hours of training in rats. Rats received one 30-minute session per day using disposable steel filiform acupuncture needles (Φ0.3 × 25 mm; Huatuo, Suzhou, Jiangsu Province,China). The entry point was Baihui acupoint (DU20, on the middle between both ears), and the needle traveled subcutaneously to Qubin acupoint (GB7, leading edge of tragus) (Figure 2). The needle was alternately rotated (180 ± 10 turns/min)manually along clockwise and counter-clockwise directions.Stimulation lasted three times, for 6 minutes each, with 6-minute intervals between stimulations. Rats in the ICH + SAc group received acupuncture at 1 cm posterior and parallel to Baihui-penetrating-Qubin needling.
Histological evaluation
Histological evaluation was performed at 7 days of the experiment. Coronal sections (5 μm) selected from the perihemorrhagic penumbra were deparaffinized in xylene and ethanol.After washing in distilled water, sections were stained with hematoxylin and eosin using a standard protocol. The perihemorrhagic penumbra was examined under a light microscope(BX53; Olympus, Tokyo, Japan) at 400× magnification to assess the injury.
Terminal deoxynucleotidyl transferase(TdT)-mediated dUTP nick end labeling (TUNEL) assay
TUNEL assay was performed at 7 days of the experiment.Coronal sections (5 μm) selected from the perihemorrhagic penumbra were deparaffinized and incubated in 0.1% Triton-X-100 at 4°C for 2 minutes, and then TUNEL reaction solution (Roche, Mannheim, Rhineland-Palatinate, Germany)in the dark at 37° C for 1 hour. After staining in 3,3′-diaminobenzidine for 5 minutes, sections were counterstained in hematoxylin. Apoptotic cells in the perihemorrhagic penumbra were observed and counted under a light microscope at 400×magnification. The apoptosis index was calculated by: number of apoptosis cells (TUNEL-positive cells)/total cells × 100%.Three randomly selected microscopy fields were used to count apoptotic cells.
Western blot assay
Western blot assay of Smo and Gli1 proteins was performed at 7 days of the experiment. Brain tissue samples were dissolved in radio immunoprecipitation assay lysis buffer containing 1%phenylmethyl sulfonylfluoride (Amresco, Atlanta, GA, USA),and centrifuged at 13,000 × g for 10 minutes at 4° C. Samples(50 μg protein) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).Membranes were blocked using 5% skimmed milk for 1 hour at room temperature, and then incubated with one of the following primary antibodies: rabbit polyclonal anti-Smo (1:1000;Abcam) or rabbit polyclonal anti-Gli1 (1:1000; Abcam) overnight at 4°C. Membranes were incubated in goat anti-rabbit secondary antibody (1:10,000; TDY Biotech, Beijing, China)for 1 hour at 37°C. Proteins bands were visualized using an enhanced chemiluminescence method (Xu et al., 2017). Images were captured and analyzed using an image analysis system(GelDoc XR+; ProteinSimple, San Jose, CA, USA). Glyceraldehyde-3-phosphate (GAPDH; Millipore, Billerica, MA,USA) was used as the internal control. Protein concentration was measured by bicinchoninic acid assay (Xu et al., 2017). The ratio of protein optical density ratio to internal control protein was calculated.
Statistical analysis
All data are expressed as mean ± SD, and analyzed using SPSS 22.0 software (IBM, Armonk, NY, USA). Data from the behavioral experiment were analyzed by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test. Data from the TUNEL assay and western blot assay were analyzed using one-way ANOVA and Student-Newman-Keuls test. A value of P < 0.05 was considered statistically significant.
Results
Baihui-penetrating-Qubin acupuncture improved motor/sensory function in a rat model of ICH
Baseline neurological function (Ludmila Belayev test score)was comparable among each group. Neurological deficits were apparent 6 hours after autologous blood injection. Ludmila Belayev test score was significantly different between the ICH group and sham group (P < 0.001). In contrast, there was no difference in Ludmila Belayev test score among the ICH + Ag,ICH + Ac, ICH + SAc, and ICH groups. On day 2, Ludmila Belayev test score was higher in the sham group than control group. While Ludmila Belayev test score was significantly higher in the ICH group compared with the control and sham groups (P < 0.001). There was a decreasing trend, albeit not statistically significant, in Ludmila Belayev test score in the ICH+ Ag and ICH + Ac groups compared with the ICH group.On days 3–7, Ludmila Belayev test score was higher in the ICH group compared with the control and sham groups (P< 0.001). Ludmila Belayev test score was lower in the ICH +Ag and ICH + Ac groups compared with the ICH group (P <0.001 or P < 0.01). Ludmila Belayev test score was higher in the ICH + SAc group than ICH + Ac group (P < 0.001 or P < 0.01 or P < 0.05; Figure 3).
Baihui-penetrating-Qubin acupuncture improved pathological injury in the perihemorrhagic penumbra in a rat model of ICH
Tissue in the control and sham groups appeared healthy, with virtually no apoptotic cells, inflammatory infiltration, or edema in neurons or interstitial spaces. However, tissue structure was disordered in the ICH group, with edema in neurons and astrocytes and among interstitial spaces. Karyopyknosis and necrotic cells were observed in the perihemorrhagic penumbra with a large number of inflammatory cells. The extent of injury was apparently lower in the ICH + SAc group, with reduced levels of edema in cells and among interstitial spaces. Further, there was still a large number of inflammatory cells and necrotic cells at the injury site. Compared with the ICH group, the degree of damage (edema, inflammatory infiltration, and cell necrosis)was markedly lower in the ICH + Ac and ICH + Ag groups(Figure 4).
Baihui-penetrating-Qubin acupuncture decreased cell apoptosis in the perihemorrhagic penumbra of a rat model of ICH
Apoptosis index was significantly lower in the ICH + Ag and ICH + Ac groups than the ICH group (P < 0.001). Apoptosis index in the ICH + SAc group was lower than the ICH group,but significantly higher than the ICH + Ag and ICH + Ac groups (P < 0.001; Figure 5).
Baihui-penetrating-Qubin acupuncture decreased Smo and Gli1 expression in the perihemorrhagic penumbra of a rat model with ICH
Smo protein levels were relatively low in the control group and sham group. Smo expression was higher in the ICH group compared with the sham group, and even higher in the ICH +Ag and ICH + Ac groups compared with the ICH group (P <0.05). Smo expression in the ICH + SAc group was not comparable with the ICH group, and significantly lower than the ICH + Ag and ICH + Ac groups (P < 0.05).
Similar to the pattern of Smo expression, Gli1 expression was significantly higher in the ICH + Ag and ICH + Ac groups than ICH group (both P < 0.05). Gli1 expression in the ICH+ SAc group was not comparable to the ICH group, but significantly lower than the ICH + Ag and ICH + Ac groups (P <0.05; Figure 6).
Discussion
Function of the Shh pathway has been widely studied in many organs and tissues, including cartilage (Teillet et al., 1998; Murtaugh et al., 1999), blood cells (Bhardwaj et al., 2001), muscle(Münsterberg et al., 1995), and teeth (Hardcastle et al., 1998).Indeed, the Shh pathway has beneficial effects on formation of these tissues. Previous studies have demonstrated that inhibiting the Shh pathway promotes apoptosis of cancer cells (Subramani et al., 2017; Xu et al., 2017), and also promotes apoptosis in traumatogenic diseases such as ischemia/reperfusion injury of skeletal muscle (Zeng et al., 2017) and kidney (Meng et al., 2016). The Shh signaling pathway also plays an important protective role in the central nervous system, promotes neurite outgrowth and synaptogenesis during stroke (Tang et al., 2017),and inhibits neuronal apoptosis induced by hydrogen peroxide(He et al., 2017).
Recovery of neurological function after stroke is largely dependent on the extent of neuronal apoptosis (Pang et al.,2017). In the present study, we show that the Shh agonist,purmorphamine, promotes recovery of neurological function and inhibits neuronal apoptosis, possibly by activating the Shh pathway in rats with ICH. Baihui-penetrating-Qubin acupuncture produced a series of effects similar to the Shh agonist, purmorphamine, including activation of Shh activity. Therefore,we speculated that activated Shh pathway contributes to the neuroprotective effect of Baihui-penetrating-Qubin acupuncture.
In conclusion, Baihui-penetrating-Qubin acupuncture after ICH can inhibit cell apoptosis and pathological injury in the perihemorrhagic penumbra, and promote recovery of motor and sensory function at 7 days, which may lead to activation of the Shh signaling pathway. However, this study has several limitations. First, neurological recovery after autologous blood injection was observed for only a limited time frame (1 week)using a single behavioral test that covers both motor and sensory function. Second, the extent of injury was assessed by only representative methods, including pathological examination by hematoxylin-eosin staining and TUNEL assay for apoptosis. Changes specific to neurons versus glial cells and astrocytes were not differentiated. Furthermore, the contributing role of the Shh pathway was only supported using a selective pharmacological activator, and not with inhibition or downregulation of the Shh pathway. Last but not least, other factors were not considered, e.g., inflammation (Qin et al., 2015) and neuronal stem cells (Jessberger, 2016). Nevertheless, we show that Baihui-penetrating-Qubin acupuncture can alleviate tissue damage in the perihemorrhagic penumbra in an animal model of ICH.
Author contributions: BZ and WZ independently designed the study.XPY, XHD and WT carried out the experiment. XWS, WWY and HL collected and analyzed the data of Ludmila Belayev test, histological evaluation, TUNEL assay and western blot assay. HW and MJS wrote the initial draft of the paper. ML assisted with refinement of the manuscript. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 30772840, 81273824, and 81473764.The funding bodies played no role in the study design, in the collection,analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study was approved by the Animal Ethics Committee of Heilongjiang University of Chinese Medicine of China (approval IACUC: 2017-10-10-01).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Juliana Dalibor Neves, Universidade Federal do Rio Grande do Sul Instituto de Ciencias Basicas da Saude, Brazil.
Additional file:
Additional Table 1: Ludmila Belayev Scores.
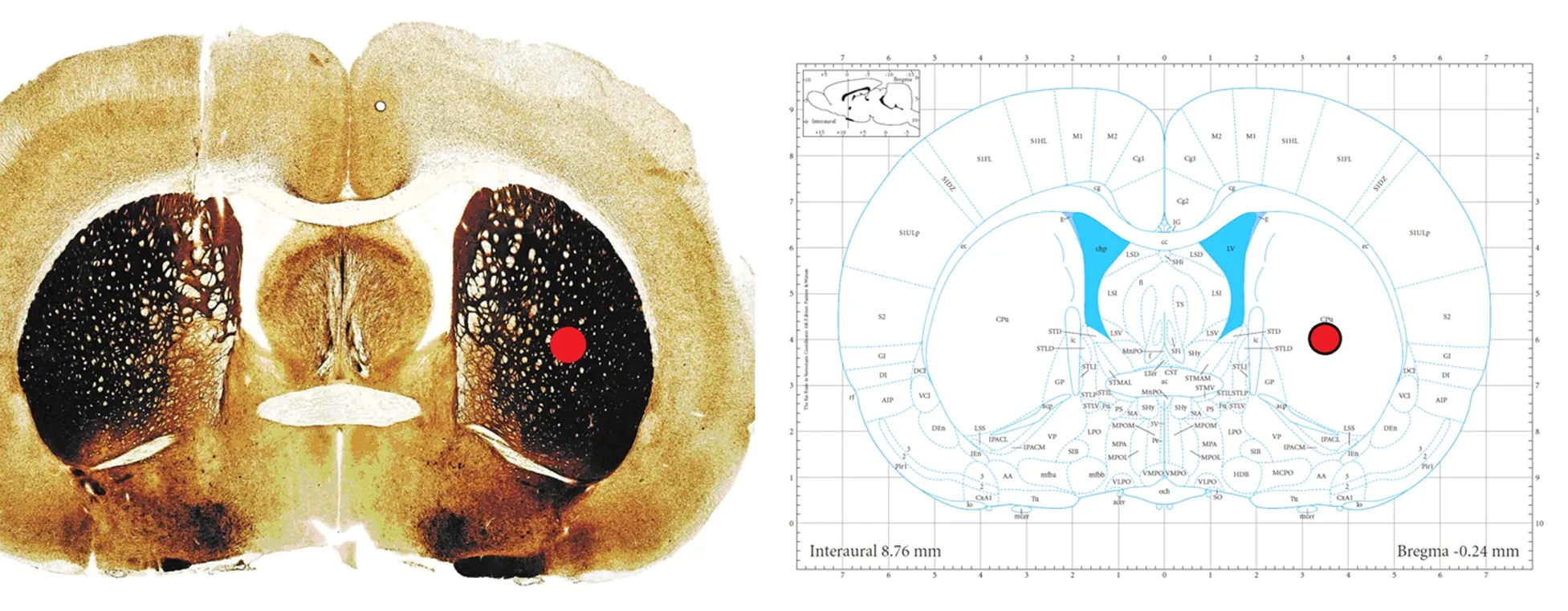
Figure 1 Location of intracerebral hemorrhage site.
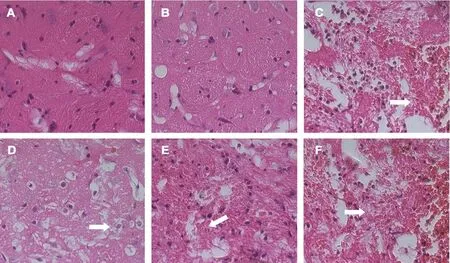
Figure 4 Effect of Baihui (DU20)-penetrating-Qubin (GB7) acupuncture on pathological injury in the perihemorrhagic penumbra of ICH rats at 7 days after surgery (hematoxylin-eosin staining).
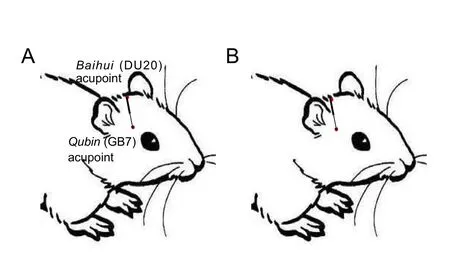
Figure 2 The Baihui (DU20)-penetrating-Qubin (GB7) acupuncture treatment method.
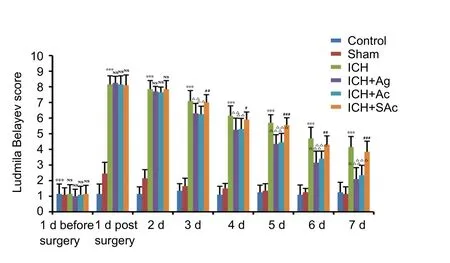
Figure 3 Effect of Baihui (DU20)-penetrating-Qubin (GB7)
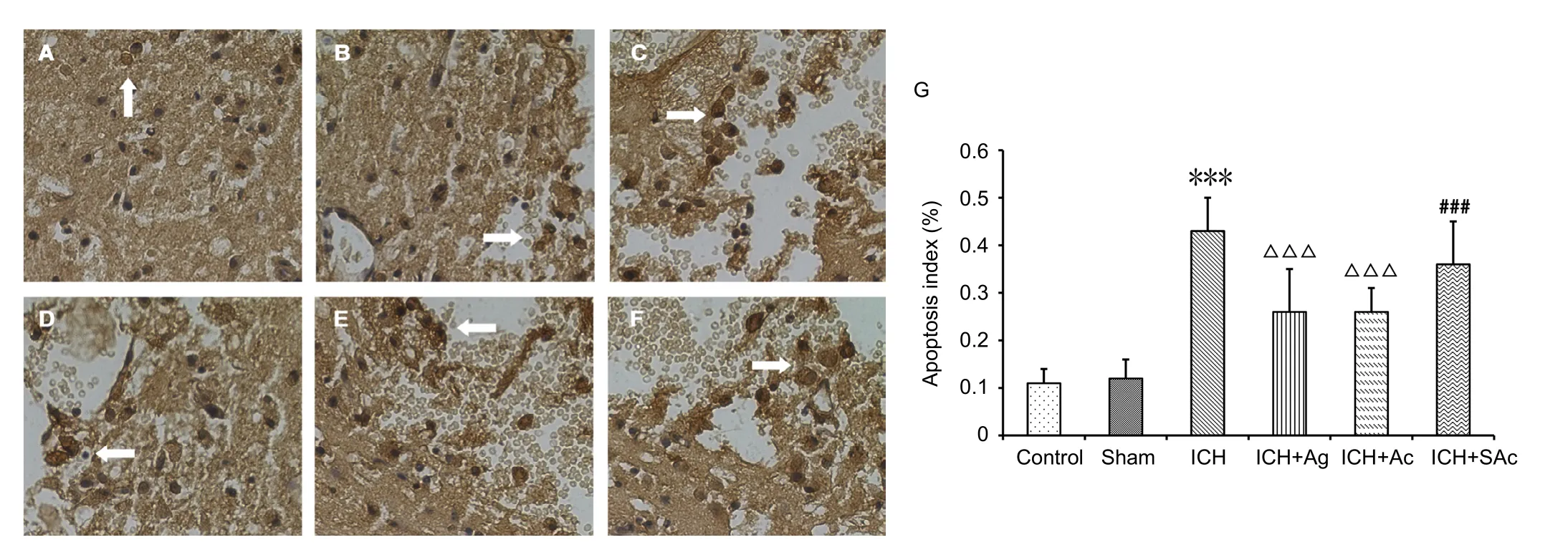
Figure 5 Effect of Baihui (DU20)-penetrating-Qubin (GB7) acupuncture on cell apoptosis in the perihemorrhagic penumbra of ICH rats at 7 days after surgery.
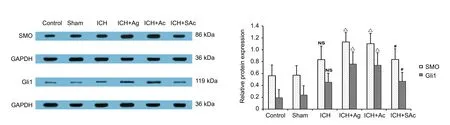
Figure 6 Effect of Baihui (DU20)-penetrating-Qubin (GB7) acupuncture on Smo and Gli1 expression in the perihemorrhagic penumbra of ICH rats at 7 days after surgery (western blot assay).
- 中国神经再生研究(英文版)的其它文章
- Seeing the wood for the trees: towards improved quantification of glial cells in central nervous system tissue
- 3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
- The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis
- Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats
- Saikosaponin a increases interleukin-10 expression and inhibits scar formation after sciatic nerve injury
- Various changes in cryopreserved acellular nerve allografts at −80° C

