Real world studies are essential for drug therapy in Parkinson’s disease
Prospective real-world data from large patient samples, which report on the long-term effectiveness of the employed different drug therapies, are rare in Parkinson’s disease (PD). The non interventional“Transdermal Rotigotine User Surveillance Study” (TRUST) trial represents such a real-world study. It investigated long-term treatment with different dopamine substituting treatment regimens in 2195 PD patients (Müller et al., 2018). Participation in TRUST meant that the treating neurologists were only asked to document and modify the dopaminergic drug regimen without any prior PD patient selection criteria. Thus this unique trial design reflects the real world of patient maintenance. The only intention was to follow patients in a 5:2:2:5:2 ratio for 1) rotigotine without levodopa, 2) other dopamine agonists without levodopa (i.e., ropinirole, pramipexole, or other oral dopamine agonist, 3) levodopa without dopamine agonists, 4) levodopa in combination with rotigotine, or 5) levodopa in combination with other dopamine agonists (Figure 1) (Müller et al., 2018). Patients were observed for ≤ 33 months. ~44% of patients received the same treatment over the full duration of their participation in the study.Beneficial effects were observed with all the different therapeutic strategies employed. No clear cut differences were found (Müller et al.,2018)
To date, the value of combination therapy in PD has not been investigated in such a large patient cohort in a real world setting over such a long interval in a prospective manner. Even the “Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease” (PD MED) register study only was an open-label, but socalled “pragmatic” randomized study. It compared levodopa-sparing therapy (dopamine agonists or monoamine oxidase (MAO)-B inhibitors with levodopa alone in newly diagnosed patients (Gray et al., 2014). Thus even in PD MED, patients were forced into a certain treatment regime following randomisation.
Generally, clinical research nowadays lacks results of real-world studies. They may provide data that are more representative of the general PD population. Instead, randomized clinical trials (RCT)are performed. They are usually limited to the assessment of a single therapeutic intervention. RCT have severe inclusion- and exclusion criteria. They often exclude certain comorbidities with concomitant necessary drug therapies, both of which are frequent in the daily maintenance of PD patients. In order to gain positive results, these trials are powered based on the outcomes of phase II proof of concept investigation. As consequence, this “trick” for the later statistical evaluation often asks for high numbers of study participants. Thus costs explode for the performance of phase III trials and accordingly for the clinical development of new drugs in general. The necessity of many study participants in conjunction with rigorous inclusion and exclusion criteria induces long intervals for recruitment. Consequently, motivational deficits of investigators become more likely with increasing pressure of sponsors and rejection of potentially interested patients but with missing eligibility. Additionally, the risk is high that intermediate change of diagnostic criteria or therapeutic improvements may prevent the consideration of these new therapeutic developments. This may be not so relevant in this artificial study world. However, it may gain importance, when the new therapeutic option enters the real world of patient maintenance under an interim development with different conditions, for instance in terms drug interactions or other safety concerns.
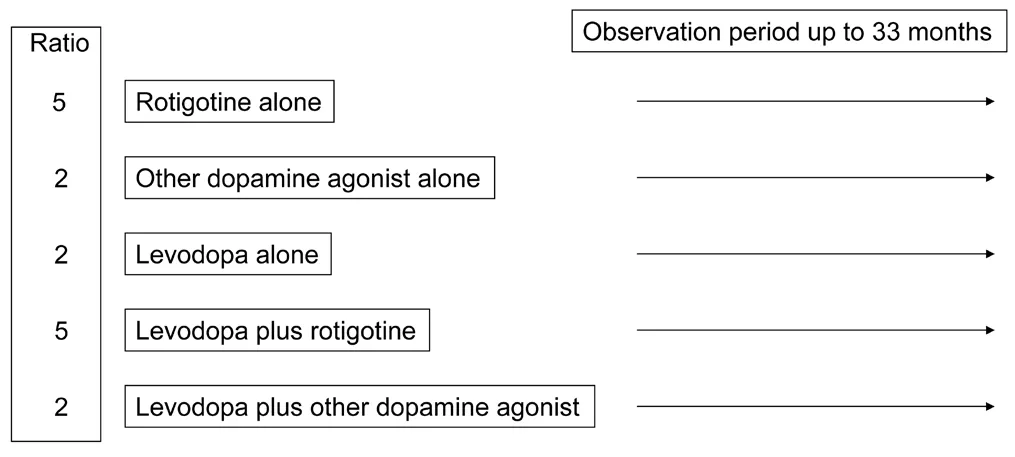
Figure 1 Design of the TRUST study.
A typical example may represent the non blinded “The Effect of Deep Brain Stimulation of the Subthalamic Nucleus (STN-DBS) on Quality of Life in Comparison to Best Medical Treatment in Patients With Complicated Parkinson’s Disease and Preserved Psychosocial Competence” (EARLY STIM) trial. It compared the effects of deep brain stimulation in a cohort of PD patients up to a age of 60 with socalled “best medical” treatment according to existing PD guidelines supervised by a committee without any direct contact to each of the participating PD patients (Schuepbach et al., 2013). Recruitment and duration of the trial lasted from 6/2006 to 12/2011. However, the value of the selected primary objective, the quality of life instrument 39-Item Parkinson’s Disease Questionnaire (PDQ-39), experienced substantial critique during the performance of the trial. The reviewers of the PDQ-39 construct even concluded that PDQ-39 outcomes should be interpreted with caution in general (Hagell and Nilsson,2009; Nilsson et al., 2010).
A further failed development - particularly in the past - is the overestimated value of RCT outcomes for the inauguration of guidelines.A RCT is normally performed in a blinded fashion in combination with a randomization procedure. In a chronic disease like PD, the RCT is limited to one therapeutic intervention. However, clinical practice often asks for an individual dosing of compounds within a combination drug regimen or adding of additional agents to achieve an optimum outcome for the patient. Moreover a design including placebo control, double-blinded randomization etc is one essential criterion for the evidence level of the performed trial and thus its impact on the guideline. When results are positive, if at all, conditions of the trial - for instance use of a certain drug dosage - are often also decisive for the later use of the drug in the real world. However efficacy of a drug may also depend on individual different preconditions.As an example, the efficacy of enzyme blockers will start to vane, if the activity of the inhibited enzyme is high. The likelihood of frustration by patients and treating physicians goes up. As a result, the real world of patient maintenance rejects an innovation. An enzyme blocker will also cause side effects, like i.e., the cheese effect due to tyramine induced hypertension, if the existing enzyme activity is low.This may be the case for instance with use of MAO-B inhibitors. Generally, MAO-B activity varies between individuals and also depends on concentrations of dopamine, which also additionally influence MAO-B enzyme activity (Müller et al., 2017a, b). Thus, application of a MAO-B inhibiting compound, such as rasagiline, selegiline or safinamide, may induce a better effect on motor behaviour with higher dosing beyond approval of authorities. Particularly, in the case of MAO-B inhibitor use in PD, phase III trials often showed safe and positive outcomes in a certain dosage. Therefore their application was restricted to a certain dosing interval. In clinical practice, these approved dosages often limit the possible better effects of the drug in the individual patient despite the fact that the compound was also tested in a higher dosage with accordingly better efficacy and same safety or in combination with other drugs. Limitations of safinamide use only in conjunction with levodopa or rasagiline application only in a 1 mg dosage may serve as examples. In the real world of PD therapy, dosing of compounds, i.e., levodopa or a dopamine agonist, is generally done an individual risk-benefit calculation with consideration of temporary occurrence of side effects in relation to the applied dose. The interaction between the individual patient and the treating physician are the essential determinant for the use of the drug. Both aforementioned drugs have admittedly a different mode of action than inhibitors of MAO-B or Catechol-O-methyltransferase (COMT), but their less limited dosing intervals enable a specific titration regimen according to the individual patients’ needs. However, particularly in the case of rasagiline, dosing of only 1 mg is allowed and performed in clinical practice, whereas enough data on the 2 mg dosing regimen are also available (Olanow et al., 2009). It remains speculative, wheth-er performance of a more individual titration regimen of rasagiline perhaps also enabled a more convincing demonstration of a putative disease modifying effect (Olanow et al., 2009). It is noteworthy, that PD patients often discuss the necessary levodopa dosage as a marker of the severity and the rate of progression of PD. They may be right and may consider the outcomes of the SELEDO (from: selegiline plus levodopa) study trial in terms of levodopa sparing as a biomarker for disease modification in the real world. In this trial curves diverged in favour for a combination of selegiline plus levodopa versus levodopa monotherapy (Przuntek et al., 1999).
In this respect, one could also additionally consider the long term outcomes of the open label Attenuation of Disease Progression with Azilect Given Once-daily (ADAGIO)-follow-up study. This study was initiated approximately 26 months after completion of the ADAGIO study (Olanow et al., 2009). 58% of the initial ADAGIO cohort and 72% of the ADAGIO completers were included. Then these patients were followed for further three years. All study participants received 1 mg rasagiline only and any other PD treatment that was deemed appropriate. This follow-up trial on the incomplete ADAGIO cohort failed to demonstrate any benefit of early-start of rasagiline treatment versus delayed application of the MAO-B inhibitor (Rascol et al., 2016). However, one must consider and should not neglect the considerable, methodological problems of the design of this follow-up study. Nevertheless, this trial may generate and support the opinion that disease modification is not enabled by MAO-B inhibition in monotherapy or in the long term during a combination therapy. More generally, MAO-B-inhibitors have been shown according to RCT outcomes to possess a positive profile in terms of prevention and symptomatic effects on motor complications. One believes that they have only a limited beneficial effect on motor impairment in PD patients, but with a good side effect profile in comparison with for instance with dopamine agonists. They may cause considerable side effects, i.e., nausea, at least temporarily with higher dosing. In the real world, it depends on the individual patient, to tolerate the frequent often only intermittent onset of side effects during dose titration. RCT designs in PD do often not consider this slow and cautious up titration regime. However a patient tailored dose escalation supports long term adherence to the treatment regimen and the PD patient remains somewhat stable in the further course of PD. In this respect, TRUST confirms that the available PD drug portfolio should be administered,combined and titrated individually adapted to patients’ symptoms and needs. TRUST also demonstrates that a continuous unaltered treatment schedule was considered appropriate and feasible for a large number of patients. Even the actual starting treatment appeared to have little influence on whether a patient continued on that treatment regimen for the duration of the study. More patients, who started on combination therapy with levodopa, remained on this treatment for the duration of the study than those, who started on dopaminergic monotherapy. Thus TRUST results reinforce the concept that dopaminergic monotherapy (dopamine agonist or levodopa) may work well up to a certain moment, but that most patients will require combination therapy with both a dopamine agonist and levodopa at some point sooner or later (Müller et al., 2018).
In this respect, TRUST outcomes may also scrutinize the tendency for too detailed treatment guidelines and restrictions in the normal maintenance of PD patients. In this regard, TRUST outcomes may also support the view that only more general recommendations make sense and that one should be cautious to esteem the a too strict fulfilment of so-called “evidence medicine based” criteria. Generally, these bureaucratic approaches privilege industry- and institution-funded, placebo controlled, mostly global studies. RCT’s have aforementioned limitations, but it is also worth to emphasize that RCT’s cannot be replaced by real world studies, only. The advantage of RCT’s is that their design allows a specific focus on potential benefits for evaluation of drugs or surgical effects. This is the true value of RCT’s. Therefore RCT’s are performed under artificial study conditions. Accordingly, the value of RCT’s for generation of guidelines is limited. Still to date, the real world finally decides, whether a new treatment approach is feasible for PD or not. In conclusion,RCT’s do not support personalized medicine approaches to add or switch treatments in order to best manage the symptoms of PD (Titova and Chaudhuri, 2017), which was the underlying concept of the TRUST trial.
Thomas Müller*
Department of Neurology, St. Joseph Hospital Berlin-Weißensee,Berlin, Germany
*Correspondence to: Thomas Müller, M.D.,th.mueller@alexianer.de or thomas.mueller@ruhr-uni-bochum.de.
orcid: 0000-0002-6799-0753 (Thomas Müller)
Accepted: 2018-06-16
doi: 10.4103/1673-5374.237118
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Jiaming Mei, Anhui Provincial Hospital, China.
Additional file: Open peer reviewer report 1.
- 中国神经再生研究(英文版)的其它文章
- Seeing the wood for the trees: towards improved quantification of glial cells in central nervous system tissue
- 3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
- The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis
- Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats
- Saikosaponin a increases interleukin-10 expression and inhibits scar formation after sciatic nerve injury
- Various changes in cryopreserved acellular nerve allografts at −80° C

