The emerging epigenetics of PARK7 and its implication in neurodegenerative disease
PARK7/DJ-1 is a multifunctional protein that acts as a regulator in stress responsive cascades. Since its original discovery as a novel oncogene, DJ-1 was further implicated in the correspondingly dubbed PARK7 autosomal recessive early onset Parkinson’s Disease variant,expanding the spectrum of its pathophysiology to neurodegenerative disease. DJ-1’s physiological roles have correspondingly expanded in the literature, effectively revealing an almost ubiquitous presence in cellular compartments and tissues. DJ-1’s functions mediate a diverse armamentarium of cytoprotective effects such as RNA binding,SUMOylation, androgen receptor binding, and regulation of mitochondrial dynamics as well as a recently discovered deglycase activity(Richarme et al., 2015).
Despite the diversity of these aforementioned pathways, DJ-1’s role appears to be that of an oxidative stress sensor, linking stress induced shifts in the intracellular milieu with the activation of antiapoptotic pathways in tandem with repair mechanisms (Wilson, 2011;Richarme et al., 2015) (Figure 1).
In this setting, DJ-1’s epigenetic roles are best examined along with its interactome members that are implicated in histone post translational modifications, i.e., acetylation/deacetylation. One of the earliest interactions was reported in 2003, indicating that DJ-1’s positive regulation of androgen receptor signaling was effected by its binding with the DJ-1 binding protein (DJBP); the latter initiated the formation of a repressive histone deacetylase complex that was inactivated by its binding by DJ-1 (Niki et al., 2003). A subsequent study by Zhang et al. (2008) described a NonO-histone acetylation/deacetylation switch regulating early atherosclerotic extracellular matrix (ECM) remodeling. This switch was reported to be controlled by the oxidative status of DJ-1’s C106 cysteine residue, regulated in turn by selective TNFa-mediated oxidation.
In silico studies from our group reported at least two more potential epigenetic mechanisms in malignant pleural mesothelioma (MPM)and in multiple sclerosis (MS) (Vavougios et al., 2018b). Whereas DJ-1’s expression was associated with deacetylation and resistance to apoptosis in malignant pleural cells in the former, acetylation and increased apoptotic signaling was found as the predominant function in peripheral blood mononuclear cells (PBMCs) in the latter (Vavougios et al., 2018b).
Further experimental evidence on DJ-1’s epigenetics and specifically, its direct implication in histone acetylation/deacetylation have emerged within the last three years, showing a tissue specific profile for the range of its physiological effects as well as their potential perturbation. The gestalt of these studies points to early oxidative stress-mediated histone post translational modifications effected by DJ-1 in the premorbid state, with tissue specific perturbations arising with the onset of the clinically identifiable disease.
An example of this modus operandi is epigenetic control exerted by DJ-1 over the prorenin receptor (PRR) expression via an histone deacetylase (HDAC)1/H3 acetylation switch in the promoter region of PRR, initiated via the oxidative modification of DJ-1 (Lee et al.,2015). This HDAC1/H3 acetylation switch is also reported as both active and DJ-1 dependent in the endothelial nitric oxide synthase(eNOS) promoter, consequently regulating vasorelaxation and by extent, blood pressure (Vavougios et al., 2018a).
At this point it would be important to outline a general paradigm for DJ-1 as it arises in the literature: its stress responsiveness would represent the afferent (Wilson, 2011) whereas the cascades it enables would be the efferent branch of a homeostatic loop (Zhang et al., 2008). In this sense, the epigenetic machinery regulated by DJ-1 would remain stress responsive, promoting cytoprotection in a tissue-specific manner. This role is further supported by the protein’s sequestration and regulation of mitochondrial homeostasis and protein synthesis, indicating a strategic in situ entrenchment proximal to potential sources of endogenous stressors (Wilson, 2011).
Aside from DJ-1’s emerging epigenetic profile, several of its interactors are increasingly recognized as potential targets of acetylation,deacetylation and methylation. Among the most salient members of its interactome, the p53 protein has been shown to regulate downstream pathways in a stress-responsive manner that is, in turn, controlled by its acetylation status (Vilas-Zornoza et al., 2011). Another example would be presented by mutant SOD1 (mtSOD1) in the setting of amyotrophic lateral sclerosis, in which selective HDAC6 modulation by mtSOD1 leads to increased tubulin acetylation and conversely, regulates mtSOD1 aggregation (Gal et al., 2013).
Re-evaluating the role of DJ-1 in the central nervous system (CNS)in light of its emerging profile as an epigenetic regulator, its emerging epigenetic profile appears to be delineated in concert with a paradigm shift in CNS therapeutics; specifically, the recognition of acetylation homeostasis (and by extent, its dysregulation) as a major node determining the advent of neurodegenerative disease. Consequently, the targeting of histone acetyltransferases (HATs) and HDACs presents itself a field of therapeutics that remains to be fully explored (Saha and Pahan, 2006). Lessons learned on failed epigenetic therapeutics would need to be incorporated in any novel approach; namely, the correct identification of the perturbed histone post-translational modification complex promoting the pathogenesis of a specific disease. Compounds failing that condition on the premises of a broader targeting spectrum (such as valproic acid) have shown little to none promise in favorably affecting cellular epigenetics in the clinical setting (Rius and Lyko, 2012).
To summarize the points discussed in this editorial, we would have to examine the emerging evidence on the epigenetic profile of the DJ-1 protein in the premises of an intricate pathophysiological basis;while DJ-1 has been proven to mediate both epigenetic events and neurodegeneration independently, the case for a causative interplay between these two aspects may currently be possible to be assessed only in multiple sclerosis. Namely, DJ-1 appears to be perturbed both in the CNS (Hirotani et al., 2008) and the periphery (Vavougios et al.,2018b). While these studies are not directly comparable, both point towards the combined effects of perturbed apoptotic signaling in response to intracellular stress. Again however, it important to stress that aside from the in silico study from our group.
Finally, it is interesting to note that, DJ-1 may already be an affected bystander of one of the available disease modifying treatments of MS,indicating that complex interactions may involve not only disease pathophysiology, but also treatment (Vavougios et al., 2018a). Taking into account the complex network of epigenetic modifications regulated by DJ-1’s interactome (Niki et al., 2003; Zhang et al., 2008),it becomes evident that both previous literature and future studies should take into account factors such as HDAC/HAT homeostasis when examining DJ-1’s implication in neurodegenerative disease.Much like its recognition as a deglycase, DJ-1’s emerging epigenetic profile may require both a reconsideration of the past as well as a more thorough planning of future research.
George D. Vavougios*, Sotirios G. Zarogiannis
Department of Neurology, Athens Naval Hospital, Athens, Greece(Vavougios GD)
Department of Physiology, Faculty of Medicine, University of Thessaly,Larissa, Greece (Zarogiannis SG)
*Correspondence to: George D. Vavougios, M.D., Ph.D.,dantevavougios@hotmail.com.
orcid: 0000-0002-0413-4028 (George D. Vavougios)Accepted: 2018-03-31
doi: 10.4103/1673-5374.237117
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Cristoforo Comi, University of Piemonte Orientale,USA.
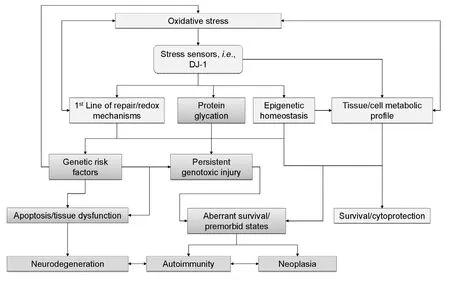
Figure 1 The epigenetic circuitry of DJ-1/PARK7. A putative model for the implications of DJ-1’s emerging epigenetic profile.
- 中国神经再生研究(英文版)的其它文章
- Seeing the wood for the trees: towards improved quantification of glial cells in central nervous system tissue
- 3′-Daidzein sulfonate sodium protects against memory impairment and hippocampal damage caused by chronic cerebral hypoperfusion
- The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis
- Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats
- Saikosaponin a increases interleukin-10 expression and inhibits scar formation after sciatic nerve injury
- Various changes in cryopreserved acellular nerve allografts at −80° C

