D-Transposition of the Great Arteries: A New Era in Cardiology
Angeline D.Opina, MD and Wayne J.Franklin, MD
lntroduction
D-transposition of the great arteries (d-TGA) is the second most common cyanotic congenital heart disease (CHD) lesion, accounting for 3–5% of all CHD, with a prevalence of 3 per 10,000 live births in the United States [1–3].Characterized by concordant atrioventricular connections and discordant ventriculoarterial connections, the aorta arises from the right ventricle, with the pulmonary artery arising from the left ventricle.This results in two parallel circuits.The “systemic” circuit returns deoxygenated blood from the systemic veins to the aorta by way of the right atrium and systemic right ventricle.The “pulmonary” circuit carries oxygenated blood from the lungs to the left atrium before it is pumped to the pulmonary arteries via the subpulmonic left ventricle (Figure 1).Associated congenital cardiac lesions include ventricular septal defects (VSDs) in 45% of cases, left ventricular outflow tract obstruction in 25% of cases, and coarctation of the aorta in 5% of cases.Intracardiac shunting, which can occur at the atrial, ventricular, or ductal level, allows mixing of deoxygenated and oxygenated blood, and is required to sustain life.
Clinical presentation in the neonatal period differs widely.On the milder end of the spectrum,patients with nonrestrictive atrial septal defects and VSDs have adequate mixing and present initially with a well-balanced circulation.Conversely,those with an intact ventricular septum are dependent on a nonrestrictive atrial septal communication to maintain adequate peripheral oxygen delivery.In the absence of a large atrial septal defect, mixing of deoxygenated and oxygenated blood is dependent on the presence of a patent ductus arteriosus.Closure of the ductus arteriosus results in profound cyanosis and rapid clinical deterioration.Therefore neonates with simple d-TGA and a restrictive atrial septum generally undergo transcatheter balloon atrial septostomy early in life, often within the fi rst few days after delivery.
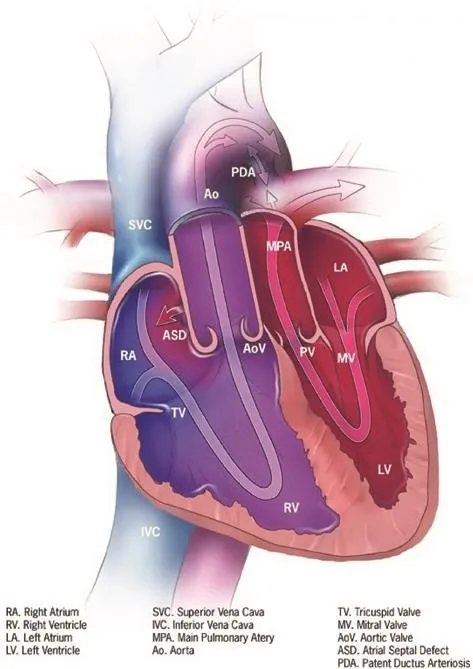
Figure 1 Transposition of the Great Arteries.
In 1969, Liebman et al.[5] described the natural history of unrepaired d-TGA in children born before the surgical era.They noted a 90% mortality rate by 1 year of age, with the worst prognosis seen in patients with simple d-TGA.The presence of a VSD and pulmonary stenosis was associated with longer lifespans, likely related to the ability for intracardiac mixing of oxygenated and deoxygenated blood in addition to protection from pulmonary overcirculation and subsequent development of pulmonary hypertension.
Early Operative History
Surgical exploration for correction of d-TGA began in the early 1950s.At that time, attempts at anatomic repair via arterial switch were universally fatal because of difficulty with coronary artery translocation [6].As a result, physiologic repair via the atrial switch procedure became the procedure of choice in the early surgical era of d-TGA repair (Figure 2).
Early atrial switch operations involved “partial”switching of the venous return by anastomosis of the right pulmonary veins to the right atrium and redirection of inferior vena cava blood fl ow to the left atrium.Using this technique in 1952, Walton Lillehei [8] performed the atrial switch operation on four babies, of whom two survived.In 1956,Baffes [9] used homograft material to join the inferior vena cava to the left atrium and anastomosed the right pulmonary veins to the right atrium.He performed this operation in 117 patients, and reported a 29% survival rate at 5 year follow-up [10].He intended to complete a second-stage procedure consisting of switching the superior vena cava and left pulmonary veins, but never performed this surgery clinically [11].

Figure 2 The Atrial Switch Procedure (Senning or Mustard).
Senning [12], in 1957, performed a successful atrial switch procedure on one patient using atrial fl aps for intra-atrial redirection of systemic and pulmonary venous return, an idea initially attributed to Harold M.Albert in 1954 [13].This procedure was thought to be technically difficult to perform in infants and small children, limiting its initial popularity among surgeons.In 1963, Mustard et al.[14] introduced a new technique for performing the atrial switch procedure by successfully using an autologous pericardial baffle to perform an atrial switch operation in addition to VSD closure in an 18-month-old girl.
After its introduction, the Mustard procedure brief l y became the technique of choice for the atrial switch operation until the 1970s.However, the Senning procedure was revived after favorable surgical results were reported by Quaegebeur et al.[15], who noted similar operative mortality between the Senning and Mustard procedures.Additionally, they highlighted a significant disadvantage of the Mustard procedure being the use of a large patch of prosthetic material and inability of the prosthetic material to grow with patients, sometimes resulting in the development of systemic and pulmonary venous baffle stenosis.
Outcomes of the Atrial Switch Operation
Patients who have undergone the atrial switch operation continue to be seen in adult CHD (ACHD)clinics worldwide and are now surpassing the fourth and fi fth decades of life.The long-term sequelae after the atrial switch operation have been well documented, and include baffle obstruction and leaks,tachyarrhythmia, sinus node dysfunction, and failure of the systemic right ventricle.
Problems with intra-atrial baffles arise after the atrial switch operation.Baffle leaks occur in up to 25% of atrial switch patients, and although they are usually small, they can pose a potential risk for paradoxical emboli or can be a cause of progressive desaturations.On the other hand, limited intra-atrial space and the use of prosthetic material with limited potential for growth can lead to baffle obstructions,which are seen in 5% of patients within a 10-year follow-up period [16].The superior limb of the systemic venous pathway is most commonly affected after the Mustard procedure, and pulmonary venous baffle obstruction is more commonly seen after the Senning procedure [17, 18].
Long suture lines and atrial scar are implicated in the common problem of arrhythmias after the atrial switch operation.Intra-atrial reentry tachycardia, a reentry pathway usually located adjacent to baffle suture lines, is most frequently seen, occurring in up to 10% of patients at follow-up at 10 years [19].The incidence of arrhythmias increases over time, with a nearly 78% arrhythmia-free survival rate at 10 years,declining to 36% at 25 years after the procedure [20].Radiofrequency ablation is commonly performed as treatment of atrial arrhythmias.However, the success rate of radiofrequency ablation is much lower than in the general population, with recurrence of atrial tachyarrhythmia in approximately one-third of patients [21].Additionally, sinus node dysfunction, bradycardia, and chronotropic incompetence occur in nearly 50% of patients within 20 years after the procedure, and are related the close proximity of suture lines and scar tissue to the sinus node [18, 22].A large European multicenter study noted the need for permanent pacemaker placement in 15% of patients during long-term follow-up after the atrial switch procedure [23].If patients meet the criteria for a permanent pacemaker, it is our preference to use a device with antitachycardia pacing capabilities because of the high risk of recurrent atrial arrhythmias.
A study published by the Congenital Heart Surgeons Society demonstrated a decline in the late survival rate over time from 96% by 1 month after the procedure to 84% at 10 year follow-up, with higher survival for the Mustard procedure versus the Senning procedure [24].Although Khairy et al.[17] also demonstrated a trend toward lower mortality after the Mustard procedure when compared with the Senning procedure, the difference was not statistically significant after meta-analysis of seven studies.A 79% survival rate at follow-up at 30 years is expected, with the major cause of death being secondary to sudden cardiac death [20].In a multicenter study performed by Kammeraad et al.[25], ventricular tachyarrhythmia was the most common arrhythmia presentation in patients who experienced sudden cardiac death or aborted cardiac death after the atrial switch operation, and risk factors for sudden cardiac death included a history of arrhythmia, atrial fl utter, or atrial fi brillation, and the need for cardiac medications.
Perhaps the biggest disadvantage of the atrial switch operation is that, in this “physiologic repair,”the right ventricle continues to pump against systemic afterload.The long-term consequence of a systemic right ventricle, well studied in patients with congenitally corrected transposition of the great arteries, also known asventricular inversion,is the increasing incidence of systemic ventricular dysfunction as age increases.Approximately 67%of patients with congenitally corrected transposition of the great arteries develop some degree of systemic ventricular dysfunction by the age of 45 years [26].In patients with d-TGA, Gilljam et al.[18] reported the development of right ventricular dysfunction in 34% of atrial switch patients within 12 year follow-up.It is unclear if tricuspid valve regurgitation is a leading cause of ventricular dysfunction or if it occurs as a result of annular dilation, but its relationship with right ventricular dysfunction is fi rmly established.In a 30-year follow-up study, moderate or severe right ventricular dysfunction was present in 12% of atrial switch patients, and moderate or severe tricuspid valve regurgitation was present in 27 and 7% of patients,respectively [20].
In the 2008 guidelines for the care of adults with CHD, the American College of Cardiology (ACC)and the American Heart Association (AHA) recommend at least annual follow-up of atrial switch patients by a cardiologist who has expertise in ACHD [27].Visits should involve at least clinical evaluation, with special attention to symptoms, oxygen saturation, and functional status.The guidelines do not delineate the frequency of diagnostic testing in this population, and it should be dependent on patient presentation.Echocardiogram is useful to evaluate systemic ventricular function and atrioventricular valve regurgitation.Transesophageal echocardiogram, MRI, or cardiac CT is useful if additional imaging is needed for more detailed evaluation of the intra-atrial baffle.ECG and Holter/event monitoring should be performed if concern for the development of arrhythmias or symptomatic bradycardia arises.
The Arterial Switch Operation
In 1975, Jatene et al.[28] performed the fi rst successful arterial switch operation (ASO) by translocating coronary artery buttons, transecting and contraposing the aorta and pulmonary artery, and reanastamosing the great vessels.Considered an “anatomic repair,” the ASO results in concordant ventriculoarterial connections and eliminates the issue of having a systemic right ventricle prone to developing systolic dysfunction (Figure 3).Improvements in the technique of translocating the coronary arteries via coronary buttons have resulted in a markedly reduced early mortality rate.Since the late 1980s,the ASO has been the preferred surgical treatment of choice for d-TGA.
Outcomes of the Arterial Switch Operation
In its infancy, the ASO had an early mortality rate of 15–28% because of myocardial ischemia secondary to coronary artery problems [28–31].In the last 3 decades, the early mortality rate after the ASO has significantly reduced to now around 2.7%in simple d-TGA and 5.3% in patients who require VSD closure in addition to arterial switch [32].The long-term postoperative survival rate is excellent, approaching 95% at 25 year follow-up, and patients typically have good functional status, New York Heart Association class I, at 20 year followup [28–31, 33–36].Additionally, ventricular systolic function is preserved in most patients with up to 30-years’ follow-up, and less than 20% develop mild to moderately depressed function [35, 37].However, the ASO can have major complications,including neoaortic root dilatation, neoaortic valve regurgitation, outflow tract obstructions, and anatomic coronary artery problems after coronary translocation.
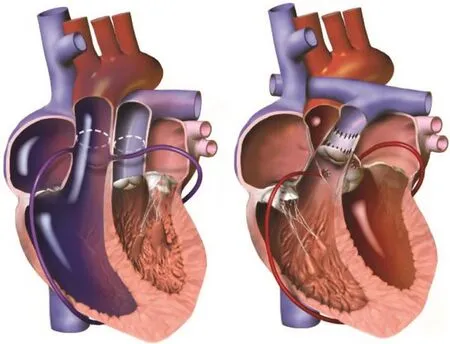
Figure 3 The Arterial Switch Procedure.
The ASO involves transection of the great vessels above the level of the pulmonary and aortic root.After reanastomosis of the great vessels, the native pulmonary root and valve, prone to dilation and valvular regurgitation when subject to systemic pressures, become the neoaortic root and valve,respectively.Progressive neoaortic root dilation and neoaortic valve regurgitation is well documented,with up to 66% of patients developing significant root dilatation (zscore >2.5) and 14% of patients developing at least mild to moderate valvular regurgitation at follow-up at up to 15 years [34].A history of aortic arch obstruction is associated with a higher frequency of aortic root dilatation and valve regurgitation [35].
Neoaortic root dilatation and valve regurgitation is common during long-term follow-up.However,less than 5% of patients require aortic root or aortic valve intervention [29, 33, 36].Conversely,the most common reason for reintervention in this patient population is right ventricular outflow tract obstruction, most commonly at the site of the pulmonary artery anastomosis and the branch pulmonary arteries, in 5–11% of patients [29, 38].
Although the ASO was largely abandoned in the early era of d-TGA repair because of difficulty with coronary artery translocation, the technique involving creation of coronary buttons to enable coronary artery manipulation resulted in significantly improved outcomes but remains a challenge for cardiac surgeons.This especially applies in the setting of coronary anomalies, specifically single ostium coronary pattern and the presence of more than one coronary ostium arising from one sinus, both of which are associated with a significantly higher risk of late reinterventions and death [39, 40].Anatomic coronary disease occurs in approximately 2–10%of patients after arterial switch [30, 36, 41].In a large Korean study, Lim et al.[33] reported normal coronary morphology or perfusion in nearly 95% of patients who underwent coronary artery evaluation after the ASO.The rate of freedom from coronary events was 88% at follow-up at 15 years, 20 years,and 22 years.Coronary artery interventions were performed in patients with coronary artery ostial stenosis and coronary compression from the main pulmonary artery.In our experience, aortic root dilatation has resulted in distortion and kinking of the proximal coronary arteries, and patients present with typical angina symptoms.
The 2008 ACC/AHA guidelines for ACHD recommend an echocardiogram to evaluate anatomy and hemodynamics every 2 years and coronary artery evaluation via stress echocardiography, CT angiography, or coronary angiography at least once [27].At our institution, functional testing with an exercise treadmill test is performed every 1–2 years, and noninvasive anatomic imaging using coronary CT or MRI is performed every 3–5 years in asymptomatic patients.In symptomatic patients,we have used stress cardiac MRI, which evaluates patients for myocardial ischemia in addition to providing excellent anatomic coronary imaging, without exposure to radiation.Although use of stress MRI is not widespread, one study has shown that stress MRI yields a higher specificity for coronary artery disease when compared with single photon emission CT in the arterial switch population [42].When coronary intervention is required,some cases of coronary artery obstruction may be amendable to percutaneous treatment but ostial and proximal obstruction frequently require surgical revascularization.
The “Switch Conversion”
After positive results from the ASO, a two-stage conversion to the arterial switch was suggested as an alternative to heart transplant in patients who develop right ventricular systolic heart failure after atrial switch.In this procedure, the pulmonary artery is fi rst banded to “retrain” the left ventricle to tolerate systemic pressures.After a period of months to years, the “switch conversion” is completed via pulmonary artery debanding, intra-atrial baffle takedown, and the ASO.After the switch conversion, studies have demonstrated preserved left ventricular function as well as reductions in tricuspid valve regurgitation, improvements in right ventricular systolic function, and worsening of New York Heart Association functional class[42–45].The results of this procedure have not been promising in patients older than 12–16 years,with the development of biventricular systolic dysfunction after pulmonary artery banding and switch conversion [45–48].Some patients do not respond well to pulmonary artery banding, and some have developed acute subpulmonic ventricular dysfunction.For others, it is unclear as to how to decide that the left ventricle is adequately “prepared” for the second stage (the ASO).Often anecdotal evidence or institutional preference comes into play.At our institution, we generally do not put patients forward for this operation if they are older than 14 years.
Conclusion
Surgical management of d-TGA has evolved rapidly since the fi rst successful atrial switch in 1957.The atrial switch operation (also known as theSenning or Mustard operation) was initially favored but it has been associated with the long-term development of right ventricular failure and rhythm disturbances.The ability to successfully translocate the coronary arteries has allowed anatomic repair, the ASO, to now be the preferred method for surgical correction.The “switch conversion operation,” which converts the prior atrial switch operation to the arterial switch,has been performed in selected pediatric patients as an alternative to heart transplant, but the outcomes when it has been performed in adolescents have been poor.Overall, the care of patients with d-TGA has evolved over the last 60 years, with impressive results.However, clinicians need to closely monitor both atrial switch and arterial switch patients through adulthood and beyond.
Conflict of lnterest
The authors declare no conflict of interest.
REFERENCES
1.Qureshi AM, Justino H, Heinle JS.Transposition of the great arteries.In: Allen HD, Shaddy RE, Penny DJ, Feltes TF, Cetta F, editors.Moss and Adams’ heart disease in infants, childrens, and adolescents:including the fetus and young adult.9th ed.Philadephia: Lippincott Williams & Wilkins; 2016.pp.1163–85.
2.Moons P, Bovijn L, Budts W,Belmans A, Gewillig M.Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium.Circulation 2010;122(22):2264–72.
3.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al.Updated national birth prevalence estimates for selected birth defects in the United States,2004–2006.Birth Defects Res Part A Clin Mol Teratol 2010;88(12):1008–16.
4.Centers for Disease Control and Prevention.(2017).Facts about dextro-Transposition of the Great Arteries | Congenital Heart Defects| NCBDDD | CDC.Retrieved from https://www.cdc.gov/ncbddd/heartdefects/d-tga.html.
5.Liebman J, Cullum L, Belloc NB.Natural history of transposition of the great arteries: anatomy and birth and death characteristics.Circulation 1969;40(2):237–62.
6.Konstantinov IE, Alexi-Meskishvili VV, Williams WG, Freedom RM,Van Praagh R.Atrial switch operation: past, present, and future.Ann Thorac Surg 2004;77:2250–8.
7.Zaluaga MA, Burgos N, Mendelson AF, Taylor AM, Ourselin A.Voxelwise atlas rating for computer assisted diagnosis: application to congenital heart diseases of the great arteries.Med Image Anal 2015;26(1):185–95.
8.Lillehei CW, Varco RL.Certain physiologic, pathologic, and surgical features of complete transposition of the great vessels.Surgery 1953;34(3):376–400.
9.Baffes TG.A new method for surgical correction of transposition of the aorta and pulmonary artery.Surg Gynecol Obstet 1956;102(2):227–33.
10.Baffes TG, Lev M, Paul MH, Miller RA, Riker WL, de Boer A, et al.Surgical correction of transposition of the great vessels: a fi ve-year survey.J Thorac Cardiovasc Surg 1960;40:298–309.
11.Marathe SP, Talwar S.Surgery for transposition of great arteries: a historical perspective.Ann Pediatr Cardiol 2015;8(2):122–8.
12.Senning A.Surgical correction of transposition of the great vessels.Surgery 1959;45(6):966–80.
13.Albert HM.Surgical correction of transposition of the great vessels.Surg Forum 1955;5:74–7.
14.Mustard WT, Keith JD, Trusler GA,Fowler R, Kidd L.The surgical management of transposition of the great vessels.J Thorac Cardiovasc Surg 1964;48:953–8.
15.Quaegebeur JM, Rohmer J, Brom AG.Revival of the Senning operation in the treatment of transposition of the great arteries.Preliminary report on recent experience.Thorax 1977;32:517–24.
16.Myridakis DJ, Ehlers KH, Allen Engle M, Myridakis D.Late Follow-up after venous switch operation (Mustard procedure) for simple and complex transposition of the great arteries.Am J Cardiol 1994;74:1030–9.
17.Khairy P, Landzberg MJ, Lambert J,O’Donnell CP.Long-term outcomes after the atrial switch for surgical correction of transposition: a metaanalysis comparing the Mustard and Senning procedures.Cardiol Young 2004;14(3):284–92.
18.Gilljam T, Eriksson BO, Solymar L, Jonsson M.Status of survivors after atrial redirection for transposition of the great arteries: a complete long-term follow-up.Acta Paediatr 1996;85(7):832–7.
19.Kanter RJ, Papagiannis J, Carboni MP, Ungerleider RM, Sanders WE,Wharton JM.Radiofrequency catheter ablation of supraventricular tachycardia substrates after Mustard and Senning operations for d-transposition of the great arteries.J Am Coll Cardiol 2000;35(2):428–41.
20.Moons P, Gewillig M, Sluysmans T, Verhaaren H, Viart P, Massin M, et al.Long term outcome up to 30 years after the Mustard of Senning operation: a nationwide multicentre study in Belgium.Heart 2004;90:307–13.
21.Gallotti RG, Madnawat H, Shannon KM, Aboulhosn JA, Nik-Ahd F,Moore JP.Mechanisms and predictors of recurrent tachycardia after catheter ablation for d-transposition of the great arteries after the Mustard or Senning operation.Heart Rhythm 2017;14:350–6.
22.Dos L, Teruel L, Ferreira IJ,Rodriguez-Larrea J, Miro L, Girona J, et al.Late outcome of Senning and Mustard procedures for correction of transposition of the great arteries.Heart 2005;91:652–6.
23.Vejlstrup N, Sørensen K, Mattsson E, Thilén U, Kvidal P, Johansson B, et al.Long-term outcome of Mustard/Senning correction for transposition of the great arteries in Sweden and Denmark.Circulation 2015;132(8):633–8.
24.Wells WJ, Blackstone E.Intermediate outcome after Mustard and Senning procedures: a study by the Congenital Heart Surgeons Society.Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2000;3:186–97.
25.Kammeraad JA, van Deurzen CH, Sreeram N, Bink-Boelkens MT, Ottenkamp J, Helbing WA,et al.Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries.J Am Coll Cardiol 2004;44:1095–102.
26.Graham TP Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H,Cetta F, et al.Long-term outcome in congenitally corrected transposition of the great arteries.J Am Coll Cardiol 2000;36(1):255–61.
27.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM,Dearani JA, et al.ACC/AHA 2008 guidelines for the management of adults with congenital heart disease.A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease): developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons.Circulation 2008;118(23):e714–833.
28.Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, et al.Anatomic correction of transposition of the great vessels.J Thorac Cardiovasc Surg 1976;72(3):364–70.
29.Fricke TA, d‘Udekem Y, Richardson M, Thuys C, Dronavalli M, Ramsay JM, et al.Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience.Ann Thorac Surg 2012;94:139–45.
30.Khairy P, Clair M, Fernandes SM,Blume ED, Powell AJ, Newburger JW, et al.Cardiovascular outcomes after the arterial switch operation for d-transposition of the great arteries.Circulation 2012;127(3):331–40.
31.Manso PH, Amaral FT, Júnior TJ,Jurca MC, Haddad J, Vicente WV,et al.Outcomes of patients after arterial switch operation: 18 years of experience in a single mediumvolume center.Pediatr Cardiol 2015;36(8):1657–61.
32.Jacobs JP, Mayer JE Jr, Mavroudis C, O‘Brien SM, Austin EH 3rd,Pasquali SK, et al.The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2017 update on outcomes and quality.Ann Thorac Surg 2017;103(3):699–709.
33.Lim H-G, Kim W-H, Lee JR, Kim YJ.Long-term results of the arterial switch operation for ventriculo-arterial discordance.Eur J Cardiothorac Surg 2013;43(2):325–34.
34.Co-Vu JG, Ginde S, Bartz PJ,Frommelt PC, Tweddell JS, Earing MG.Long-term outcomes of the neoaorta after arterial switch operation for transposition of the great arteries.Ann Thorac Surg 2013;95:1654–9.
35.Baruteau AE, Vergnat M, Kalfa D,Delpey JG, Ly M, Capderou A, et al.Long-term outcomes of the arterial switch operation for transposition of the great arteries and ventricular septal defect and/or aortic arch obstruction.Interact Cardiovasc Thorac Surg 2016;23(2):240–6.
36.Kempny A, Wustmann K, Borgia F, Dimopoulos K, Uebing A, Li W,et al.Outcome in adult patients after arterial switch operation for transposition of the great arteries.Int J Cardiol 2013;167:2588–93.
37.Tobler D, Williams WG,Jegatheeswaran A, Van Arsdell GS,McCrindle BW, Greutmann M, et al.Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries.J Am Coll Cardiol 2010;56:58–64.
38.Shim M-S, Jun T-G, Yang J-H,Park PW, Kang IS, Huh J, et al.Current expectations of the arterial switch operation in a small volume center: a 20-year, single-center experience.J Cardiothorac Surg 2016;11(1):34.
39.Pasquali SK, Hasselblad V, Li JS,Kong DF, Sanders SP.Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: a meta-analysis.Circulation 2002;106(20):2575–80.
40.Moll M, Michalak KW, Sobczak-Budlewska K, Moll JA, Kopala M, Szymczyk K, et al.Coronary artery anomalies in patients with transposition of the great arteries and their impact on postoperative outcomes.Ann Thorac Surg 2017;104(5):1620–8.
41.Ou P, Khraiche D, Celermajer DS,Agnoletti G, Le Quan Sang KH,Thalabard JC, et al.Mechanisms of coronary complications after the arterial switch for transposition of the great arteries.J Thorac Cardiovasc Surg 2013;145:1263–9.
42.Tobler D, Motwani M, Wald RM,Roche SL, Verocai F, Iwanochko RM, et al.Evaluation of a comprehensive cardiovascular magnetic resonance protocol in young adults late after the arterial switch operation for d-transposition of the great arteries.J Cardiovasc Magn Reson 2014;16(1):98.
43.Daebritz SH, Tiete AR, Sachweh JS, Engelhardt W, von Bernuth G,Messmer BJ.Systemic right ventricular failure after atrial switch operation: midterm results of conversion into an arterial switch.Ann Thorac Surg 2001;71:1255–9.
44.Maeda T, Sakamoto T, Nagashima M, Hiramatsu T, Yamazaki K.Long-term outcome of arterial switch operation conversion after failed Senning/Mustard procedure.Ann Thorac Surg 2016;102:1573–9.
45.Van Son JAM, Reddy VM,Silverman NH, Hanley FL,Francisco S.Surgery for congenital heart disease regression of tricuspid regurgitation after two-stage arterial switch operation for failing systemic ventricle after atrial inversion operation.J Thorac Cardiovasc Surg 1996;111:342–7.
46.Mavroudis C, Backer CL.Arterial switch after failed atrial baffle procedures for transposition of the great arteries.Ann Thorac Surg 2000;69:851–7.
47.Poirier NC, Yu JH, Brizard CP,Mee RBB, Mavroudis C, Fraser CD.Long-term results of left ventricular reconditioning and anatomic correction for systemic right ventricular dysfunction after atrial switch procedures.J Thorac Cardiovasc Surg 2004;127(4):975–81.
48.Benzaquen BS, Webb GD, Colman JM, Therrien J.Arterial switch operation after mustard procedures in adult patients with transposition of the great arteries: is it time to revise our strategy? Am Heart J 2004;147(3):e8.
 Cardiovascular Innovations and Applications2018年2期
Cardiovascular Innovations and Applications2018年2期
- Cardiovascular Innovations and Applications的其它文章
- His Bundle Pacing: Rebirth of an lmportant Technique for Pacing the lntrinsic Conduction System
- Depression in Adults with Congenital Heart Disease: Prevalence, Prognosis,and lntervention
- Traditional Chinese Medicine ls Widely Used for Cardiovascular Disease
- The Fontan Circulation: Contemporary Review of Ongoing Challenges and Management Strategies
- Heart Transplantation for Adult Congenital Heart Disease: Overview and Special Considerations
- Atrial Arrhythmias lncluding Atrial Fibrillation in Congenital Heart Disease: Mechanisms,Substrate ldentification and lnterventional Approaches
