Effect of industrial microwave irradiation on the physicochemical properties and pyrolysis characteristics of lignite☆
Guoshun Zhou ,Qunxing Huang *,Ben Yu Hui Tong ,Yong ChiJianhua Yan
1 State Key Laboratory of Clean Energy Utilization,Institute for Thermal Power Engineering,Zhejiang University,Hangzhou 310027,China
2 Everbright Environmental Research Institute(Nanjing)Limited,Nanjing 211100,China
3 Kunshan Jiuhua Electronic Equipment Factory,Suzhou,215300,China
1.Introduction
Approximately 45%-47%of world solid fossil fuel reserves are low rank brown coal or lignite which are especially abundant in Australia,Germany,northern America,Russia and China.Compared with other old black coal,lignite has relative high content of volatiles and low sulfur and is more reactive[1].However,its high moisture content(25%-60%),greater tendency towards spontaneous ignition,and re-adsorption properties have restricted its commercial utilization[2-5].Previous research has shown that when untreated lignite is fed directly into a combustion furnace,around 20%to 25%of its calorific value is consumed for internal moisture evaporation[6-8]and the overall boiler efficiency can be increased by about 2.6%by reducing the coal moisture by 5%[9].
To improve lignite combustion efficiency,many chemical(solvent extraction,supercritical water extraction,and so on)and physical(hot air drying,microwave drying and so on)methods have been developed to reduce the water content.Very recently,microwave irradiation based on lignite drying has received intensive interests[10,11].Microwave is electromagnetic wave with a frequency range of 300 MHz to 300 GHz[12].Pickleset al.[13]compared the drying rate of microwave and conventional oven drying,and they concluded that the microwave drying rates were about 35 times higher than conventional drying.Tahmasebiet al.[14]investigated the microwave drying efficiency of coal particle samples with different sizes,ash composition under different wave intensity.They found that unlike conventional heating,the drying rate increased and the drying time was reduced as the particle size increased.Although microwave drying owns relative high energy efficiency and is capable of rapid drying,concern has been raised on the non-uniformity of temperature field inside the material.These concerns can be partly alleviated by using microwave operating at lower frequency.Comparing to that of 2450 MHz microwave,the penetration of microwave energy at 915 MHz is about three times deeper and 915 MHz generators can provide up to 100 kW from a single unit[15].Moreover,the overall energy conversion efficiency of 915 MHz generators is about 15%-20%higher than that of a 2450 MHz generator.Very recently,Gbenga A.Olatunde investigated the one-pass drying of rough rice with a 915 MHz microwave dryer and they concluded that the use of microwave drying to improve heat rice yield could increase the current value of rice and benefit the rice industry[16].
Currently,the performance of 915 MHz microwave on lignite drying has not been well studied and information about the physicochemical properties and pyrolysis characteristics of irradiated lignite is very limited.In this paper,typical Chinese lignite samples were dried with a pilot-scale microwave oven working at915 MHz.After drying,the physicochemical properties including pore structure,surface oxygen functional groups and re-adsorption behavior were comparatively studied with that of the samples dried with traditional hot air.The pyrolysis characteristics of the dried coal were also analyzed by TG-FTIR and the effect of microwave irradiation on the composition of pyrolysis oil was examined with GC-MS.The results of this paper can provide valuable information to evaluate the possibility of lignite drying through high power microwave irradiation.
2.Experimental
2.1.Lignite sample
Lignite samples studied in this paper were collected from Wulagaicoal mining plant of Inner Mongolia where over70%of Chinese lignite reserves are located.The proximate and ultimate analysis data,along with heating value and mineral composition are listed in Table 1.As can be seen,Wulagai lignite is very typical lignite with a moisture and oxygen content of 33.8%and 17.8%,respectively.The samples were ground and sieved to three size fractions(0-1 mm,1-2 mm and 2-4 mm)before tests.

Table 1 Proximate,ultimate and mineral content analyses of Wulagai lignite
2.2.915 MHz microwave drying setups
The 915 MHz microwave drying oven was provided by Kunshan Jiuhua Electronic Equipment Factory,Suzhou,China,as shown in Fig.1.The system consisted of a microwave generator(10-30 kW)and inducer,drying chamber(920 mm×905 mm×785 mm)and nitrogen protecting system which was used for keeping dried environment inert.During the test,the microwave power was set to 10 kW and 1 kg of the coal with three different particle sizes was respectively tested repeatedly.N2was used to protect the sample from burning and carrying away the moisture desorbed from the sample.For comparison,hot air drying experiment was conducted in a DHG-9053 electric heated oven with cavity dimensions of 415 mm×370 mm×345 mm(Lantian Apparatus Co.,Ltd.,Hangzhou,China).To keep the energy consumption per unit mass of coal dried by hot air the same as that for microwave dried sample,80 g of the sample with three different kinds of particle sizes was also placed in a drying chamber and the temperature of the drying chamber was kept at105°C with a power of 0.8 kW.Moisture loss was measured by taking out and measuring the sample on a digital balance.When the sample reached a constant weight,equilibrium moisture content was assumed to be reached.
2.3.Pore structure
The specific surface area and pore volume of the samples were measured by a Quantachrome Autosorb-1-C N2adsorption porometer.
2.4.Surface oxygen functional groups
XPS spectrum was used to retrieve surface oxygen functional groups and vacuum Generator ESCALAB MARK II with Al Kαnonmonochromatic radiation was used.Before analysis,samples were ground into fine powders and pressed into tablets.During analysis,energy correction was used to account for sample charging based on the carbon(1 s)peak at 284.8 eV[17].
2.5.Re-adsorption and surface wettability
The re-adsorption tests were carried out with a SH-045E thermostatic and humidistat chamber(Lantian Apparatus Co.,Ltd.,Hangzhou,China)at a constant temperature of 40°C and a relative humidity of 90%.The mass of the sample was measured every 150 min with a BAS224S-CW balance(0.1 mg)until the equilibrium condition was achieved.
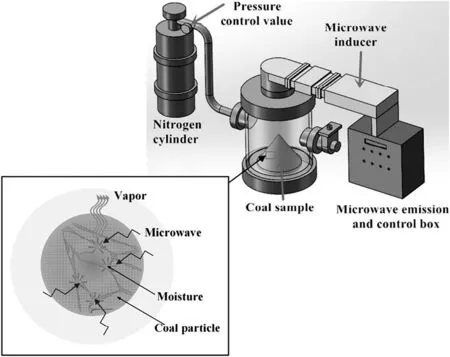
Fig.1.Experimental apparatus for microwave irradiation.
2.6.Pyrolysis characteristics
The pyrolysis of dried sample was carried out with a thermogravimetric analyzer(NETZSCH STA409 C/3F),which was linked to a Nexus 670 infrared spectrometer from Nicolet(FTIR).About 10 mg of dried sample was placed in a crucible and pyrolyzed under 60 ml·min-1N2flow at a heating rate of 30 °C·min-1from 40 °C to 800 °C.A high precision balance could automatically record the mass changing and FTIR was used for evolved gases analysis.Tarspecies were analyzed with a GC-MS(Trace GC,ISQ MS,Thermo Scientific Co.)equipped with a TR-5MS capillary column(30 m length,0.25 mm inner diameter,and 0.25 μm film thickness).
3.Results and Discussion
3.1.Drying kinetics
The drying curves of Wulagai lignite dried by 915 MHz microwave and hot air are shown in Fig.2,where the moisture content at various time intervals was recorded.For microwave drying,the drying time for the coal with particle sizes of0-1 mm,1-2 mm,and 2-4 mm was respectively 15 min,13 min and 12 min,indicating that the drying rate increased with the particle size increased.Same phenomenon was also observed by Tahmasebiet al.[14],and they explained that samples with larger particle size obviously had smaller surface area,and power absorption per unit surface area for microwave drying increased with smaller surface area.For hot air drying,the drying time for the coal with particle sizes of 0-1 mm,1-2 mm,and 2-4 mm was 90 min,100 min and 100 min,respectively,showing that the drying time of microwave irradiation was reduced by 83%,87%,and 88%of the hot air drying time for the coal with particle sizes of 0-1 mm,1-2 mm,and 2-4 mm.
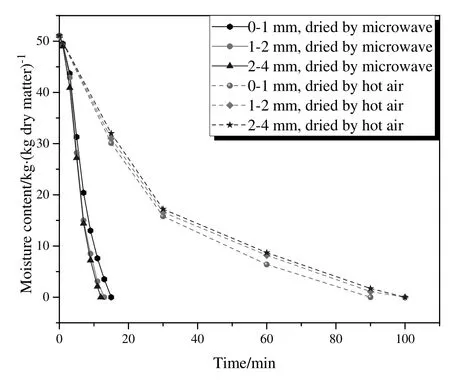
Fig.2.Drying curves of air and microwave dried coal with different particle sizes.
3.2.Effect of microwave treatment on pore structure
Fig.3 gives the SEM micrographs(3000×)of raw coal and the coal dried by hot air and microwave irradiation.It illustrated that after drying,small pores were formatted which was more obvious for microwave irradiated samples.The N2adsorption results were shown in Table 2.The total specific surface area and specific pore volume for microwave irradiated sample were 3.06 m2·g-1and 0.0081 cm3·g-1,respectively.They were 1.7 and 1.8 times larger than those of the coal dried with hot air.Chenget al.[18]have studied the drying behavior of another typical lignite in China(Shenhua coal)using three different microwave powers of 280 W,420 W and 700 W,and the operation time was 20 s,40 s,and 60 s.In comparison,the temperature of the hot air was 300 °C,450 °C,and 600 °C,and the heating time was 0.5 h,1 h,and 2 h.They found that the specific surface area of microwave irradiated coal was lower than that of hot air dried coal.One possible explanation is that the experimental conditions were different.In this paper,the power of microwave irradiation was 10 kW,which was much higher than theirs and the temperature of hot air was 105°C,which was lower than theirs.So,the evaporation rate of the water contained in the coal dried by microwave was much higher than that of the water contained in the coal dried with hot air,and a larger volumetric space was needed.Then,the specific surface area of the microwave irradiated coal was higher than that of the hot air dried one.

Table 2 Total specific surface area and specific pore volume of Wulagai lignite dried by microwave and hot air
3.3.Effect of microwave treatment on surface functional groups
XPS spectra of the coal dried by hot air and microwave which can provide detailed chemico-structural information of organic carbon and oxygen[17,19-21]are shown in Fig.4.It's obvious that the oxygen content in the coal dried by microwave was lower than that of air dried one.As listed in Table 3,the C/O ratios deduced from the XPS spectrum by calculating the carbon and oxygen peak areas were respectively 0.61,0.68,and 0.75 for raw coal,hot air dried coal and microwave dried coal.It indicated that the oxygen functional groups were destroyed partially during microwave irradiation.

Fig.3.SEM micrographs(3000×)of raw coal(a),hot air dried coal(b)and microwave dried coal(c).

Fig.4.XPS spectrum of the coal dried by microwave and hot air.

Table 3 XPS results and carbon-oxygen ratios of three types of coal
To determine the changing tendency in different types of oxygen functional groups during drying,the effect of oxygen on the XPS carbon(1 s)signal of adjacent carbon atoms was further analyzed[21,22],and the carbon(1 s)signal of the dewatered coal was resolved from four peaks at 284.8,286.3,287.5,and 289.0(±0.1)eV.The curve- fitting of different components for the coal dried by microwave and hot air is shown in Fig.5.Kelemenet al.[21]and Zhou[23]declared that the 284.8 eVpeak represents contributions from aromatic and aliphatic carbons(e.g.,C--C,C--H),which do not interact with the oxygen atoms.The 286.3 eV peak represents carbon bound to one oxygen by a single bond(e.g.,C--O,C--OH).The 287.5 eV peak represents a carbon that is bound to oxygen by two bonds(C=O and O--C--O).The 289.0 eV peak corresponds mainly to a carbon that is bound to oxygen by three bonds(O=C--O).The polynomial fitting coefficients show that the fitting curve agreed well with the experimental curve,and the peak area of the aromatic and aliphatic carbons in the coal dried by microwave was larger than that of the coal dried with hot air.The percentage of different types of carbon contained in dried coalis shown in Fig.6.The fraction of aromatic and aliphatic carbons increased from 62.0%of the coal dried with hot air to 74.8%of microwave dried one.Moreover,the fractions of the three other types of carbon that were bound to oxygen decreased.The percentage of carbon atoms bound to oxygen atoms by a single bond decreased from 19.2%to 13.6%.The carbon bound to oxygen by dual bonds decreased from 11.9%to 8.2%,and carbon bound to oxygen by triple bonds decreased from 6.9%to 3.4%.Fig.7 represents the oxygen(1 s)signal of the dewatered coal which was resolved by three peaks at 531.3,532.8,and 534.1(±0.3)eV,and the meaning of each fitted peak was also shown.The overall proportion of each type of oxygen was identical to the fitting result of the carbon(1 s).The oxygen(1 s)results combined with carbon(1 s)show that C--O is the main form of oxygen functional groups,showing the same results as Zhou[23].
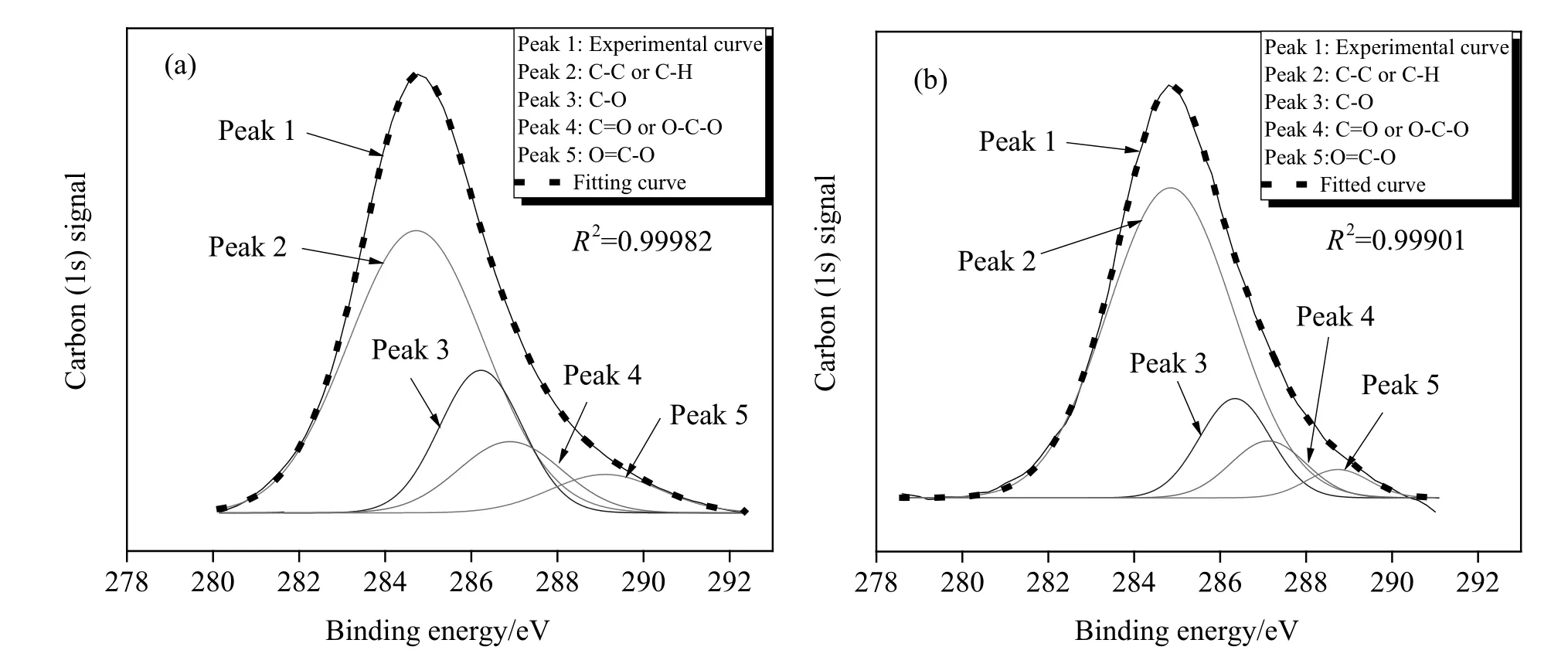
Fig.5.XPS carbon(1 s)spectrum and curve resolution into different components for the coal dried by microwave and hot air.(a)Coal dried by hot air.(b)Coal dried by microwave.
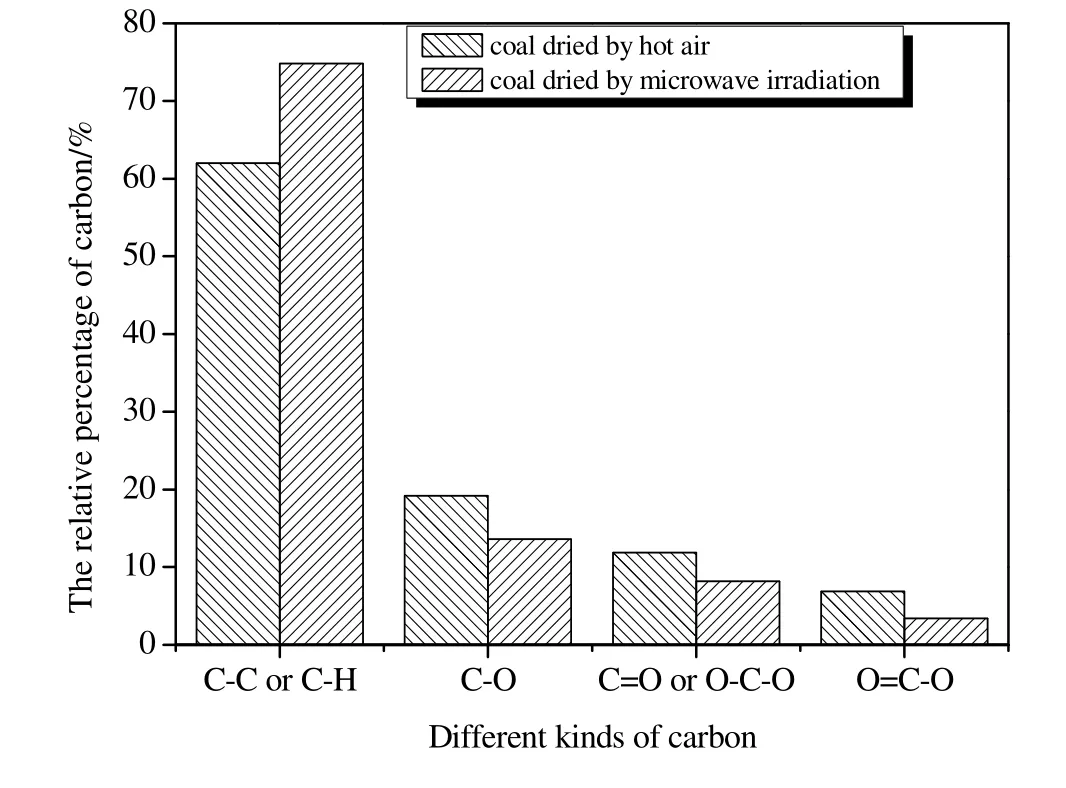
Fig.6.Relative percentage of different types of carbon for the coal dried by microwave and hot air.
3.4.Effect of microwave treatment on re-adsorption rate
Pore structure and chemical composition can strongly affect the readsorption and wettability of the coal[24,25].The average readsorption ratios accompanied with the errors of three measurements are shown in Fig.8.It shows that the average re-adsorption ratios of the sample dried by microwave and hot air were respectively 15.3%and 19.0%in the first 2.5 h,representing that re-adsorption mostly occurred in the beginning period after the sample was exposed to the humid atmosphere.The equilibrium content of the sample dried by microwave was 17.7%,which was 4.1%lower than that of hot air dried one,and the reduced moisture content can greatly improve the overall boiler efficiency if microwave dried coal is used as boiler feed stock.

Fig.7.XPS oxygen(1 s)spectrum and curve resolution into different components for the coal dried by microwave and hot air.(a)Coal dried by hot air.(b)Coal dried by microwave.
3.5.Effect of microwave treatment on pyrolysis characteristics
Previous study shows pore structure and oxygen functional groups have strong influence on the products during the coal pyrolysis for producing chemical materials[26].TG-FTIR was used to analyze the coal pyrolysis kinetics and evolved gases.Fig.9 shows the TG/DTG curves,accompanied with the temperature of three typical conversion rates of the dried samples.It reveals that the residual mass of the coal dried by microwave and hot air was 60.2%and 59.8%,respectively,suggesting that the conversion rate of microwave dried sample was higher than that of air dried sample.The figure also indicates that,at the same conversion rate,the corresponding temperature of microwave dried coal was higher than that of the coal dried with hot air.As the conversion rate and corresponding temperature were known,then the activation energy of both dried coal could be evaluated.
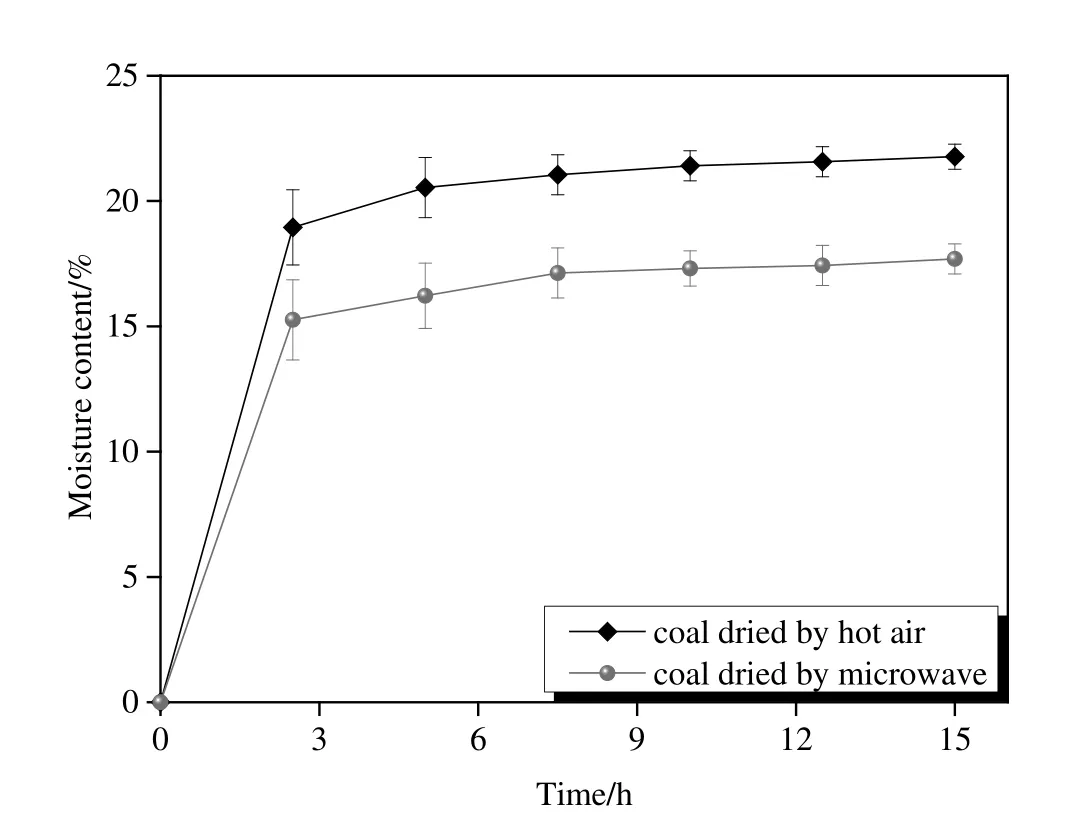
Fig.8.Re-absorbed moisture content of the sample dried by microwave and hot air.
Many investigators suggested that coal pyrolysis at a high heating rate was a first order reaction[27-29]which could be simply expressed as:

HereAis pre-exponential factor;Eis activation energy;Tis temperature;tis time;Ris gas constant,and α is conversion rate which could be expressed by:

whereW0is the original mass of the coal;Wtis the mass at timet;andWfis the final mass at the end of pyrolysis.
As the heating ratebwas constant during the experiment,andb=dT∕dt,then Eq.(1)could be integrated as:


Fig.9.TG/DTG curves of the coal dried by microwave and hot air with a heating rate of 30 K·min-1.

Fig.10.Plotofln(-ln(1-α)∕T2)vs.1/T of coal pyrolysis calculated by three step integral method.
For the temperature range of coal pyrolysis,the expression of ln[AR(1-2RT∕E)∕bE]is essentially constant and a straight line could be obtained when the left side of Eq.(3)was plottedversus1/T[30].The activation energyEcould be obtained from the slope while preexponential factorAcan be determined by the intercept.Fig.10 shows the typical plot of ln[-ln(1-α)∕T2]versus1/T,indicating that the reaction could be described by three independent first order reactions.The fitting results are also shown in Fig.10,and the corresponding values ofEandAare listed in Table 4.It represents that the activation energy of coal became higher after microwave drying,which can beexplained that the content of oxygen functional groups contained on the surface of microwave dried coal was less than that of air dried coal,and it increased the difficulty of coal pyrolysis.
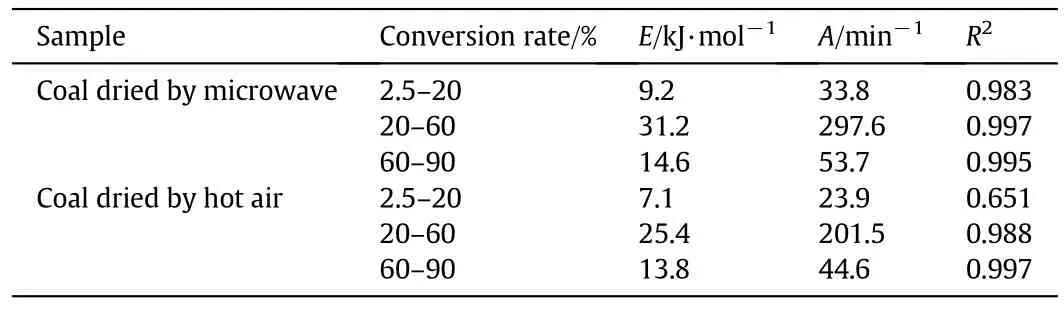
Table 4 Kinetic parameters of coal pyrolysis
The IR spectra of the evolved gases were also analyzed during the coal pyrolysis,and the absorption intensity of typical four kinds of evolved gases during the coal pyrolysis is shown in Fig.11.It reveals that for both kinds of the coal,the changing tendency of the absorption intensity of evolved gasesversusthe temperature was nearly the same.It was also found that the absorption intensity of CO2,CO,and CH4,which can be produced by the oxygen functional groups,became weaker after microwave drying,indicating that the total gas yield produced by air dried coal was larger than that of microwave dried one.
The tar was collected while the dried coal was pyrolyzed in a tubular fixed bed furnace at the same condition with the TG-FTIR experiment,and results are shown in Table 5.It represents that the char yields for both the two kinds of dried coal were similar TG-FTIR analysis.But the tar yield of microwave dried coal was much higher than that of the hot air dried one,which could be explained by two factors:On the one hand,the coal dried with hot air contained much more oxygen functional groups which could easily be decomposed as light gases.On the other hand,the pore structure of the coal expanded largely during the microwave drying process.Therefore,the sample had a larger surface area and inner paths,then the volatiles could leave the char layer quickly,and the secondary reaction was partially prohibited[31].Oppositely,for the coal dried with hot air,volatiles could not go out immediately and the pressure in the pore increased which can also restrict the production of tar[32].

Table 5 Tar,char and gas yield of the dried coal pyrolyzed from 40 °C to 800 °C with a heating rate of 30 K·min-1

Fig.11.Gas release profile during dried coal pyrolysis with a heating rate of 30 K·min-1.(a)Coal dried by microwave.(b)Coal dried by hot air.

Fig.12.GC-MS total ion chromatograms of the tars generated from the dried coal pyrolysis with a heating rate of 30 K·min-1.
Fig.12 shows GC-MS ion chromatograms of the tars generated from the dried coal pyrolysis,and typical fifteen different kinds of substances between the coal dried by microwave and hot air were also shown.In order to examine the effect of microwave on the tar composition,the tar components were separated into three types according to their molecular weight and chemical properties[33-35]:Polycyclic aromatic hydrocarbons(PAHs)which includes naphthalene,methylnaphthalenes,biphenyl,acenaphthylene, fluorene,phenanthrene,anthracene,benzo[g]quinoline,methylphenanthrenes,11H-benzo[a] fluorine,benzo[a]anthracene,chrysene and benzo[a]pyrene;BTEX including Benzene,toluene,ethylbenzene,and xylene isomers;Miscellaneous hydrocarbons(HCs)represented ethers,esters,and furans.The relative content of an identified compound can be evaluated by the variation in the normalized area values,and they can be summed into the three types of substances.According to the statistics,the percentage of each substance groups is shown in Table 6.It indicates that Miscellaneous HCs were the main composition of the tar for both microwave and hot air dried coal.The relative mass fractions of Miscellaneous HCs for the coal dried with hot air and microwave decreased from 72.1%to 66.9%.The weight proportion of PAHs decreased from 26.4%to 22.7%for the coal dried with hot air and microwave irradiation and correspondingly,the BTEX content of air dried coal was lower than that of microwave dried one during the pyrolysis process.

Table 6 Relative content of different substance groups in the tar produced from the coal pyrolysis
4.Conclusions
The industrial microwave was firstly used for the drying of lignite,and results show that,comparing to traditional hot air dying,915 MHz microwave irradiation could effectively reduce the drying time and largely change the physicochemical properties and pyrolysis characteristics of lignite.For the studied Wulagailignite,the drying time of microwave irradiation was reduced by 83%,87%,and 88%of the hot air drying time for the coal with particle sizes of 0-1 mm,1-2 mm,and 2-4 mm,respectively.After microwave drying,the specific surface area increased from 1.84 m2·g-1to 3.06 m2·g-1and pore volume increased from 0.0046 cm3·g-1to 0.0081 cm3·g-1,respectively.The C/O ratio increased from 0.68 to 0.75,and the re-adsorption ratio decreased from 21.8%to 17.7%,showing weakened hydrophilicity.During the pyrolysis process,the yield of tar largely increased from 1.3%to 8.5%and the gas production increased correspondingly.The composition of tar was also analyzed,it indicated that Miscellaneous hydrocarbons(HCs)were the main component of the tar,and microwave irradiation can reduce the fraction of polycyclic aromatic hydrocarbons(PAHs)from 26.4%to 22.7%.
[1]W.G.Willson,D.Walsh,W.Irwinc,Overview of low-rank coal(LRC)drying,Coal Prep.18(1997)1-15.
[2]Y.Yang,X.Jing,Z.Li,X.Liu,Y.Zhang,L.Chang,Effect of drying conditions on moisture re-adsorption performance of dewatered lignite,Dry.Technol.31(2013)1430-1437.
[3]C.Z.Li,Some recent advances in the understanding of the pyrolysis and gasification behaviour of Victorian brown coal,Fuel86(2007)1664-1683.
[4]A.Tahmasebi,J.Yu,Y.Han,X.Li,A study of chemical structure changes of Chinese lignite during fluidized-bed drying in nitrogen and air,Fuel Process.Technol.101(2012)85-93.
[5]X.Jing,K.Jing,Z.Li,X.Liu,Y.Zhang,L.Chang,W.Bao,Thermal effect during moisture re-adsorption of dewatered lignite,J.Therm.Anal.Calorim.119(2015)2187-2194.
[6]D.J.Allardice,L.M.Clemow,G.Favas,W.R.Jackson,M.Marshall,R.Sakurovs,The characterisation of different forms of water in low rank coals and some hydrothermally dried products,Fuel82(2003)661-667.
[7]M.Karthikeyan,J.V.M.Kuma,C.S.Hoe,D.L.Y.Ngo,Factors affecting quality of dried low-rank coals,Dry.Technol.25(2007)1601-1611.
[8]M.Karthikeyan,W.Zhonghua,A.S.Mujumdar,Low-rank coal drying technologies—current status and new developments,Dry.Technol.27(2009)403-415.
[9]N.Sarunac,E.Levy,Use of Coal Drying to Reduce Water Consumed in Pulverized Coal Power Plants,Energy Research Center,Lehigh University,Bethlehem,2005.
[10]E.Lester,S.Kingman,The effect of microwave pre-heating on five different coals,Fuel83(2004)1941-1947.
[11]M.S.Seehra,A.Kalra,A.Manivannan,Dewatering of fine coal slurries by selective heating with microwaves,Fuel86(2007)829-834.
[12]R.Vadivambal,D.S.Jayas,Changes in quality of microwave-treated agricultural products—a review,Biosyst.Eng.98(2007)1-16.
[13]C.A.Pickles,F.Gao,S.Kelebek,Microwave drying of a low-rank sub-bituminous coal,Miner.Eng.62(2014)31-42.
[14]A.Tahmasebi,J.Yu,X.Li,C.Meesri,Experimental study on microwave drying of Chinese and Indonesian low-rank coals,Fuel Process.Technol.92(2011)1821-1829.
[15]Y.Kumar,Application of microwave in food drying,Int.J.Eng.Stud.Tech.Approach1(2015)9-24.
[16]G.G.Atungulu,D.L.Smith,S.A.Wilson,H.Zhong,S.Sadaka,S.Rogers,Assessment of one-pass drying of rough rice with an industrial microwave system on milling quality,Appl.Eng.Agric.32(2016)417-429.
[17]S.Kelemen,P.Kwiatek,Quantification of organic oxygen species on the surface of fresh and reacted argonne premium coal,Energy Fuel9(1995)841-848.
[18]J.Cheng,J.Zhou,Y.Li,J.Liu,K.Cen,Improvement of coal water slurry property through coal physicochemical modifications by microwave irradiation and thermal heat,Energy Fuel22(2008)2422-2428.
[19]T.Grzybek,R.Pietrzak,H.Wachowska,X-ray photoelectron spectroscopy study of oxidized coals with different sulphur content,Fuel Process.Technol.77(2002)1-7.
[20]S.Kelemen,K.Rose,P.Kwiatek,Carbon aromaticity based on XPS-II to II*asterisk signal intensity,Appl.Surf.Sci.64(1993)167-173.
[21]S.R.Kelemen,M.Afeworki,M.L.Gorbaty,A.D.Cohen,Characterization of organically bound oxygen forms in lignites,peats,and pyrolyzed peats by X-ray photoelectron spectroscopy(XPS)and solid-state 13C NMR methods,Energy Fuel16(2002)1450-1462.
[22]M.Kozlowski,XPS study of reductively and non-reductively modified coals,Fuel83(2004)259-265.
[23]J.Zhou,Study on the distribution of O-containing functional groups in low rank coals and its removal methods,Ph.D.Thesis,China University of Mining&Technology(Beijing),China,2014.
[24]Y.Fei,Y.Artanto,L.Giroux,M.Marshall,W.R.Jackson,J.A.MacPhee,J.P.Charland,A.L.Chaffee,D.J.Allardice,Comparison of some physico-chemical properties of Victorian lignite dewatered under non-evaporative conditions,Fuel85(2006)1987-1991.
[25]H.Choi,C.Thiruppathiraja,S.Kim,Y.Rhim,J.Lim,S.Lee,Moisture readsorption and low temperature oxidation characteristics of upgraded low rank coal,Fuel Process.Technol.92(2011)2005-2010.
[26]T.L.Liu,J.P.Cao,X.Y.Zhao,J.X.Wang,X.Y.Ren,X.Fan,Y.P.Zhao,X.Y.Wei,In situ upgrading of Shengli lignite pyrolysis vapors over metal-loaded HZSM-5 catalyst,Fuel Process.Technol.160(2017)19-26.
[27]P.R.Solomon,M.A.Serio,R.M.Carangelo,J.R.Markham,Very rapid coal pyrolysis,Fuel65(1986)182-194.
[28]M.Versan Kök,E.Özbas,O.Karacan,C.Hicyilmaz,Effect of particle size on coal pyrolysis,J.Anal.Appl.Pyrolysis45(1998)103-110.
[29]P.Solomon,D.Hamblen,Z.Yu,M.Serio,Network models of coal thermal decomposition,Fuel69(1990)754-763.
[30]Q.Liu,H.Hu,Q.Zhou,S.Zhu,G.Chen,Effect of inorganic matter on reactivity and kinetics of coal pyrolysis,Fuel83(2004)713-718.
[31]X.Wang,H.Chen,K.Luo,J.Shao,H.Yang,The influence of microwave drying on biomass pyrolysis,Energy Fuel22(2008)67-74.
[32]C.Sathe,J.I.Hayashi,C.Z.Li,Release of volatiles from the pyrolysis of a Victorian lignite at elevated pressures,Fuel81(2002)1171-1178.
[33]Q.Huang,Y.Tang,S.Lu,X.Wu,Y.Chi,J.Yan,Characterization of tar derived from principle components of municipal solid waste,Energy Fuel29(2015)7266-7274.
[34]M.E.Sanchez,J.A.Menendez,A.Dominguez,J.J.Pis,O.Martinez,L.F.Calvo,P.L.Bernad,Effect of pyrolysis temperature on the composition of the oils obtained from sewage sludge,Biomass Bioenergy33(2009)933-940.
[35]L.P.L.M.Rabou,R.W.R.Z.Zwart,B.J.Vreugdenhil,L.Bos,Tar in biomass producer gas,the Energy research Centre of the Netherlands(ECN)experience:an enduring challenge,Energy Fuel23(2009)6189-6198.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
