Adaptation of intestine-based microbial functions to bioethanol production
Zhuojun Ying*,Xidong Zhang
Nanjing Foreign Language School,No.30 East Beijing Road,Nanjing 210008,Jiangsu,China
1.Introduction
In 2016,America initiated the “Microbiome”project that was compared withProject Apollo,in which extensive studies have been conducted on the gutmicrobiome due to its importance to human health[1].Many scientists are interested in the genome and function elucidation to further understand the gut microbes,while what interests us most is the intestine that provides a physiological active habitat for the microbes.Not only does the habit at enable them to accomplish a series of life activities such as growth,proliferation,death and repair,it also facilitates the evolution of dense and diversified microbial communities that are highly active,persistent and self-repairable throughout life span[2,3].This inspires us to establish high-efficient continuous bio-manufacturing processes to produce a wide variety of chemicals.
An intestine-based process may be superior to traditional biomanufacturing processes regarding the following points:
(1)The inner wall of the small intestine is covered with wrinkles and finger-like villi,which dramatically increase the total surface area of the small intestine by approximately 600-fold[5],and supports a highly diversified microbial community at a high cell density.It is reported that the human intestinal ecosystem comprises approximately 300 to 500 bacterial species,and the number of bacteria within the gut is approximately 10 times that of all of the cells in the human body[5,6],with densities approaching 1012cells·ml-1of luminal contents[2].These cell densities are 2-4 orders of magnitude higher than that achieved in traditional fermentation liquid.
(2)The interactions between the intestine surface and microbes mediate resilient microbial colonization[5,6],which enables microbial species to be established as entrenched residents within the intestine.For instance,during illness humans tend to use a variety of antibiotics which can greatly destroy the intestinal microbes.However,the intestinal microbes will soon return to normal levels after the illness.This indicates that the microbes inhabiting in an intestine are highly persistent and have the ability of self-repairing.
(3)Intestinal microbes possess huge metabolic capacity.As we know,digestion of lignocellulosic biomass like grass by herbivore animal is entirely accomplished by the intestinal microbes.Assuming a 100-kg pig can feed an average of 3-kg lignocellulosic biomass every day,estimated at a digestion ratio of 80%and an intestine volume of 6 L,the digestion efficiency will be 16 g·L-1·h-1,which is10-to 20-fold higher than that of current processes for lignocellulose degradation[7].
These unique functions enable the intestinal microbial systems to behave like a high-efficient “perpetual-motion machine”that runs throughout life span.Maybe by designing novel industrial processes simulating the intestinal system,we could overcome the drawbacks commonly encountered in current bioprocess,such as cell degeneration and autolys is caused by unfavorable liquid conditions which hamper the construction of continuous process and reduce productivities[8-9].
Therefore,inspired by the functions of the intestinal microbes,we speculate that an intestine similar reactor can be constructed to achieve a continuous ethanol fermentation process.Hence,in this study,the pig small intestine was explored as an immobilization material for industrialSaccharomyces cerevisiaefor the first time and its performances and implications during continuous bioethanol production were also investigated.
2.Materials and Methods
2.1.Pretreatment of pig small intestines
Small intestines were obtained at the Fanxing food market(Pukou,Nanjing)from one-year old pigs.They were first rinsed using tap water to remove the intestinal contents.Then they were cut into pieces(3 cm×3 cm)and weighed(wet mass).It was determined that 60-g wet intestine had an area of around 120 cm2(including both sides).Different methods were investigated to process and sterilize the pig small intestines before they were used in fermentation:(1)immersing in 0.1 mol NaOH for 3 h then rinsed several times in sterile water to thoroughly remove residual NaOH;(2)immersing in 75%(v/v)ethanol solution for 3 h then rinsed several times in sterile water to thoroughly remove residual ethanol;(3)autoclaving at 121°C for 15 min;(4)lyophilizing in a vacuum refrigerator for one day to dry.These pretreated small intestines were added into fermentation mediums as immobilization carriers.Scanning electron microscopy(SEM)observation was performed as previously described[9].
2.2.Ethanol production
IndustrialSaccharomyces cerevisiae1308 was supplied by Tian-Guan Group(Henan,China).The culture and fermentation conditions were the same as previously reported[9].For intestine-based cell immobilization,320 cm2·L-1of pig small intestine was added to a 500-ml sterilized Erlenmeyer flask containing 200 ml of fermentation medium.Then,20 ml of seed culture was added to the sterilized culture medium and incubated at 100 r·min-1and 30 °C to induce natural cell adhesion for 48 h.After that,the fermentation broth was decanted and the small intestine was rinsed in sterile water gently to remove planktonic cells and used for ethanol fermentation.Free cells were cultured as same as the cells for immobilization,but collected by centrifuging at 5000 r·min-1or 10 min and used for ethanol fermentation.The repeated batch fermentations were first carried out in batch mode.At the end of each batch(namely,no glucose was detected),the fermented broth was drained and only the carrier with immobilized cells was retained in the flask.Then,the same amount of the fermentation medium was immediately replaced to start the next cycle.All experiments were carried out in triplicate.Glucose and ethanol were analyzed using the methods previously described[10].
3.Results
3.1.Effects of pretreatment and amount of intestines on fermentation performance
Different methods were investigated to sterilize the intestine as well as possibly remove lipid or some other organic compounds.Different amounts of intestine used in fermentation medium in term of surface area(cm2·L-1)were also studied.As shown in Fig.1,generally,the larger amounts of intestine were used,the faster the fermentation turned out to be.When 480 cm2·L-1of lyophilized or autoclaved intestine was used,the both the glucose consumption and ethanol formation were apparently accelerated,and the fermentation period was 8 h shorter than that when 160 cm2·L-1of intestine was used.This indicated that the pig small intestine had a positive effect on the fermentation and could be potentially used for surface-based cell immobilization.Although 480 g·L-1of intestine obtained the best performances,there was no significant difference compared to that of 320 g·L-1of intestine at the late fermentation period(Fig.1:ABC).Considering the cost and the difficulty of subsequent processing,320 g·L-1of intestine could be an optimal choice.At an amount of 320 g·L-1,the initial glucose(184 g·L-1)could be consumed completely,although the fermentation speed varied depending on how the intestine was pretreated.The final ethanol titer was all around(85 ± 2.2)g·L-1,giving the same ethanol yield of(0.46±0.06)g·g-1.These yields were the same as the yield obtained using free cells,but lower than the yields obtained in repeated batch fermentations(see below),suggesting that it is the repeated use of cells that contributes to the improvement in yield.

Fig.1.Effects of pretreatment and amount(cm2∙L-1)of pig small intestine on fermentation performance.
As shown in Fig.1,lyophilized intestine achieved the best performance,while NaOH-treated intestine achieved the worst performance.This difference may be attributed to the difference in surface morphology after pretreatment.Observation with scanning electron microscope(Fig.2:A1B1)revealed that lyophilized intestine was characterized by a loose and porous structure,while the NaOH-treated intestine was characterized by a more compact and smoother surface structure.Since a loose and porous structure is more favorable for cell adsorption and adherence,the intestine might adsorb more cells in the fermentation process.Indeed,SEM showed that most of the lyophilized intestine was completely covered by a multilayer cell community at the end of the fermentation,much denser than that on the NaOH-treated intestine(Fig.2:A4B2).Since simple lyophilization of the intestine gave the best performances,no weak acid or other chemicals for pretreatment was further investigated.Also,pretreatment with special chemicals like NaOH and acid can cause significant increase in cost.
In addition,temporal observation of the pig small intestine during fermentation revealed that cells were dispersedly appeared at the early stage(6 h).Then they appeared in large clusters(16 h),and finally covered the whole area in multilayers(32 h)(Fig.2).This indicated that the cells adhering onto the intestine retained the ability of self duplication,which was important for achieving high cell densities.Also,some extra-cellular polymeric substance(EPS)was observed to connect cells at early stages(Fig.2:A3).This indicated that the cells were immobilized on the intestine and hold together in a natural way by self-produced matrix.Altogether,these results demonstrated that the pig small intestine requiring no special treatment was a favorable material forS.cerevisiaeimmobilization.
3.2.Intestine-based cell immobilization for continuous ethanol production
Currently,industrial bioethanol is typically generated from submerged fermentation.However,during submerged fermentation,cells are dispersed in a free form in a liquid medium,which can easily disrupt the cells and make it hard to collect and recycle the cells.As a result,submerged fermentation is generally operated in a batch mode(Fig.3A).
Here,we examined the potential of the intestine-immobilized cells for continuous utilization.Results showed that the intestineimmobilized cells can be continuously used for a long time.Compared to the free cells that could be used only once,the intestineimmobilized cells were able to be used repeatedly and continuously over 140 h without performance degradation(Fig.3B).More importantly,the reaction speed of the intestine-immobilized cells grew more rapidly over time.The fermentation period was dramatically shortened from 24 h to a final 10 h.Furthermore,since the cells could be used repeatedly,more substrate would be used for ethanol production rather than biomass synthesis.The average yield of ethanol obtained from intestine-immobilized cells was(0.48 ± 0.05)g·(g glucose)-1,4.3%higher than the yield obtained from free cells[(0.46 ± 0.06)g·(g glucose)-1].Morphology study with SEM indicated that after 140 h of fermentation,almost the entire intestine surface was covered with thick multilayers of cells,with little surface could be spotted(Fig.4).
3.3.Comparison of repeated batch ethanol fermentation with yeast cells immobilized on various carriers
The performances of the intestine-based process in this study were compared to that of other well-known cell immobilized processes.As shown in Table 1,due to the significantly accelerated fermentation speed,the average productivity for ethanol in this study reached(8.1 ± 0.28)g·L-1·h-1,which was much higher than the productivity obtained in other processes that ranged from 1.43 to 6.28 g·L-1·h-1.This suggested that compared to other cell immobilization materials,intestine had some unique features and could serve as a superior carrier for cell growth.In addition,an average of 170 g·L-1glucose was used for each repeated batch in this study,and the glucose could be completely consumed by the intestine-immobilized cells and a yield of(0.48 ± 0.05)g·g-1was obtained.Except the study using calcium alginate to immobilize cells wherein a yield of 0.50 g·g-1was obtained,all other studies obtained a yield between 0.45 and 0.48 g·g-1.The theoretical ethanol yield to glucose is 0.51 g·g-1.The yield of(0.48 ± 0.05)g·g-1is close to the theoretical yield and was a relatively high yield compared to the yields obtained in other studies.Typically,the ethanoltiters ranged from37.8 to 104.3 g·L-1in different studies.Here we obtained an ethanol production of(81.2±2.4)g·L-1,which is a moderate level.Overall,these results indicated that the intestine had some advantages over previously employed materials for cell immobilization and ethanol production.

Fig.2.SEM of S.cerevisiae cells adhering to pig small intestine.
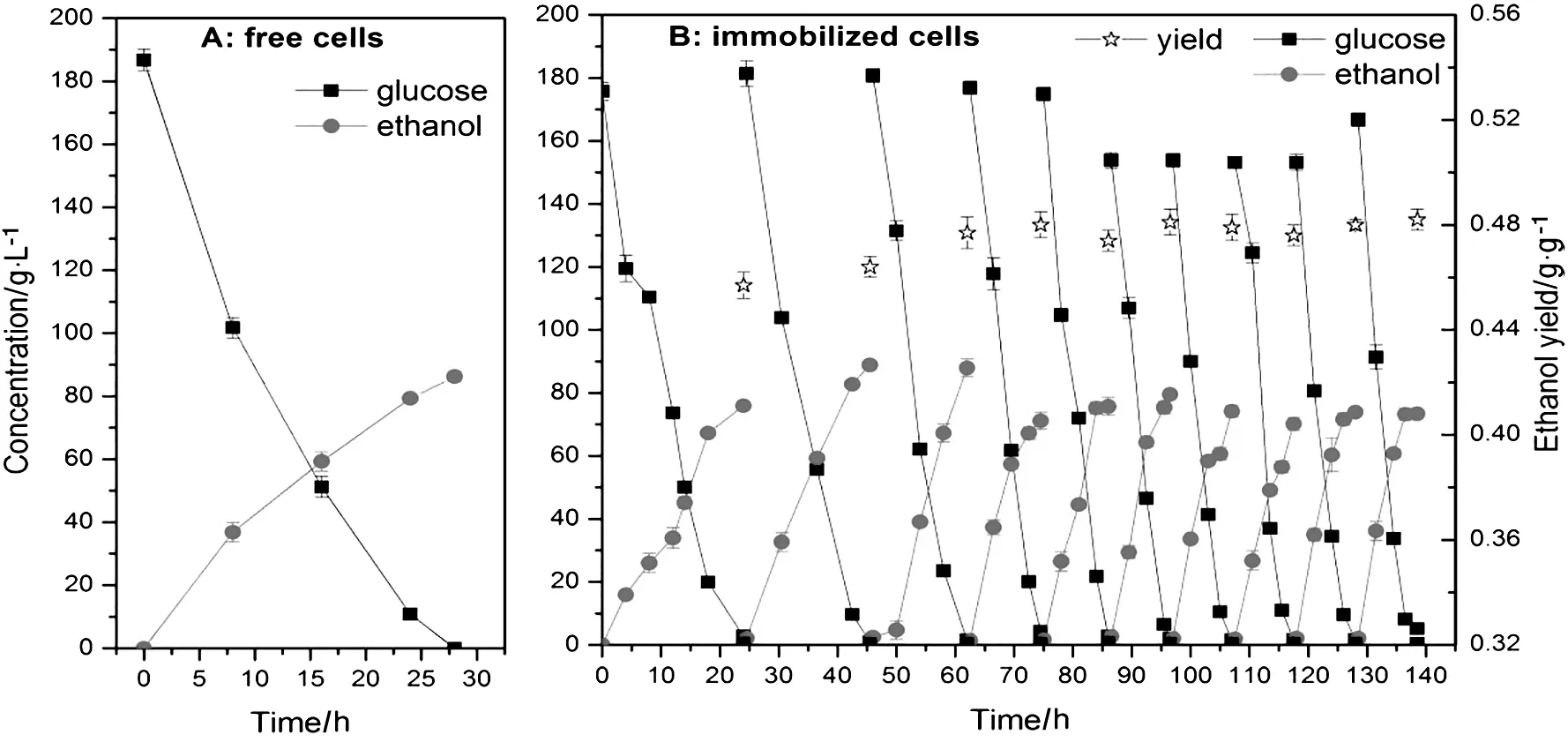
Fig.3.Comparison of ethanol production using free cells(A)or intestine-immobilized cells(B).
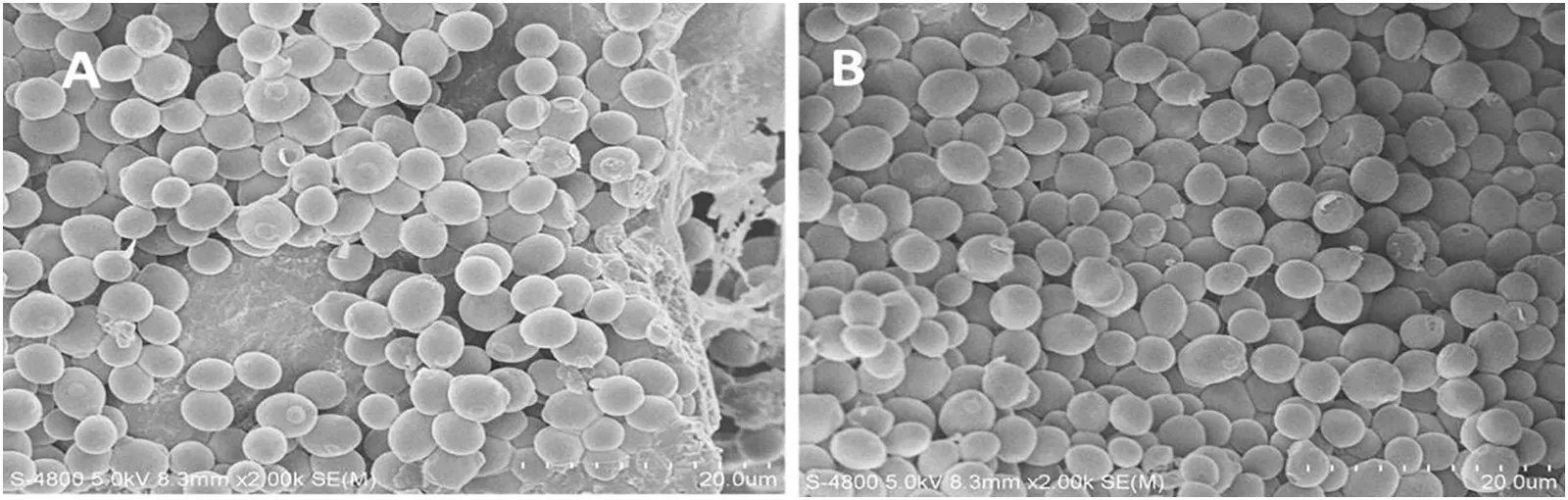
Fig.4.Morphology of the cells after 140 h of fermentation.A,little intestine surface was spotted;B,almost the entire surface was covered with thick multilayers of cells.
4.Discussion
In this study,we demonstrated that small intestine could be an advantageous material for cell immobilization.The intestine does not need special pretreatment,suggesting that the intact surface structure is important for cell adherence.As observed by SEM,the extracellular polymeric substance(EPS)was found on the intestine-immobilized cells.This EPS was typically synthesized by the cells themselves to promote their adherence to the solid surface.Therefore,they can form aggregates and build a multi-layer architecture,which can dramatically increase their tolerance to unfavorable conditions[11].Thus,with no aid of chemical fixation and exogenous matrix,the cells can be retained onto the intestine by a self-immobilization manner.As revealed by the SEM observation over time,the cells communities on the intestine evolved from small sets to large clusters to multilayers,indicating that the cells retained their self-duplication function.As we know,due to the toxicity of the high-titer ethanol in the fermentation broth,the cells would be inactivated and lost to some extent.However,the results in this study showed that the intestine-immobilized cells can be used continuously without degradation on fermentation performance.This suggested that the cell communities on the intestine can be refreshed by self-repairing:dead cells fall off,and new cells born.Overall,the results showed that thein vitrointestine-immobilized cells during industrial fermentation well retained theirin vivoproperties such as self immobilization,self-duplication,high persistence and high metabolicactivity in an animal intestine.All these unique features enabled the intestine-based fermentation to run continuously at a high reaction speed.

Table 1 Comparison of repeated batch ethanol fermentation with yeast cells immobilized on various carriers
By adaptation of biological methods and systems found in nature to engineering design,bionics can contribute a lot to the development of technology[18].In this study,we demonstrated that bionics can be a powerful way to overcome the drawbacks encountered in traditional submerged fermentation.In China,there are a total of about700 million pigs.People seldom eat pig intestine and most of the intestine is discarded as waste in the meat market.So the availability as well as the cost of intestine is not a big concern.Also,the intestine can be prepared for use by simply washing.However,our other experiments indicated that the intestine could be degraded when used for some mold,although no obvious degradation of the intestine was observed forS.cerevisiae.This might be becauseS.cerevisiaedid not produce enzymes that could degrade the intestine,and the high ethanol titer it produced could also prevent the growth of other microbes.Future study should further dissect the interaction mechanisms between cells and the intestine surface structure,as well as the molecular basis of EPS formation and potential change in the cell's life span.The elucidation of surface mechanism and basis of cellular physiological changes can provide scientific principles for bionic design of various materials that better suit our industrial demands(i.e.,highly available,easily prepared and resistant to bio-degradation).These bio-inspired design and construction will make a variety of bio-manufacturing processes more economically favorable and environmentally sustainable.
5.Conclusions
It was demonstrated for the first time that,pig intestine could be an effective material for self-immobilization of industrial strains such asS.cerevisiae.The intestine-based biosystem well retained itsin vivocell functions such as self-duplication,self-repairing and high metabolic activities,and could be applied to continuous production of biofuel for a long term.This study should inspire the development of bionic industrial processes.Future design of more elaborate bionic materials will make bioprocesses more economically favorable and environmentally sustainable.
Acknowledgements
The authors thank National Engineering Research Center for Biotechnology(Nanjing)for providing the platform to conduct the experiments,and thank Professor Hanjie Ying from Nanjing Tech University for his help with experimental design.Also,thank Ph.D Dong Liu and Ph.D Wei Zhuang for their help with experimental details.
[1]J.W.Debelius,Y.Vazquez-Baeza,D.McDonald,Z.Xu,E.Wolfe,R.Knight,Turning participatory microbiome research into usable data:lessons from the American Gut Project,J.Microbiol.Biol.Educ.17(2016)46-50.
[2]J.L.Sonnenburg,L.T.Angenent,J.I.Gordon,Getting a grip on things:how do communities of bacterial symbionts become established in our intestine?Nat.Immunol.5(2004)569-573.
[3]S.M.Lee,G.P.Donaldson,Z.Mikulski,S.Boyajian,K.Ley,S.K.Mazmanian,Bacterial colonization factors control specificity and stability of the gut microbiota,Nature501(2013)426-429.
[5]P.B.Eckburg,E.M.Bik,C.N.Bernstein,E.Purdom,L.Dethlefsen,M.Sargent,S.R.Gill,K.E.Nelson,D.A.Relman,Diversity of the human intestinal microbial flora,Science308(2005)1635-1638.
[6]F.Guarner,J.R.Malagelada,Gut flora in health and disease,Lancet361(9356)(2003)512-519.
[7]A.Bayané,S.R.Guiot,Animal digestive strategiesversusanaerobic digestion bioprocesses for biogas production from lignocellulosic biomass,Rev.Environ.Sci.Bio-Technol.10(1)(2011)43-62.
[8]M.McIntyre,J.K.Eade,P.W.Cox,C.R.Thomas,S.White,D.R.Berry,B.McNeil,Quantification of autolysis inPenicillium chrysogenumby semiautomated image analysis,Can.J.Microbiol.47(4)(2001)315-321.
[9]D.Liu,Y.Chen,F.Y.Ding,T.Zhao,J.L.Wu,T.Guo,H.F.Ren,B.B.Li,H.Q.Niu,Z.Cao,et al.,Biobutanol production in aClostridium acetobutylicumbiofilmreactor integrated with simultaneous product recovery by adsorption,Biotechnol.Biofuels7(1)(2014)1-13.
[10]Y.Chen,Q.Liu,T.Zhou,B.Li,S.Yao,A.Li,J.Wu,H.Ying,Ethanol production by repeated batch and continuous fermentations bySaccharomyces cerevisiaeimmobilized in a fibrous bed bioreactor,J.Microbiol.Biotechnol.23(4)(2013)511-517.
[11]H.C.Flemming,J.Wingender,The biofilm matrix,Nat.Rev.Microbiol.8(9)(2010)623-633.
[12]A.M.Pacheco,D.R.Gondim,L.R.Goncalves,Ethanol production by fermentation using immobilized cells ofSaccharomyces cerevisiaein cashew apple bagasse,Appl.Biochem.Biotechnol.161(1-8)(2010)209-217.
[13]A.Rattanapan,S.Limtong,M.Phisalaphong,Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons,Appl.Energy88(12)(2011)4400-4404.
[14]P.Ariyajaroenwong,P.Laopaiboon,P.Jaisil,L.Laopaiboon,Repeated-batch ethanol production from sweet sorghum juice bySaccharomyces cerevisiaeimmobilized on sweet sorghum stalks,Energies5(4)(2012)1-14.
[15]L.Laopaiboon,P.Laopaiboon,Ethanol production from sweet sorghum juice in repeated-batch fermentation bySaccharomyces cerevisiaeimmobilized on corncob,World J.Microbiol.Biotechnol.28(2)(2012)559-566.
[16]J.Yu,Z.Xu,T.Tan,An novel immobilization method ofSaccharomyces cerevisiaeto sorghum bagasse for ethanol production,J.Biotechnol.129(3)(2007)415-420.
[17]T.R.Kannan,G.Sangiliyandi,P.Gunasekaran,Improved ethanol production from sucrose by a mutant ofZymomonas mobilislacking sucrases in immobilized cell fermentation,Enzym.Microb.Technol.22(3)(1998)179-184.
[18]Y.Bar-Cohen,Biomimetics-using nature to inspire human innovation,Bioinspir.Biomim.1(1)(2006)1-12.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
