The occurrence,fate,and distribution of natural and synthetic hormones in different types of wastewater treatment plants in Iran
Mohammad Mehdi Amin ,Bijan Bina ,Afshin Ebrahimi,Zeynab Yavari*,Farzaneh MohammadiSomayeh Rahimi
1 Environment Research Center,Research Institute for Primordial Prevention of Non-communicable Disease,Isfahan University of Medical Sciences,Isfahan,Iran
2 Department of Environmental Health Engineering,School of Health,Isfahan University of Medical Sciences,Isfahan,Iran
3 Student Research Committee,Isfahan University of Medical Sciences,Isfahan,Iran
1.Introduction
In the last decade,steroid estrogens(SEs),as one group of endocrine disrupting compounds(EDCs),have received growing attention from environmental researchers.These emerging contaminants include natural estrogens,such as estrone(E1),17β-estradiol(E2),and synthetic steroid 17α-ethynil estradiol(EE2)[1].In most cases,the presence of these pollutants in water is originated from sewage treatment plants(STPs)and is regarded as a threat for aquatic life due to their estrogenic activities[2].The adverse effects of SE on various species are powerful even at low-level concentrations(ng·L-1).As an example,when these compounds enter the aquatic environment,they can interfere with the reproduction,feminization,and vitellogenin production of fishes[3].
For assessing the ultimate fate of these emerging pollutants in WWTPs,it is necessary to determine their possible pathways:from the point when humans and animals release them up to the point when they end up in different environmental compartments.The sewage and sludge of the municipal,hospital,livestock,and industrial wastewater treatment plants are the main entrance routes of micropollutants into the environment.Hence,the numerous studies around the globe are dedicated to survey the fate and removal rates of hormones by different treatment processes in sewage treatment plants(STPs)[2,4-8].The occurrence and fate of hormones in the influent and the effluent of WWTPs around the world have a wide range.To state an example,the values of E1,E2,and EE2 that were measured in influents ranged from 2.4-116,4-150 and n.d-14.4(expressed in ng. L-1),respectively.Pauwelset al.reported that the concentrations of SE in the hospital and the domestic WWTP samples were between 0.2 to 114 ng EE2·L-1and 0.2 to 58 ng,respectively[9].In addition,the common concentrations of these compounds in effluent were n.d-96,n.d-30,and n.d-5 for E1,E2,and EE2,respectively[2,10,5,11,7].
The removal efficiency of estrogenic compounds during conventional WWTPs is insufficient and imperfect.Nevertheless,researchers have illustrated a wide range from 19 to 98%for E1,62 to 98%for E2 and 76 to>90%for EE2[12,3,13].Based on these results,biodegradation and sorption followed by the removal of the excess sludge are regarded as two main processes that can be considered for the elimination of the estrogens during wastewater treatment processes[1,14].
To the best of the researchers knowledge,except for one recent study by Mohagheghian[13],no steroid estrogen monitoring has been done previously in different wastewater treatment plants in IRAN.Therefore,in the current study,we determined the amount of estrogens in the influent and effluent wastewater and assessed their occurrence and fate in several of WWTPs.A total of 5 municipal WWTPs,two rural WWTPs,one hospital WWTP,one livestock WWTP,and one commercial WWTP were selected for this study.The target analytes were estrone,17β-estradiol,and 17α-ethinyl estradiol that were extracted by the new pre-concentration technique,Dispersive Liquid Liquid Microextraction(DLLME),and detected by gas chromatography,followed by mass spectrometry(GC-MS).
2.Material and Methods
2.1.Chemical and materials
The target steroidal compounds were estrone(CAS 53-16-7,>99%),17β-estradiol(CAS 50-28-2,>98%),17α-ethynilestradiol(CAS 57-63-6,>99%),internal standardn-OP(99%,CAS:1806-26-4,liner compound),pyridine,and derivatization reagentN-Obis(trimethyl)trifluoroacetamide(BSTFA),containing 1%of trimethylchlorosilane(TMCS)which were purchased from “Sigma Aldrich®”,Germany.Individual stock solutions for E1 and E2 in 200 mgL-1and 100 mgL-1for EE2 were supplied in methanol and were kept at 4°C prior to use.
2.2.Sample collection
Table 1 depicts the relevant information of the selected WWTPs in this study.The Grab samples were collected in glass bottles from the influent(crude sewage)and the effluent( final treated)of 5 municipal WWTPs(conventional activated sludge and aerated lagoon),2 rural WWTPs(Aerated lagoon),a livestock WWTP(conventional activated sludge with wetland),a Commercial WWTP(MBBR),and a hospital WWTP(conventional activated sludge).Sludge samples in CAS process were derived from the thickener where the primary and secondary sludge were homogenized.Sampling site in MBBR was at the waste sludge of the sedimentation tank.The daily sludge loads were measured through the flow rate and the content of solids in the influent.The sludge samples were poured into the clean containers and transported to the laboratory immediately.The fractions of E1,E2,and EE2 in sludge phase were measured according to Qingling Zeng and Kaushalya C.Wijekoon[15,16].All samples were stored at 4°C before the analysis.
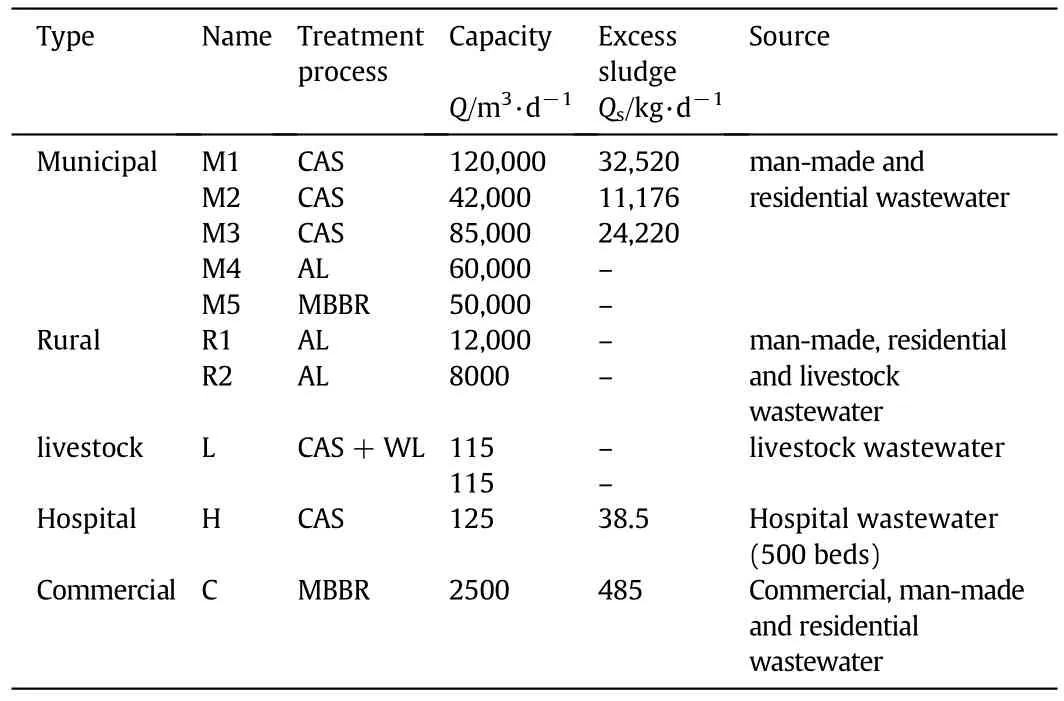
Table 1 Information of WWTPs surveyed in this research
2.3.Analytical procedures
2.3.1.Extraction of estrogens
The dispersive liquid-liquid micro extraction(DLLME)was carried out for the extraction of E1,E2,and EE2[17].Wastewater samples were filtered through glass fiber filters(GF/F,0.45 μm,Whatman).Afterwards,a volume of 5.0m sample was poured into a 10ml capped tube with a conical bottom and spiked with 10μl ofn-OP as internal standard.Under the optimal conditions,100μl of chloroform(extractive solvent)and 500 μ of methanol(dispersive solvent)were quickly injected into the tube.A cloudy solution was formed and centrifuged for 5 min at5000 r∙min-1.The lower phase was removed by a micro syringe and transferred into a 2mlvial.The extract was evaporated to dryness under a gentle flow of nitrogen.Finally,the dry residue was ready for the derivatization process.
2.3.2.Derivatization
As steroids consist of both carbonyl and of hydroxyl groups,derivatization is necessary for the enhancement of chromatographic separation.In fact,derivatization decreases the polarity and increases the volatility thermal stability of analytes[18].Brie fly,10μl of BSTFA+1%TMCS and 20μl pyridine were added to dry residue and heated at 70 C for 30min in a water bath[19].Pyridine prevents the interconversion between EE2 and E1 during the derivatization process[20].The derivatives were cooled to room temperature,and finally 3μl was injected to GC-MS for analysis.All experiments were performed in triplicates.
2.3.3.Instrumental measurement
The final derivatives were analyzed by a gas chromatograph(7890A Agilent Technologies,USA)interfaced with a mass spectrometry(5975C series)(GC-MS).The HP-5MS column was used(30 m×0.25 mm,0.25 μm),and helium carrier gas was maintained at a constant flow rate of 1ml·min-1.The GC column temperature was programmed from 100 to 200 °C(10 °C· min-1),200-260 °C(15 °C· min-1),and 260-300 °C(3 °C· min-1)and was held at 300 °C for 2 min.Data acquisition was performed in selected ion monitoring(SIM)mode fromm/z,50-600 for qualitative and quantitative analysis[21].Sample injection(3μl)was in 1:10 split mode.Fig.1 illustrates the chromatogram of estrone,17-β estradiol,and 17-α ethynil estradiol.
2.3.4.Identification and confirmation criteria
Qualitative analysis of target compounds was conducted based on retention time and the mass-to-charge(m/z)ratio values.The molecular ions[M]+withm/z342,416,and 425 correspond to E1,E2 and EE2,respectively[22].Quantitative analysis was conducted using internal standard calibration.The calibration curves were determined for E1,E2,and EE2,individually.The concentration ranges of analytes for drawing the calibration curves were between 1 and 10μg·L-1(for the influent samples)and 1-10 ng·L-1(for the effluent samples).Linear regression analysis was run and high correlation coefficients were obtained(r2>0.98)in all experiments.Identification and quantitation ions,limit of detection(LOD),limit of quantification(LOQ),and extraction recovery of the target analytes are shown in Table 2.
3.Results
3.1.Concentrations of estrogenic steroids in in fluent of WWTP
In this study,the concentration of natural and synthetic hormones was measured in the influent,effluent,and sludge samples in ten WWTPs with different types of treatment processes.The results of the analysis of the target compounds(E1,E2,and EE2)are summarized in Table 3.The concentrations of natural estrogens(E1 and E2)were detected in the raw sewage influent of all wastewater samples.
In the hospital(4.5 and 63 μg. L-1)and the livestock(94 and 85 μg. L-1)wastewater,maximum values of these compounds were observed,compared to other STPs.In the livestock,hormones promote growth and fertility and enhance breastfeeding.Hence,their wastewater usually contains high levels of SE.In the report by Ramanet al.(2004),the maximum concentrations of E1 and E2 in dairy and swine wastewater were determined 56 and 40 μg∙L-1,respectively.Zhenget al.also found that the levels of these estrogens were below one μg∙L-1[23].This inconsistency with the results of this study might be related to the lack of any pretreatment for this wastewater.In the hospital,like in the livestock,the wastewater had relatively high levels of estrogens.These levels are contradictory to the findings of other studies on hospital wastewater[24].In addition,Pauwelset al.reported that the concentrations of SE in the hospital and domestic WWTPs samples were between 0.2 to 114 ng EE2·L-1,and 0.2 to 58 ng E1·L-1[9].
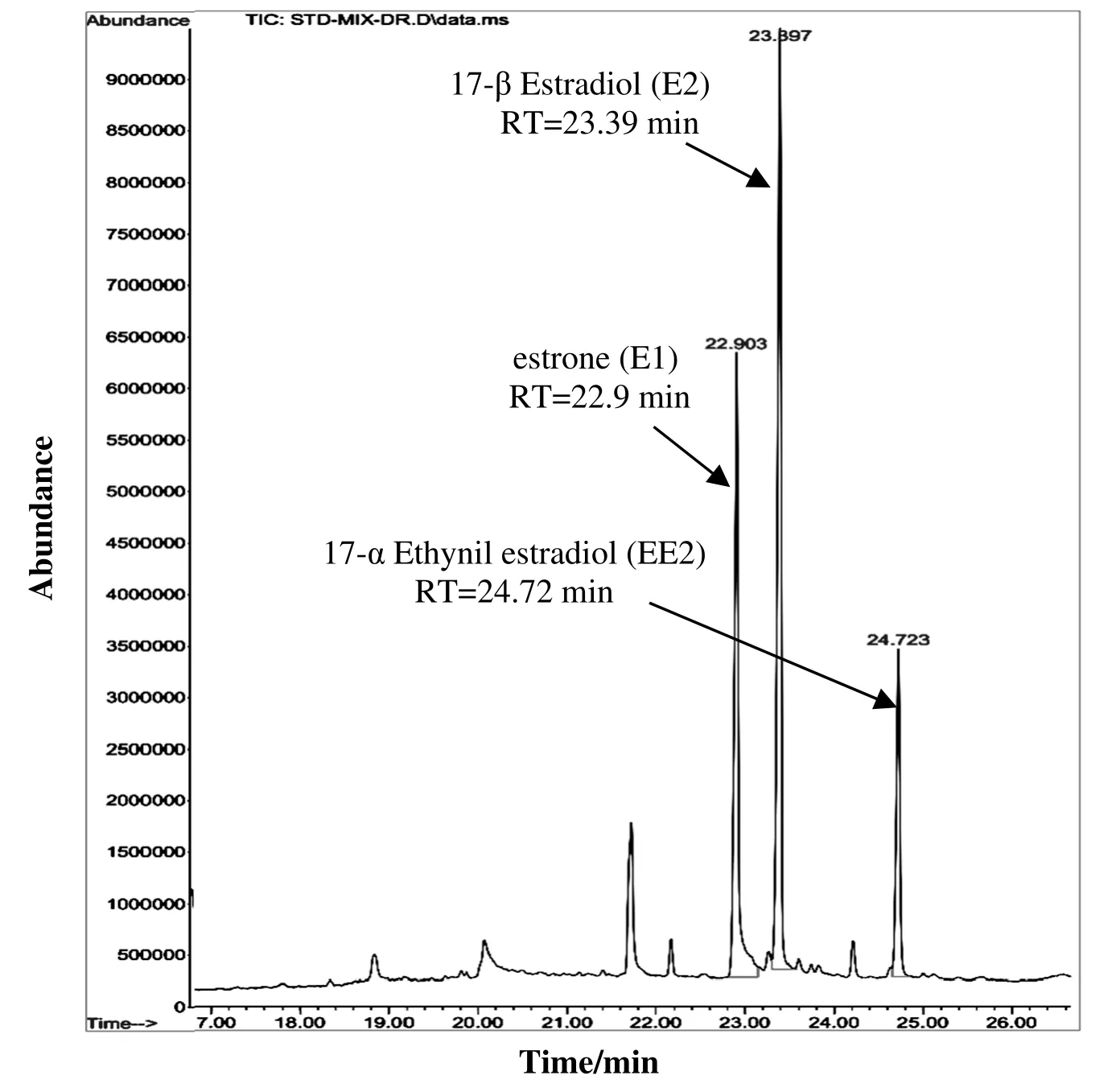
Fig.1.GC-MS(SIM)chromatograms of E1,E2,and EE2.
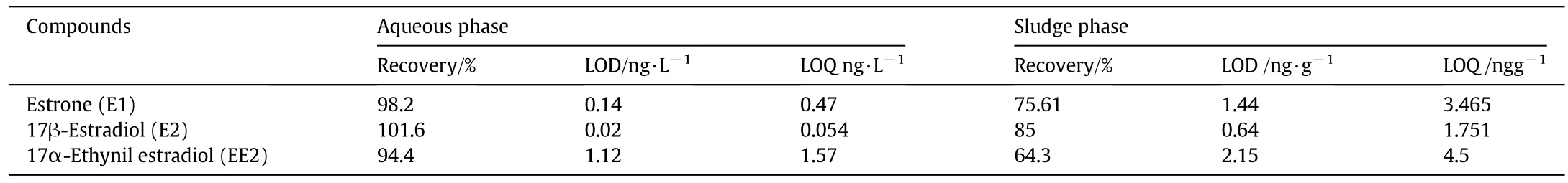
Table 2 LOD,LOQ,and Recoveries of E1,E2,and EE2
In the domestic wastewater(municipal and rural),E1(1.12-2.56 μg·L-1)and E2(11-35 ng·L-1)were monitored in all samples.EE2(n.d-2.6 μg·L-1)had relatively low concentrations compared with the natural estrogens.E1 and E2 in lower quantities are two main compounds found in the crude sewage corresponding to those mainly excreted in human.The synthetic estrogen EE2 is the main ingredient in oral contraceptive pills and the concentration is very low.Thus,this chemical was not detected in all samples.The current data agree well with the results of Yiqi Zhouet al.who surveyed the concentrations of estrogens in the largest WWTPs in Beijing.In their study,the level of SE in the influent varied from 11.6 to 1.1×102ng·L-1,3.7 to 1.4× 102ng·L-1,no detection(n.d)to 7.6 × 102ng· L-1and n.d to 3.3× 102ng·L-1for E1,E2,E3,and EE2,respectively[7].In the commercial WWTP,extremely low concentrations were observed in the influent.The low level of estrogens in this WWTP influent may be due to the low population density and low volume of wastewater.Because limited research has been done on the occurrence of hormones in the commercial wastewater,it is difficult to compare the findings in the current study with other studies.
The excretion of SE in species is a function of sex,age,physiological,and reproductive state[4].Meanwhile,multiple important factors can influence the presence of high-level estrogens in the raw influent,including,higher population density,birth rate,sampling condition,per capita consumption,and so on[2,25,7].There are also other reports yielding similar results with the findings of the current study.Numerous studies conducted in other countries have detected average values of E1,E2,and EE2 of 0.025-0.132 μg·L-1,4-22 ng·L-1and<0.001-0.013 μg·L-1,respectively[26,27].Likewise,Mohagheghianet al.[13]reported that the concentrations of E1,E2,and EE2 in the influents of STPs in Tehran ranged from 6.54-18.76 ng·L-1,1.02-8 ng·L-1,and 4.18-11.76 ng·L-1,respectively.In the same vein,Won-Jin Simet al.,who assessed the fate of estrogens in different STPs,showed that the highest concentration of steroids are attributed to the livestock,municipal,hospital,and pharmaceutical wastewater in the influent samples[6].
3.2.Concentrations of estrogenic steroids in the effluent of WWTP

2 EE ts ef f i cien E2 co tion rp so Ad E1 2 EE E2 ge Slud E1 k oc lg 3.24 3.25------2.68 n.d k d lg 2.69 2.70------2.13 n.d k oc lg 3.34 3.47------3.84 3.38 k d lg 2.78 2.92------3.29 2.82 k oc lg 3.18 3.32------3.12 3.08 k d lg 2.63 2.76------2.57 2.52 g 1 C s/μg·(k ds)-2010------10 0 6 g 1 C s/μg·(k ds)-1710------458 g 1 val C s /μg·(k ds)-0 1287------30 0 30 mo 8459067 0.00.3.7.9.1.5.0.2.38 Re /%97938679907872820.00 TP WW 2 in d EE 2,an,E E1 of alyses an Ta ble 3from Data 2 EE E2 ater Wastew E1 t en Treatm process name pe Ty nt1 Ef f l ue g·L-/n valIn f l uent1 g·L-/n Remo/%Ef f l uent1 g·L-/n nt1 valIn f l ue g·L-/n mo Re /%nt1 Ef f l ue/μg·L-nt 1 f l ue In /μg·L-0.04 0.02 0.36 0.20 0.10 0.10 0.11 n.d 0.74 n.d 1.85 0.33 2.60 0.98 1.00 0.46 0.38 0.18 4.20 n.d.2.0.2.1.4.0.1.6.2.1 5996774291 91899592999189979792 2.81.20.81.96 0.12.53.81.52.31.2 321117251928356385.2 15 5029.7.2.5.2.7.3.2.3.1.8 8652 88859359977572898295 0.00 974 0.0059 0.00 0.05 7225 0.00 0.03 0.02741 100.80 0.00 0.08 0.051 0.09 0.14 0.09 0.13 0.098 944.52 0.09 WL CA SSS CA CA AL BR MB AL AL S+CA CA SBR MB M1M2M3M4M5R1R2LHC l nicipa ital ercial Mu ral stock Ru live sp Ho mm Co
According to the data illustrated in Table 3,like the influent,the concentration of E1 and E2 were detected in all effluent samples,except for EE2.Regarding the removal efficiency in different processes,the effluents had relatively low concentrations of estrogens.Nevertheless,the highest concentrations of estrogens were observed in the livestock and the rural wastewater.
In the municipal effluent,E1(0.05-0.5μg·L-1),E2(0.1-2.8 ng·L-1),and EE2(0.04-0.36 μg·L-1)were analyzed in all samples.Results from many previous have shown that the values of estrogens in the municipal effluents were lower than 1 μg·L-1[6].However,the results of this study contradict with these previous results.To state an example,Mohagheghianet al.[13]reported that the concentrations of E1,E2,and EE2 in effluents of WWTPs in Tehran were 1.04-4.99 ng·L-1,0.5-2.20 ng·L-1,and 0.5-2.58 ng·L-1,respectively.
As in the livestock effluent,much higher concentrations of E1(10 μg·L-1)and E2(1.5 ng·L-1)were detected than those detected in other WWTPs.In a study by Won-Jin Sim,mean concentrations of E1 and E3 in the L-effluent were observed as 0.169 and 0.2 μg·L-1,respectively[6].It seems the levels of estrogens in the current study were somewhat different,compared with other findings.
The Hospital effluent also showed relatively high levels of estrogenic compounds.E1(0.8 μg·L-1),E2(0.77 ng·L-1),and EE2(0.34 μg·L-1)were measured in the sample.Pauwelset al.showed that the concentrations of natural hormones in the hospital effluents were at ng·L-1[9].
Although in the rural wastewater the values of E1,E2,and EE2 were similar to the levels of municipal wastewater,these concentrations were observed to be relatively high values(E1(1.2-2.5 μg·L-1),E2(28-35 ng·L-1)and EE2(0.38-0.46μg·L-1)).Similar to the influents,much lower concentrations of estrogens were measured in the effluents of commercial wastewater.Even though there are not any regulations imposed on the residual concentration of emergency pollutants in receiving waters,the effluent of WWTPs usually contains a low concentration of these micropollutants to some extent.
3.3.Removal efficiency of hormones during different treatment processes
In this study,the removal efficiency of two natural and one synthetic estrogens was detected in various WWTPs,including conventional activated sludge(CAS),aerated lagoon(AL),moving bed bioreactor(MBBR),and conventional activated sludge with wetland(CAS+WL).These WWTPs exhibited moderate to high elimination rates of the target compounds with average removal efficiency of 88%,98.3%,and 82%for E1,E2,and EE2,respectively.Among WWTPs,the high elimination rate of the target compounds was observed in the MBBR,followed by the CAS+WL,CAS and AL.In particular,the eliminations of MBBR were relatively firm and usually higher than 95%for all steroids.
Fig.2 summarizes the data for the influent and effluent concentrations and the removal efficiency of E1,which was acquired from different treatment processes.Accordingly,the MBBR process showed the highest removal rate of E1(>96%).Thus,the CAS has been the most efficient process with the removal percentage of 82%-94%.The CAS+WL and AL processes with 89%and 67%-79%have lower efficiency.Previous studies have reported a wide variation for the removal rate of these compounds.For example,Andersenet al.observed that the removal of E1 and E2 in WWTPs in Germany was more than 98%[28].In contrast,D'Ascenzoet al.reported that the elimination rate of E1 in a similar type of STP in Italy was 61%[12].In another study by Barontiet al.,the removal efficiency of E1,E2,and EE2 was 61%,87%,and 85%in an AS treatment plant[29].
The removal rate,influent,and effluent concentrations of E2 are depicted in Table 3 and Fig.3.In general,the removal efficiency of E2 was higher than E1 and EE2 in all WWTPs.All the processes showed the removal efficiencies exceeding 95%.That is why E2 has been typically regarded as a compound that degrades microorganisms easily[1].The elimination rate in these processes was 97%for CAS+WL,99%for MBBR,89%-97%for CAS,and 89%-91%for AL.Hashimotoet al.found that the removal rate of E2(85.7%)was notable,but for E1(55.9%)there was a negative trend[30].There are also other reports suggesting the removal efficiency of 90%-99%for E2 and E3[31,4,32,25,33].According to Terneset al.in aerobic batch experiments,E2 was oxidized to E1 more than 95%.Generally,the removal efficiency ofE1 is lower compared to E2[34].One reason might be that at the first stage of degradation,the E2 is converted to E1 and oxidized by heterotrophic microorganisms in wastewater.The current data for the biotransformation of E2 to E1 are supported by Jurgenset al.(2002)who showed that the E2 was mineralized to E1 according to the first order kinetics.
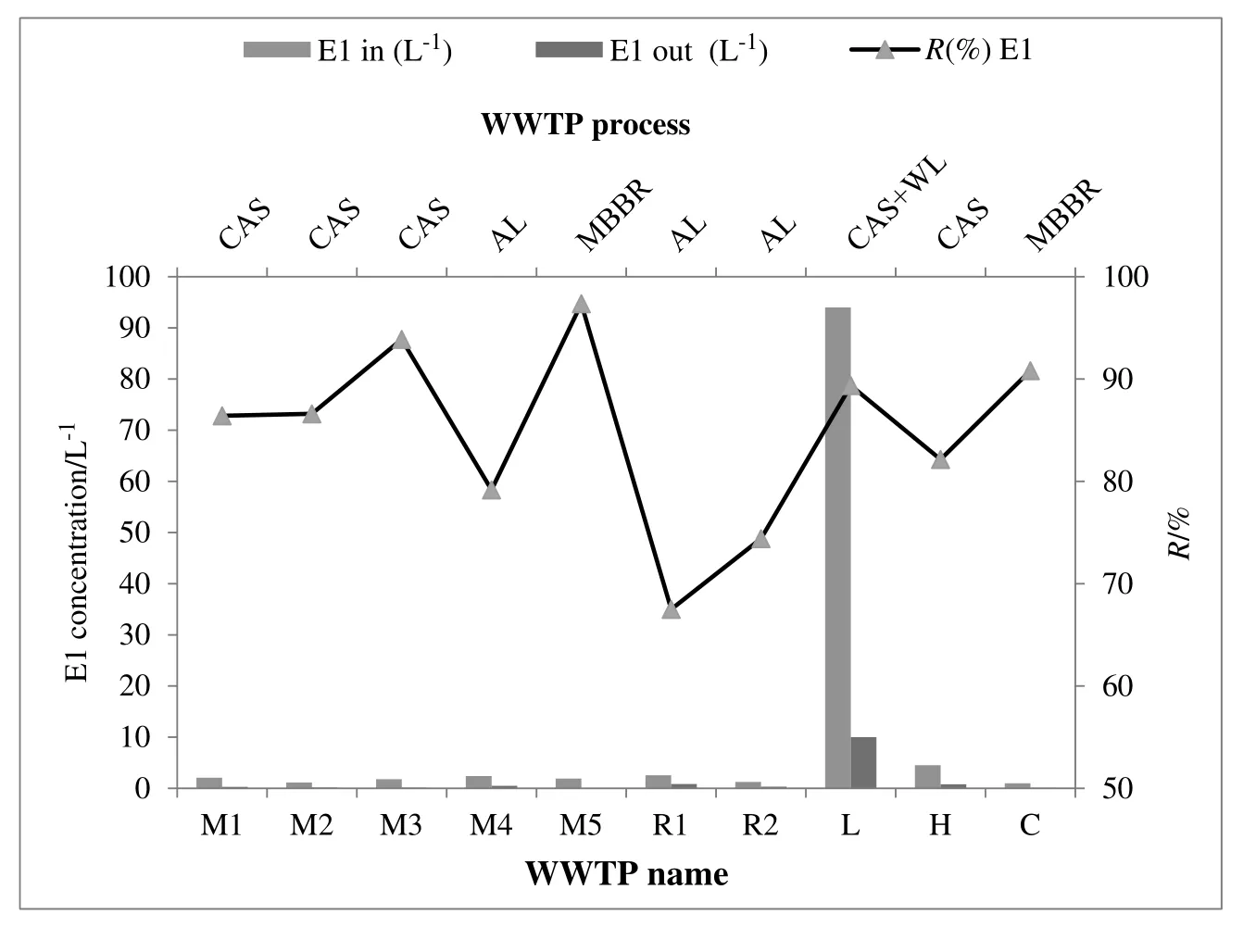
Fig.2.Removal efficiency of E1 in different treatment processes.
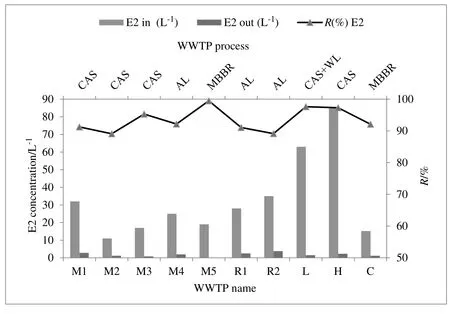
Fig.3.The removal efficiency of E2 in different treatment processes.
The removal efficiency of EE2 was considerably reduced(90%)in MBBR.Slightly lower elimination rates(80%-86%and 72%-79%)were observed at CAS and AL,respectively.EE2 experienced inefficient(average 35.8%)and variable removals during the wastewater treatment process.This evidence indicates that the EE2 is the most insistent estrogen for microbial degradation.These results are presented in Fig.4.
As mentioned above,the excellent removal(>90%)of these emerging pollutants was obtained at MBBR.One would reasonably expect that the enhancement efficiency of estrogen concentrations in MBBR compared to other processes would be ascribed to sorption onto biomass and the conformance biotransformation.This was presumably due to the presence of acclimatized film degrading microorganisms(heterotroph and autotroph)on media.Falåset al.[37]concluded that in a MBBR-AS process,the biologicalremoval of EDCs could be enhanced because of the presence of media.
Aside from the chemical properties of the micropollutants,the removal efficiencies in WWTPs have been postulated to depend on some operational parameters,such as sludge retention time and hydraulic retention time.Kreuzingeret al.demonstrated that the high SRT can promote the condition for slow growing bacteria that is responsible for the microbial degradation of EDCs,compared with biological processes with low SRT[35].
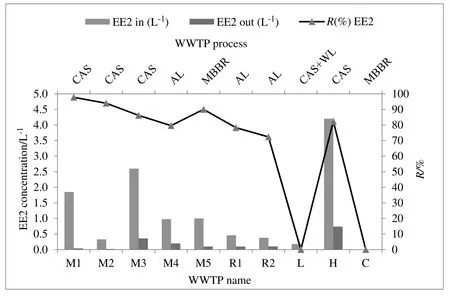
Fig.4.The removal efficiency of EE2 in different treatment processes.
In the AL process,the removal efficiency of E1,E2,and EE2 were 60%-74%,96%-98%,and 72%-78%,respectively.In this process,the concentration of MLSS in aeration tank is low,making this system unsuitable for attenuating estrogens successfully.On the other hand,given the low SRT and the lack of nitrification and denitrification,the rate of biodegradation of estrogens declined.The low removal rate in this process showed that an advanced process is essential to achieve the low concentration of micropollutants in discharge[36,37].
In WWTPs with biologicaltreatment and trickling filter,low elimination rate of E2(70%)and E1(30%)were obtained.Besides,AS has a high efficiency removal of more than 85%for E2 and 60%for EE2 and E1[12].Cargouetet al.(2004)reported that the elimination rates of estrogenic compounds in AS were 40%-70%,while other researchers found that the removal efficiency was more than 85%for E2,E3,and EE2 and about 60%for E1[29,10].
The elimination rate of estrogens CAS+WL was higher than other process,except for MBBR.The moderately high to low value of lgKow(2.54.0)in estrogen compounds indicates their tendency to partition with solid phase of sludge particles.Therefore,in this system,beside biodegradation in CAS,sorption of target analytes by roots of plants in wetland is regarded as a secondary process,which is responsible for reduction of the substances concentration.
3.4.Fate of E1,E2 and EE2 by activated sludge and MBBR processes
To prohibit the entrance of the emerging pollutants to the environment,it is imperative to assess their fate during different wastewater treatment processes.Comparative studies have found that the fate of EDCs in WWTPs is affected by their individual characteristics and some operation parameters,such as hydraulic retention time(HRT)and solid retention time(SRT)[36,38].
In the current study,the monitoring of steroid estrogens in two processes,CAS and MBBR,was accomplished by mass balance calculations.The mass balance of these processes consists of the input,output,and excess sludge.The mass balance equations are given in Eqs.(1)and(2).

whereQinputandQoutputare the flow rates of the influent and the effluent(m3·d-1),CinfluentandCeffluentare the influent and effluent concentrations as μg·L-1(ng·L-1),respectively.Wbiodegradationmass flow of biodegraded pollutants as(mg·d-1),andWexcesssludgeis the mass flow of steroid estrogens in the excess sludge(g·d-1).
Similar studies have revealed that the biodegradation,adsorption,air stripping,and photolysis are four possible pathway for EDCs removal within the wastewater treatment process[28,12,1].The value of volatilization in substances was determined by Henry's law constant(kH).This parameter is a partitioning of dissolved compounds in water and in air.Compounds withkHbetween 10-2to 10-3mol(m3·Pa)-1usually have high tendency to volatilization.In contrast,this coefficient for E1,E2,and EE2 is 6.2,6.3,and 3.8×10-7,respectively.Therefore,estrogenic hormones have small H and their removal from volatilization can be neglected[3].Moreover,the microbial degradation and sorption are propound to be the most substantial contribution in the decomposition of these compounds[39,28].
Considering the mass balance calculation,the fate of E1,E2,and EE2 in these processes is depicted in Fig.5.The fractions of E1,E2,and EE2,which were biodegraded,adsorbed and residual,are listed in Table 3.
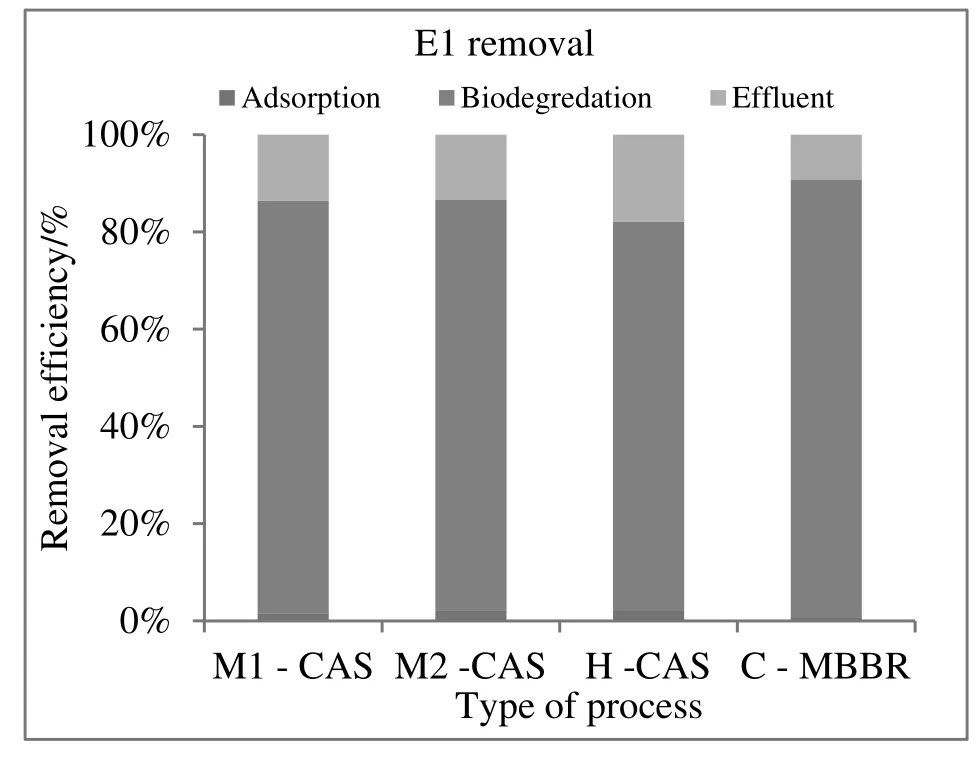
Fig.5.Contribution of biodegradation,adsorption,and release of E1,E2,and EE2 at different STPs.
The contribution of sorption by the excess sludge for E1 is 0.6%-2.1%.About 80%-90%was biodegraded and the ultimate effluent discharged was 2.2%-17.6%(Fig.5).Only 0.1%,out of the total influent of E2,was released with the effluent.About 99.0%was biodegraded,and 0.1%-0.2%was removed by sorption on the excess sludge(Fig.6).For EE2,approximately 75%-95%was eliminated by biodegradation.In contrast,the fraction(2.9%-8.1%)that sorbed on the excess sludge,2.2%-17.6%of the EE2,was released with the effluent(Fig.7).

Fig.6.Contribution of biodegradation,adsorption,and release of E2 at different STPs.
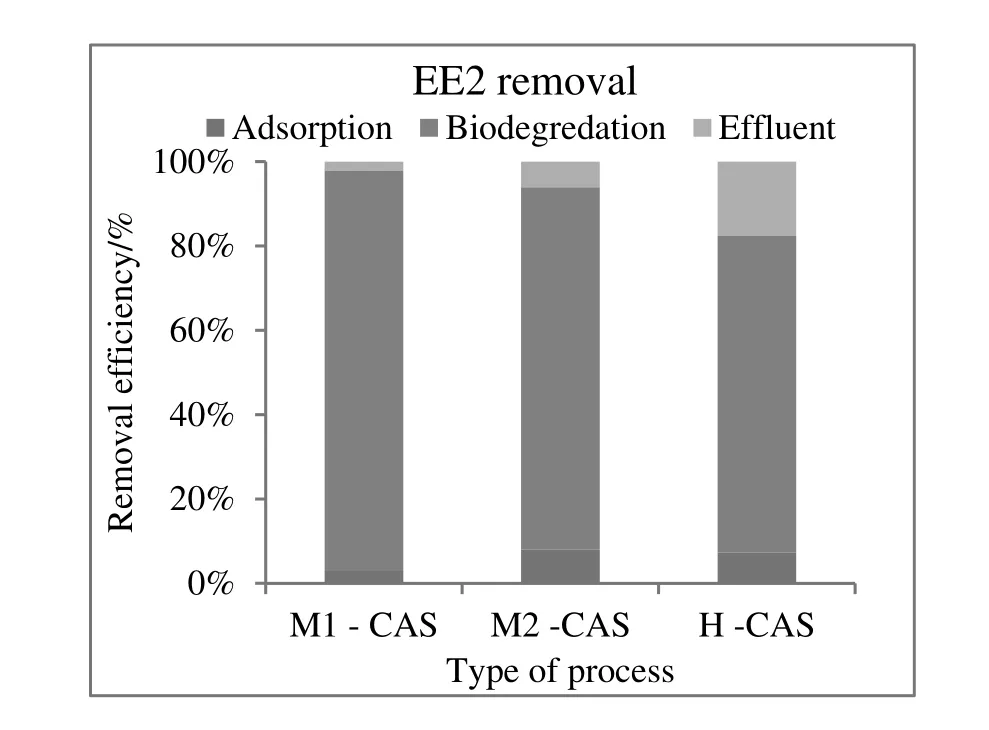
Fig.7.Contribution of biodegradation,adsorption,and release of EE2 at different STPs.
As went above,biodegradation is the major route for the attenuation of estrogens in the biological treatment process.According to the data in Table 3,more than 70%of the total natural and synthetic estrogens were biodegraded.This conclusion is in agreement with studies conducted to understand the occurrence of estrogenic compounds(E1,E2,and EE2)in WWTPs.These studies reported that roughly 50%-95%of estrogens could be removed in conventional activated sludge plants[40,41,42].Interestingly,similar studies,which were based on the mass balance of hormone in the influents and effluents of WWTPs,identified just 2%to 8%of hormones accounted for sorption to sludge[7,11,43,34].The current data is in accordance with the results of Andersenet al.'s study which showed that the fraction of SE that is sorbed to sludge was relatively lower than degradation[28].Although microbial degradation plays a key role in the removal of estrogens in these processes,sorption to sludge was also significant for the synthetic estrogen(EE2).Nevertheless,the importance of these mechanisms depends on the effectivity of the treatment process and operational conditions.
3.5.The distribution coefficients
During the process of wastewater treatment,the decline concentration of hormones in liquid phase(MLSS)was attributed to biodegradation.Adsorption of estrogens onto sludge was determined by solid-water distribution coefficient(kd),which is the ratio of steroid concentration in solid and water phase(Eq.(3))[3].In addition,by the normalization of thekdcoefficient to organic carbon content of the sludge,the obtainedkocindicated that the organic carbon content is the main source for adsorption of organic matter.

These coefficients(kdandkoc)were calculated and summarized in Table 3.

IfCsis the concentration in the solid phase(ng·g-1),Cwis the concentration in aqueous phase(ng·L-1),focis the fraction of organic carbon(%),andkdis the distribution coefficient expressed as(L·kg-1).
The elimination fraction of target analytes by sorption onto sludge was calculated using thekdvalue.These distribution coefficients were calculated for each SE individually and summarized in Table 3.These values correspond to lgkdof 2.54-2.76 for E1,2.67-2.82 for E2 and 2.85-2.94 for EE2.The behavior of sorption in AS process is possible based on this coefficient.Additionally,the portion of sorption as a removal mechanism of steroids could be evaluated.The EE2 has high hydrophobicity compared to E1 and E2.However,for natural estrogens with a low hydrophobicity,biodegradation plays a crucialrole.Therefore,sorption to sludge is important for the elimination from wastewater.Nevertheless,for the synthetic estrogen(EE2),sorption to sludge was also significant.These results are in line with findings from several other studies wherein it was found that the sorption has minor contribution,compared to biodegradation,to omission of steroids[44-46].
In this study,the lgkocof E1,E2,and EE2 was 4.50,2.8,and 2.41,respectively.This small fraction of estrogen sorption could be attributed to the low concentration of MLSS in aeration tank or characteristics of these compounds.Estrogen hormones get negative charge by losing the phenolic hydroxyl group.Charge repulsion between negatively charged compounds and solid phase in sludge can be prohibited by the elimination of natural and synthetics hormones.These results concur with those by Claraet al.(2004)who reported that the lgkdfor the estrogen E2 was 2.84(2.64-2.97)and 2.84(2.71-3.00)for the estrogen EE2.
4.Conclusions
In this study,the occurrence and fate of natural and synthetic estrogens(E1,E2,and EE2)were evaluated in different municipal,rural,hospital,livestock,and commercial WWTPs.The concentrations of all estrogens,except for EE2,were higher than those reported in other similar studies.Based on the calculations of the mass balance,the main pathway for removal of natural and synthetic estrogen in STP is biodegradation and sorption in excess sludge.The elimination fraction of target analytes by sorption onto sludge was calculated using the distribution coefficients(kdandkoc).
Acknowledgments
This article is the result of a PhD dissertation approved in the Isfahan University of Medical Sciences(IUMS).The authors wish to express their deep gratitude to the Vice Chancellery of Research of IUMS for their financial support of the Research Project#394774.
[1]C.Moschet,J.Hollender,Microbial Degradation of Steroid Hormones in the Environment and Technical Systems,Swiss Fderal Institute of Technology,Institute of Biogeochemistry and Pollutant Dynamics(IBP-ETH),Zurich,2009.
[2]S.Combalbert,G.Hernandez-raquet,Occurrence,fate,and biodegradation of estrogens in sewage and manure,Appl.Microbiol.Biotechnol.86(2010)1671-1692.
[3]H.Hamid,C.Eskicioglu,Fate of estrogenic hormones in wastewater and sludge treatment:A review of properties and analytical detection techniques in sludge matrix,Water Res.46(2012)5813-5833.
[4]T.De Mes,G.Zeeman,G.Lettinga,Occurrence and fate of estrone,17β-estradiol and 17α-ethynylestradiol in STPs for domestic wastewater,Rev.Environ.Sci.Biotechnol.4(2005)275-311.
[5]P.Biochemistry,Y.F.United,N.Educational,endocrine disrupting compounds removal from wastewater,a new challenge wastewater,a new challenge,Process Biochem.41(2015)525-539.
[6]W.Sim,J.Lee,S.Shin,K.Song,J.Oh,Chemosphere assessment of fates of estrogens in wastewater and sludge from various types of wastewater treatment plants,Chemosphere82(2011)1448-1453.
[7]Y.Zhou,J.Zha,Z.Wang,Occurrence and fate of steroid estrogens in the largest wastewater treatment plant in Beijing,Environ.Monit.Assess.184(2012)6799-6813.
[8]I.G.Lange,A.Daxenberger,B.Schiffer,H.Witters,D.Ibarreta,H.H.D.Meyer,Sex hormones originating from different livestock production systems:Fate and potential disrupting activity in the environment,Anal.Chim.Acta473(2002)27-37.
[9]B.Pauwels,H.Noppe,H.De Brabander,W.Verstraete,Comparison of steroid hormone concentrations in domestic and hospital wastewater treatment plants,J.Environ.Eng.134(2008)933-936.
[10]M.Carballa,F.Omil,J.M.Lema,C.Garc,I.Rodr,Behavior of pharmaceuticals,cosmetics and hormones in a sewage treatment plant,Water Res.38(2004)2918-2926.
[11]H.Rasmus,M.Hansen,J.Kjølholt,F.Stuer-lauridsen,T.Ternes,B.Halling-sørensen,Assessment of the importance of sorption for steroid estrogens removal during activated sludge treatment,Chemosphere61(2005)139-146.
[12]G.D'Ascenzo,A.Di Corcia,A.Gentili,R.Mancini,R.Mastropasqua,M.Nazzari,R.Samperi,Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities,Sci.Total Environ.302(2003)199-209.
[13]A.Mohagheghian,R.Nabizadeh,A.Mesdghinia,N.Rastkari,A.H.Mahvi,M.Alimohammadi,M.Yunesian,R.Ahmadkhaniha,S.Nazmara,Distribution of estrogenic steroids in municipal wastewater treatment plants in Tehran,Iran,J.Environ.Health Sci.Eng.12(2014)1-7.
[14]Y.Luo,W.Guo,H.H.Ngo,L.D.Nghiem,F.I.Hai,J.Zhang,S.Liang,X.C.Wang,A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment,Sci.Total Environ.473-474(2014)619-641.
[15]Y.Luo,A sponge-based moving bed bioreactor for micropollutant removal from municipal wastewater,Ph.D.Thesis,University of Technology,Sydney,2014.
[16]Q.Zeng,Y.Li,S.Yang,Sludge retention time as a suitable operational parameter to remove both estrogen and nutrients in an anaerobic-anoxic-aerobic activated sludge system,Environ.Eng.Sci.30(2013)161-169.
[17]J.López-Darias,M.Germán-Hernández,V.Pino,A.M.Afonso,Dispersive liquidliquid microextraction versus single-drop microextraction for the determination of several endocrine-disrupting phenols from seawaters,Talanta80(2010)1611-1618.
[18]Z.L.Zhang,A.Hibberd,J.L.Zhou,Optimisation of derivatisation for the analysis of estrogenic compounds in water by solid-phase extraction gas chromatography-mass spectrometry,Anal.Chim.Acta577(2006)52-61.
[19]Y.Yu,L.Wu,Analysis of endocrine disrupting compounds,pharmaceuticals and personal care products in sewage sludge by gas chromatography-mass spectrometry,Talanta89(2012)258-263.
[20]A.Shareef,M.J.Angove,J.D.Wells,Optimization of silylation usingN-methyl-N-(trimethylsilyl)-trifluoroacetamide,N,O-bis-(trimethylsilyl)-trifluoroacetamide andN-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide for the determination of the estrogens estrone and 17α-ethinylestradi,J.Chromatogr.A1108(2006)121-128.
[21]R.Liu,J.L.Zhou,A.Wilding,Microwave-assisted extraction followed by gas chromatography-mass spectrometry for the determination of endocrine disrupting chemicals in river sediments,J.Chromatogr.A1038(2004)19-26.
[22]R.Liu,J.L.Zhou,A.Wilding,Simultaneous determination of endocrine disrupting phenolic compounds and steroids in water by solid-phase extraction-gas chromatography-mass spectrometry,J.Chromatogr.A1022(2004)179-189.
[23]W.Zheng,S.R.Yates,S.A.Bradford,Analysis of steroid hormones in a typical dairy waste disposal system,Environ.Sci.Technol.42(2)(2008)530-535.
[24]W.J.Sim,J.W.Lee,S.K.Shin,K.B.Song,J.E.Oh,Assessment of fates of estrogens in wastewater and sludge from various types of wastewater treatment plants,Chemosphere82(2011)1448-1453.
[25]K.Mcavoy,B.Lane,Occurrence of estrogen in wastewater treatment plant and waste disposal site water samples,Clearwaters38(3)(2008)28-37(fall).
[26]C.H.U.A.H.Uang,D.A.L.S.Edlak,Analysis of estrogenic hormones in municipal wastewater effluent and surface water using enzyme-linked immunosorbent assay and gas chromatography/tandem mass spectrometry,Environ.Toxicol.Chem.20(2001)133-139.
[27]T.A.U.Ternes,P.Kreckel,J.Mueller,Behaviour and occurrence of estrogens in municipal sewage treatment plants-II.Aerobic batch experiments with activated sludge,Sci.Total Environ.225(1999)91-99.
[28]H.Andersen,H.Siegrist,B.Halling-Sorensen,T.A.Ternes,Fate of estrogens in a municipal sewage treatment plant,Environ.Sci.Technol.37(2003)4021-4026.
[29]Chiara Baronti,Roberta Curini,Giuseppe DAscenzo,Antonio Di Corcia,Alessandra Gentili,R.Samperi,Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water,Environ.Sci.Technol.34(2000)59-66.
[30]T.Hashimoto,T.Murakami,Removal and degradation characteristics of natural and synthetic estrogens by activated sludge in batch experiments,Water Res.43(2009)573-582.
[31]K.C.Wijekoon,F.I.Hai,J.Kang,W.E.Price,W.Guo,H.H.Ngo,L.D.Nghiem,The fate of pharmaceuticals,steroid hormones,phytoestrogens,UV- filters and pesticides during MBR treatment,Bioresour.Technol.144(2013)247-254.
[32]L.Barreiros,J.F.Queiroz,L.M.Magalhães,A.M.T.Silva,M.A.Segundo,Analysis of 17-β-estradiol and 17-α-ethinylestradiol in biological and environmental matrices —a review,Microchem.J.126(2016)243-262.
[33]B.C.Lee,J.Y.Jung,H.K.Kim,Evaluation of estrogenic activity and measurement of EDCS in wastewater treatment plants,J.Ocean Univ.China5(2006)351-356.
[34]T.A.Ternes,M.Stumpf,J.Muller,K.Haberer,R.-D.Wilken,M.Servos,Behaviour and occurrence of estrogens in municipal sewage treatment plants:I,Investig.Ger.Canada Brazil,Sci.Total Environ.225(1999)81-90.
[35]N.Kreuzinger,M.Clara,B.Strenn,H.Kroiss,Relevance of the sludge retention time(SRT)as design criteria for wastewater treatment plants for the removal of endocrine disruptors and pharmaceuticals from wastewater,Water Sci.Technol.50(2004)149-156.
[36]E.B.Estrada-Arriaga,P.N.Mijaylova,Influence of operational parameters(sludge retention time and hydraulic residence time)on the removal of estrogens by membrane bioreactor,Environ.Sci.Pollut.Res.18(2011)1121-1128.
[37]P.Falås,P.Longrée,J.La Cour Jansen,H.Siegrist,J.Hollender,A.Joss,Micropollutant removal by attached and suspended growth in a hybrid biofilm-activated sludge process,Water Res.47(2013)4498-4506.
[38]Y.K.K.Koh,T.Y.Chiu,A.R.Boobis,M.D.Scrimshaw,J.P.Bagnall,A.Soares,S.Pollard,E.Cartmell,J.N.Lester,Influence of operating parameters on the biodegradation of steroid estrogens and nonylphenolic compounds during biological wastewater treatment processes,Environ.Sci.Technol.43(2009)6646-6654.
[39]G.G.Ying,R.S.Kookana,Y.J.Ru,Occurrence and fate of hormone steroids in the environment,Environ.Int.28(2002)545-551.
[40]M.Auriol,Y.Filali-Meknassi,C.D.Adams,R.D.Tyagi,T.N.Noguerol,B.Piña,Removal of estrogenic activity of natural and synthetic hormones from a municipal wastewater:efficiency of horseradish peroxidase and laccase from Trametes versicolor,Chemosphere70(2008)445-452.
[41]B.Pauwels,H.Noppe,H.De Brabander,W.Verstraete,Comparison of steroid hormone concentrations in domestic and hospital wastewater treatment plants,J.Environ.Eng.134(2008)933-936.
[42]L.Gunnarsson,M.Adolfsson-Erici,B.Björlenius,C.Rutgersson,L.Förlin,D.G.J.Larsson,Comparison of six different sewage treatment processes-reduction of estrogenic substances and effects on gene expression in exposed male fish,Sci.Total Environ.407(2009)5235-5242.
[43]A.Joss,H.Andersen,T.Ternes,P.R.Richle,H.Siegrist,Removal of estrogens in municipal wastewater treatment under aerobic and anaerobic conditions:consequences for plant optimization,Environ.Sci.Technol.38(2004)3047-3055.
[44]X.Chen,J.Hu,Adsorption of natural estrogens and their conjugates by activated sludge,Water Air Soil Pollut.206(2010)251-261.
[46]Y.Zhang,J.L.Zhou,Removal of estrone and 17α-estradiol from water by adsorption,Water Res.39(2005)3991-4003.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
