Temperature-dependent aggregation of bio-surfactants in aqueous solutions of galactose and lactose:Volumetric and viscometric approach
S.Chauhan*,Vivek Sharma,Maninder Kaur,Poonam Chaudhary
Department of chemistry,Himachal Pradesh University,Shimla 5,India
1.Introduction
Bile salts(BS)are vitalcomponents in human digestion,primarily for the digestion of amphipathic bio-molecules such as glycerides,fatty acids,cholesterol,lipids and aid in absorbing fat soluble vitamins such as vitamins A,D,E,Ketc.[1-4].These are commonly engaged as permeability enhancers owing to their ability to increase drug penetration through various biological membranes by interacting with phospholipids in cell membranes and in certain cases they have been employed as therapeutic agents for treatment of liver and metabolic disorders[5,6].Chemically,BS has a rigid and slightly curved hydrophobic tetracyclic steroid ring system with a few hydrophilic groupsi.e.--OH/--COOH[7].Their remarkable physiological and physicochemical properties result in their applications as natural detergents,emulsifiers,wetting agents and solubilizers in the fields of drug delivery,pharmaceutics and food industries[8-10].The functions of BS have been related to their ability to self-aggregate and solubilize in aqueous medium.
On the other hand,saccharides are the mostabundantbio-molecules found in nature,which are water soluble and easily digestible.These are polyhydroxy aldehydes/ketones with well-defined orientations and show peculiar hydration in aqueous solution.Their derivatives are widely distributed in various forms of life as essential moieties such as glycoproteins,glycolipids,nucleic acidsetc.and play key role in the immune system,blood clotting,fertilization[11]etc.These are major source of energy required for metabolic processes of living organisms;the energy is being released as a product of their oxidation.They also act as co-solutes for solubilizing biological macromolecules in food industries[12,13].Several carbohydrates have been used for preparation of antibiotic and anticancer drugs due to their biological importance related to protein-glycoconjugate interactions and intercellular recognition events[14].
The micellar properties of BS have been extensively studied by various conventional as well as spectroscopic techniques including surface tension,electrical conductivity,dynamic light scattering,small angle neutron scattering(SANS),viscosity,density,speed of sound, fluorescence,UV-Visible,NMR,FTIR measurementsetc.[15-22].These experimental methods are useful in providing sensitive information about ion-solvent interaction,ion-ion association and solvent structure.By virtue of presence of various groups,carbohydrates can interact well with bile salts and may affect their aggregation process.This fact,together with the hydrophobic interactions between bio-surfactants and long hydrocarbon chains of saccharides lead to spontaneous association in water to form self-organized assemblies(micelles)of BS.However,further aggregation and stabilization of these micelles in aqueous solution occurs through attractive forces such as hydrogen bonding between hydrophilic groups[23,24].Thus,it would be interesting to study the effect of carbohydrates on micellization behavior and their interactions with these bio-active molecules.
So the main aim of present work is to clarify the aggregation and interactions of sodium cholate and sodium deoxycholate in aqueous solutions of saccharides,galactose and lactose along with the effect of temperature using density,speed of sound and viscosity measurements in the temperature range(293.15-313.15)K at an interval of 5 K.The data have been used to evaluate volumetric,compressibility,viscometric and acoustical parameters to get an idea about the interplay of various interactions prevailing in the studied ternary(bio-surfactant+saccharide+water)system.
2.Experimental
2.1.Materials
The bio-surfactants,sodium cholate and sodium deoxycholate,both of purity<98%,obtained from SD fine Chem Ltd.were used after recrystallization by following the procedure mentioned in literature[25].Lactose and galactose,both of A.R.grade and purity<99%were purchased from SD fine Chem Ltd.and used as received.Doubly distilled water having conductivity(κ)in the range(2-3)× 10-6S·cm-1and pH 7.0 at 25°C was collected from Millipore-Elix system and used for all the experiments.The chemical representation of various samples used has been given in Fig.1.
2.2.Equipment and experimental procedure
2.2.1.Density and speed of sound measurements
Density and speed of sound measurements were performed with a high precision digital Density and Sound Velocity Analyzer-5000(DSA-5000)supplied by Anton Paar Gmbh,Austria.The instrument has been calibrated periodically with two fluidsi.e.dry air and distilled water over a temperature range(293.15-313.15)K.This two-in-one instrument has been equipped with a density cell and a speed of sound cell;both the cells are thermally controlled by intrinsic Peltier thermostat.The sample is injected into a U-shaped glass tube that is electronically excited to vibrate atits characteristic frequency.This characteristic frequency depends upon the density of the sample.The working frequency for the measurement of speed of sound is~3 MHz[26].The uncertainties in the density measurements were found to lie well within 0.297 kg·m-3while those in speed of sound data were found to be better than 0.249 m·s-1.The precision in temperature of the DSA-5000 was±0.001 K.The density(ρ)and speed of sound(u)of NaC and NaDC in the concentration range(4.0 to 22.0)and(1.0-10.0)mmol∙kg-1respectively in the presence of 0.5%,1.0%and 1.5%(w/v)aqueous solutions of galactose and lactose have been measured in the temperature range(293.15-313.15)K at an interval of 5 K.
2.2.2.Viscometric measurements
Viscosity studies have been carried out by using jacketed Ostwald viscometer having flow time 348 s for distilled water at 25°C.The viscometer was subjected to calibration before use at 25°C using solvents,water(η =0.891 mPa⋅s),dioxane(η =1.19 mPa⋅s)and DMSO(η =2.01 mPa⋅s).The reproducibility of the measurements of viscosity was within ±0.02 mPa⋅s.A high precision water bath fitted with a digital temperature controlled device with an uncertainty of 0.01 K supplied by Narang Scientific Works(NSW)Pvt.Ltd.New Delhi was used for temperature control.
3.Results and Discussion
3.1.Density and speed of sound measurements
The density and speed of sound data for bio-surfactants,NaC and NaDC in aqueous solutions of galactose and lactose have been documented in Tables 1 and 2.The results from Table 1 show that ρ values rise with increase in concentration of both bile salts and saccharides.Addition of solute results in ionic hydration,solute-solute and solutesolvent interactions present in solution leading to the shrinkage in volume.Comparatively,the values are greater for lactose than galactose which is in accordance with the molecular mass of the saccharides.Further,density of aqueous solutions of NaC is more than that of NaDC.The presence of more number of hydroxyl groups in NaC results in higher magnitude of hydrogen bonding as well as hydrophilic interactions thereby decreasing the volume of the system and hence density increases[27].However,the lowering of density values with rise in temperature may be attributed to greater thermal energy acquired by the molecules than the interaction energy due to the destruction of water structure.Also,temperature increment favors the increase of kinetic energy and volume expansion and therefore,leads to decrease in density[28].
On the other hand,speed of sound(u)of aqueous solutions of bile salts increases with increase in concentration of bile salts as well with temperature(Table 2).The association between components of the ternary system may be responsible for the increase in speed of sound[29].The values have been found to be more for NaC as compared to NaDC.
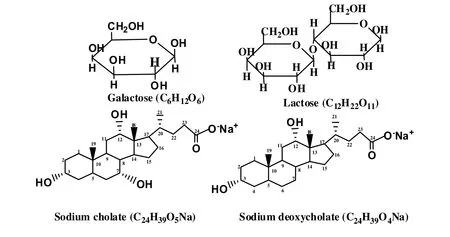
Fig.1.Chemical formulae and structures of samples used.
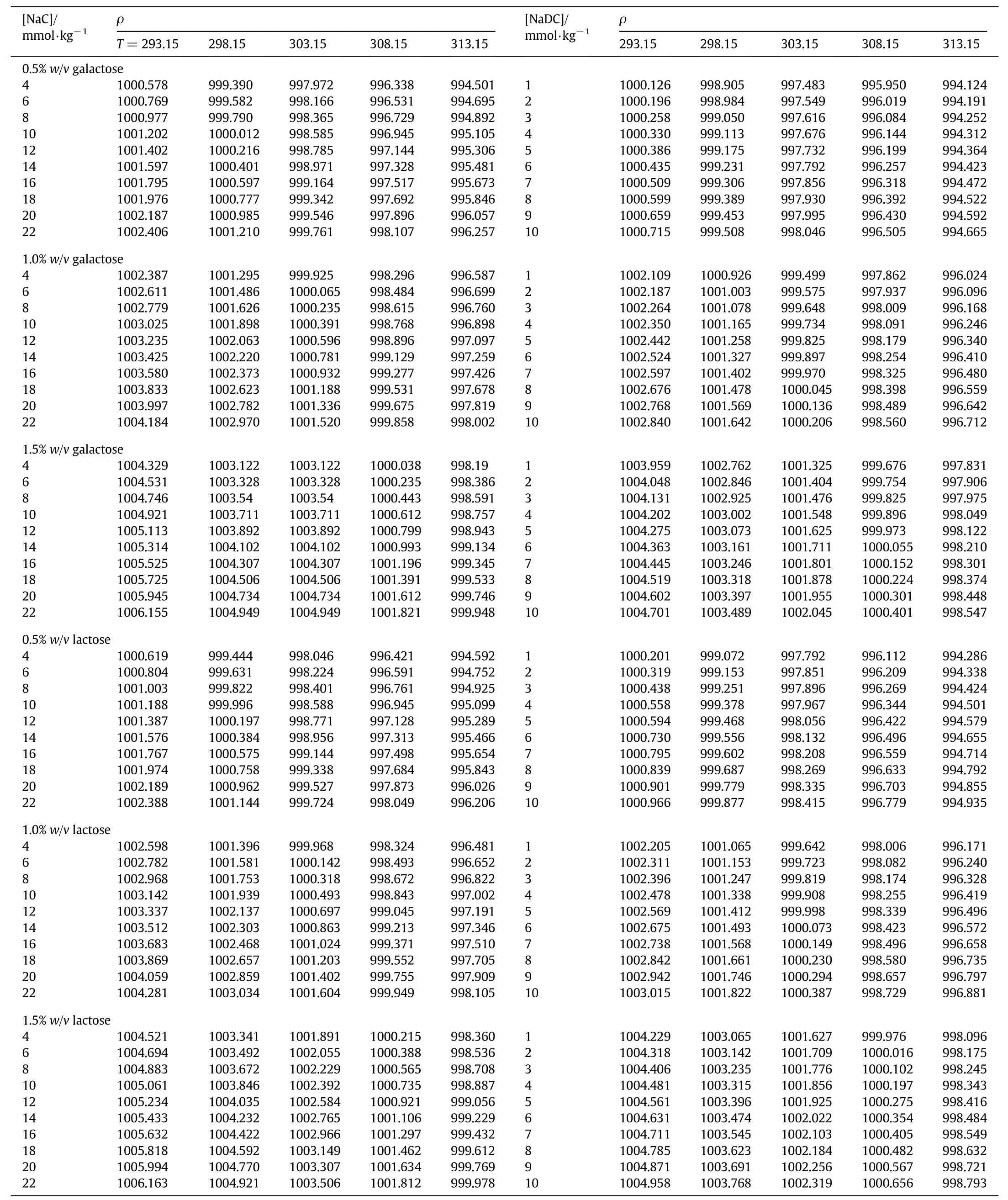
Table 1 Density,ρ (kg·m-3)of NaC and NaDC in aqueous solutions of galactose and lactose 0.5%,1.0%,1.5%(w/v) at different temperatures(T/K)
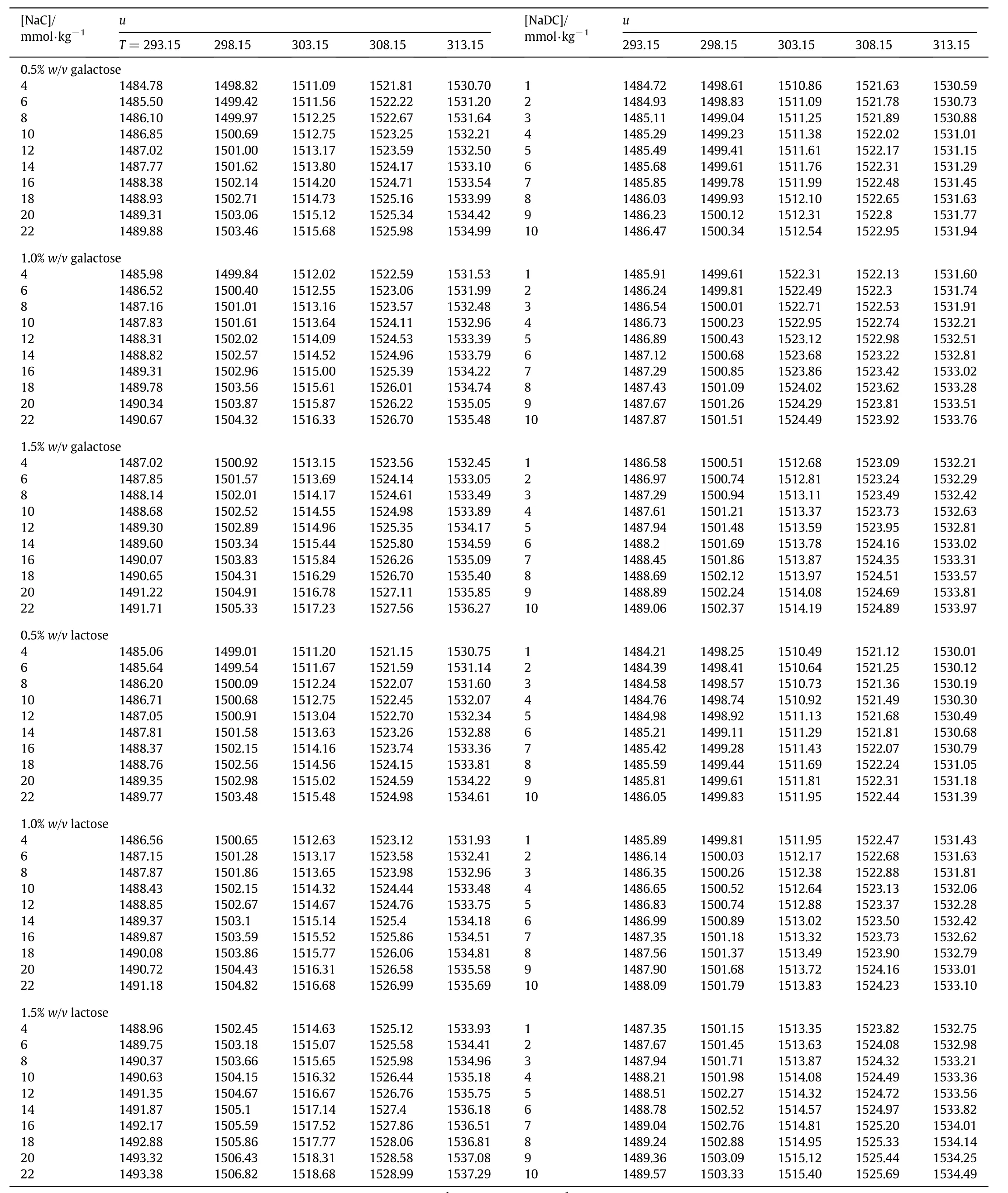
Table 2 Speed of sound,u(m·s-1)of NaC and NaDC in aqueous solutions of galactose and lactose 0.5%,1.0%,1.5%(w/v)at different temperatures(T/K)
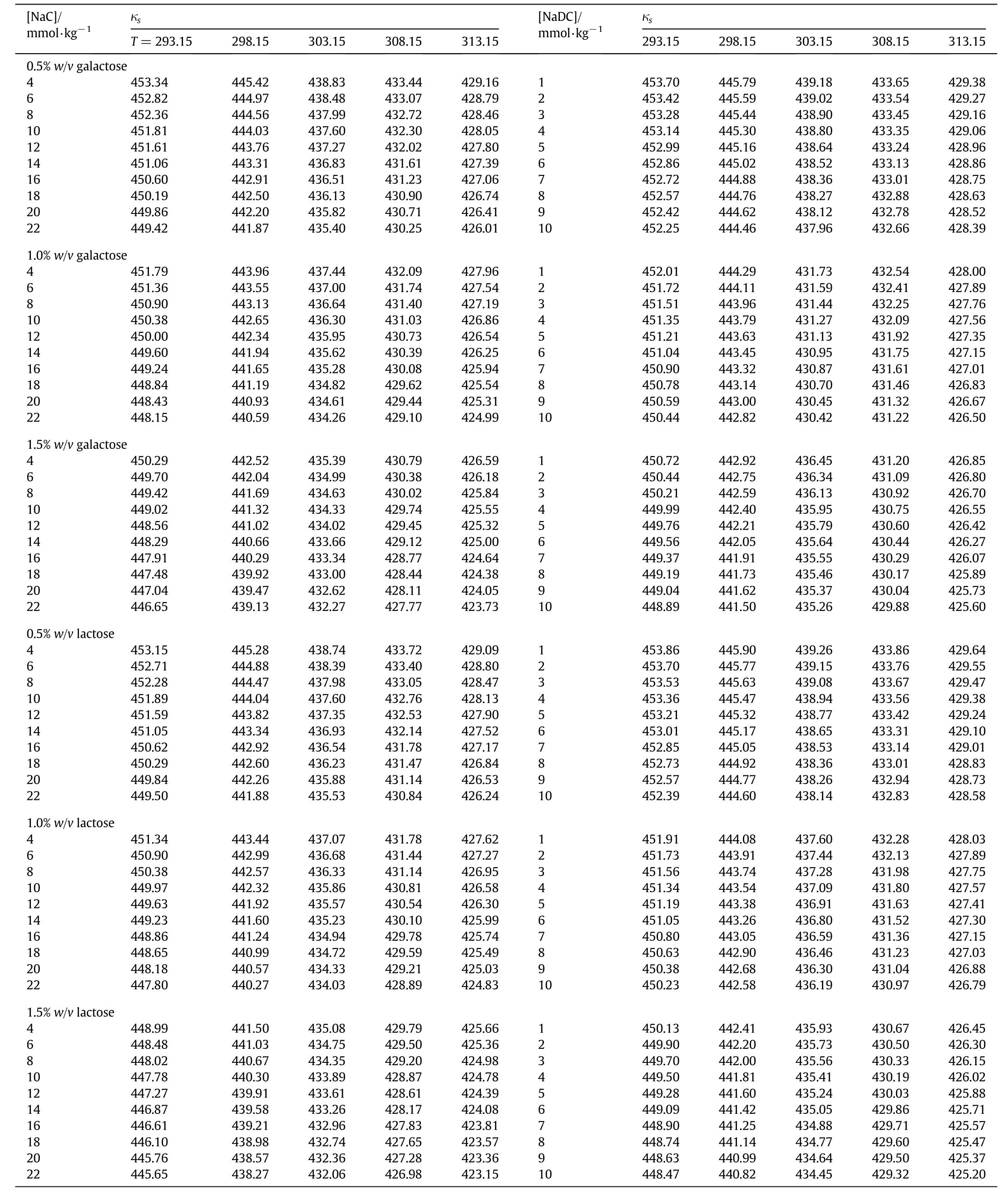
Table 3 Isentropic compressibility,κs(TPa-1)of NaC and NaDC in aqueous solutions of galactose and lactose 0.5%,1.0%,1.5%(w/v)at different temperatures(T/K)
Density(ρ)and speed of sound(u)data have been used to derive various volumetric and compressibility parameters such as apparent molar volume(Vφ),isentropic compressibility(κs),apparent molar adiabatic compression(κs,φ)etc.All these parameters absolutely depend on the solvent environment around the solute species and are known to contain information pertaining to the structural consequences of solute-solvent interactions[26-28].The isentropic compressibility(κs)has been calculated using the relation[30]:

The compressibility of micellar solutions is a measure of internal pressure due to compactness arising from solute-solvent interactions in the system and has been proposed to depend on the compressibility of hydrocarbon core and the interactions between the head groups.However,κsis likely to be dependent on the variation of the counter ion binding and the hydrophilicity of the head group.The compressibility data for bile salts in aqueous solutions of galactose and lactose has been summarized in Table 3.From the data,it has been found that κsvalues decrease with increase in temperature and concentration of BS.This lowering in the compressibility of system implies enhanced molecular association and hydrogen bonding between solute and solvent molecules.Such a sharp decrease may also provide an insight for aggregation within the surfactant molecules signifying more compactness of the solution which makes it incompressible to greater extent[30,31].On the other hand,κsdecreases with increase in the percentage of saccharides and follows the order:0.5>1.0>1.5 whereas among different saccharides,the following order has been observed:galactose>lactose.This order may arise due to the structural differences in two saccharides.However,for NaC,compressibility is lower than NaDC,which may be attributed to the presence of extra hydroxyl group in NaC.These types of observations are characteristic of electrolytic behavior of surfactants[32,33].
The apparent molar volume(Vφ)has been calculated using the relation[30]:


Fig.2.Representative plots of apparent molar volume,Vφ as a function of[NaC](a)and[NaDC](b)in aqueous solution containing 0.5%(w/v)galactose at 293.15 K(■),298.15 K(),303.15 K(),308.15 K()and 313.15 K().
wheremis the molality of the solution;Mis the molecular weight of bio-surfactant;ρ and ρ0are the density of the solution and solventi.e.aqueous solutions of saccharides,respectively.In present ternary(water-saccharide-BS)system,the following different types of interactions may take place which are expected to affect hydration of BS and hence the values ofVφ:
i.Ionic-hydrophilic interactions between the ions(COO-,Na+)of solute and hydrophilic sites(--OH,--C=O and--O--)of saccharides.
ii.Hydrophobic interactions between the alkyl chains of the saccharides and hydrophobic part of the solute(NaC and NaDC).
iii.Hydrophobic-ionic interactions between the hydrophobic groups of saccharides and the ionic part of NaC/NaDC.
iv.Hydrogen bonding between saccharide and water molecules.
According to co-sphere overlap model[34],hydrophobic interactions are supposed to increase the electrostriction in the surfactant system resulting in the destruction of structured water and hence decrease inVφvalues.On the other hand,interactions of types(i,iii and iv)weaken the electrostatic interactions and hence enhance the water structure by promoting hydrogen bonding leading to increased values ofVφ.In the present case,Vφvalues of bile salts in aqueous solutions of saccharides at different temperatures are positive and show dependence on solute concentration(NaC/NaDC)as can be seen from Fig.2.The curvi-linear nature of these graphs at low concentrations become linear as surfactant concentration is increased.The contributing factors responsible for the present trend inVφvalues may be due to the release of structured water around the hydrophobic tails,electrostatic repulsions between head groups of the surfactant and the release of water molecules from the counter ion as a result of binding to the micelle[35,36].
Apparent molar volume,(Vφ)decreases with increase in percentage of saccharides whereas,on comparing among saccharides the following order has been observedi.e.galactose>lactose(Table S1 of supplementary material).This is due to deeper penetration of lactose into micellar interior due to hydrophobic interactions(increase in hydrophobic carbon chain)with the micelle.AlsoVφis lower for NaDC than NaC due to greater hydrophobic character.
The value of apparent molar is entropic compression(κs,φ)has been calculated using the relation[30]:


Fig.3.Representative plot of apparent molar isentropic compression(κs,φ)as a function of[NaC](a)and[NaDC](b)in aqueous solution containing 0.5%(w/v)galactose at 293.15 K(■),298.15 K(),303.15 K(),308.15 K()and 313.15 K().
where κ0the isentropic compressibility for the solvent;κsis the isentropic compressibility for the solution;Vφis the apparent molar volume,and ρ0is the density of solvent.The κs,φvalues,thus obtained have been reported in Table S2 of supplementary material and Fig.3 represents the behavior ofκs,φas a function of concentration of BS in aqueous solutions containing 0.5%(w/v)galactose at different temperatures.The apparent molar isentropic compression values of NaC and NaDC in aqueous solutions of saccharides are negative which imply that the water molecules around BS are less compressible than in the bulk solution.However,with rise in concentration of bile salts as well as temperature,the κs,φvalues increase probably due to the self-association of surfactant which results in the release of some water molecules from the counterion upon the binding to the micelles making the system more compressible[30].The non-linear behavior in κs,φvalues at higher saccharide concentration(Table S2)gives an idea for strong solute-solvent interactions[37-39].
3.2.Viscometric studies
Viscosity(η)of bio-surfactants,NaC and NaDC in different percentages of aqueous solution of galactose and lactose(0.5,1.0 and 1.5)%w/vhas been calculated by using the relation:

where ηois viscosity of solvent,toandtis time of flow of solvent and solution,respectively,and ρoand ρ is density of solvent and solution,respectively.The values of viscosity have been documented in Table 4.The increment in temperature may result into inflation in kinetic energy of the molecules and ions present in the solution,which in turn reduces intermolecular interactions leading to a decrease in η values[45,46].
Further viscosity data have been studied in the form of relative viscosity(ηr)[45]using relation:

where ηoand η are the viscosity of the pure solvent and solution respectively.The relative viscosity of both NaC and NaDC initially rise with small increments up toCMC.However,a relatively larger increase in ηrvalues is noticed in post-micellar region as shown in Fig.4.This observation,therefore,leads to the conclusion that there exist significant interactions between bile salts and saccharides leading to promoted micellization of BS in aqueous solutions of saccharides[40,41].
Interestingly,similar results have been obtained from viscous relaxation time(τ)values(Table S4 of supplementary material).Relaxation time is the time taken for the excitation energy to appear as translational energy and it depends on temperature and impurities.Viscous relaxation time,τ has been calculated using relation[42]:

Viscous relaxation time when plotted against concentration of these bio-surfactants shows almost linear behavior in small increments,however,decreases with rise in temperature(Fig.5).This is mainly due to the structural relaxation processes occurring in the system due to the re-arrangement of the molecules[43].The observation also con firms the existence of intermolecular interactions in presentternary BS+saccharide+water(B/S/W)system.
3.3.Derived parameters
The values of density,speed of sound and viscosity have been further used to calculate various derived parametersviz.free volume(Vf),internal pressure(πi)and molar cohesive energy(MCE)of NaC and NaDC in aqueous solution of saccharides which provide another evidence of interactions between bile salts and aqueous saccharides.The molar volume(Vm)is defined as the volume occupied by 1 mol of a substance at a given temperature and pressure and is calculated from the relation[44]:

where ρ is the density of the solution;Mis the average molecular weight of the solution which is defined as:=+mass of solute whereM12=x1M1+x2M2is average molecular weight of solvent;hereM1andM2are the molecular weights,andx1andx2are mole fractions of the solvent components(saccharides+water).TheVmvalues for NaC and NaDC in saccharides(Table S5 of supplementary material)are positive,which rise with increase in concentration of both the surfactants and percentage of saccharides and among various saccharides follows the order:lactose>galactose.This happens because of the fact that molecular weight is directly proportional to the molar volume and at higher temperatures;there is loosening of packing in NaC and NaDC molecules eventually leading to more intermolecular spacing[44].Another contributing factor could be thermal energy which facilitates the molecular separation in the liquid mixtures leading to an increase in molar volume[45].
Free volume,(Vf)is the effective volume referred as the void space between the moleculesi.e.volume present as holes of monomeric size,due to irregular packing of molecules.TheVfhas been calculated by using following equation[46]:

where η anduare viscosity and speed of sound,respectively,K′=4.28×109is constant and is independent of nature of liquid.The observed increase inVfvalues(Table 5)with concentration and temperature may be attributed to the dispersion forces,steric hindrance of component molecules,unfavorable geometric fitting and electrostatic repulsions.
Another parameter calculated is internal pressure(πi)whose variation gives some reliable information regarding the nature,molecular arrangement and describes strength of forces existing between molecules of solute in the solution.It is measure of forces that contribute to cohesion of the liquid systemi.e.dispersion,ionic and dipolar force and defined as the resultant of forces of attraction and repulsion between solute and solvent molecules of the solution.The value of πihas been calculated by relation[47]:

where packing factor(b)is assumed to be 2 in liquid systems;RandTare the gas constant and temperature(K)respectively.From Table 6,it can be inferred that πivalues are positive and decrease with rise in concentration of bile salts as well as the percentage of saccharides.The reduction in internal pressure may be due to the loosening of cohesive forces leading to breaking up the structure of the solvent or increase in intermolecular interactions among bile salt molecules leading to micellization process,which ultimately disturbs the structural arrangement of the solvent system.
The molar cohesive energy is a measure of attraction between various components of solution and has been computed by the relation[43]:

Increase inMCEwith bile salt concentration particularly aboveCMCaccounts for the enhancement of the structure forming tendency of the solvent molecules.Also the values are higher for NaDC than NaC and between two saccharides;it follows the order:lactose>galactose(Table S6 of supplementary material).
4.Conclusions
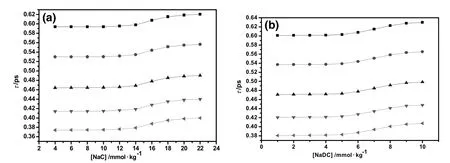
Fig.5.Representative plots of relaxation time(τ)as a function of[NaC](a)and[NaDC](b)in aqueous solution containing 0.5%(w/v)galactose at293.15 K(■),298.15 K(),303.15 K(),308.15 K()and 313.15 K().
Table 5 Free volume,V f(m3·mol-1)of NaC and NaDC in aqueous solutions of different percentages of galactose and lactose 0.5%,1.0%,1.5%(w/v)at different temperatures(T/K)
Table 6 Internal pressure,πi(Pa)of NaC and NaDC in aqueous solutions of different percentages of galactose and lactose 0.5%,1.0%,1.5%(w/v)at different temperatures(T/K)
Volumetric and viscometric analyses of(bio-surfactant+water+saccharide)systems have substantiated the fact that in the presence of saccharides micellization of bio-surfactants has been enhanced.The disaccharide(lactose)has shown more impact on micellar properties of bile salts as compared to monosaccharide(galactose),owing to the presence of more hydrophobic region in the former which results better into diffusion of the molecule into micellar core.The trends of volumetric and compressibility parameters may arise from i)dehydration of structured water around the hydrophobic tails,ii)electrostatic repulsions between head groups of the surfactant and iii)dehydration of counterion as a result of binding to the micelle.Further,lowering in viscosity values with temperature may be attributed to the increase in speed of molecules/ionspresentin the system thereby reducing interactions between them.The study of such(BS+aqueous saccharide)systems may be helpful in estimating their applications in the areas of biological and industrial interest.
Acknowledgement
S.Chauhan and Maninder Kaur thank UGC,New Delhi for financial assistance under the project(F.No.42-249/2013/SR)and award of Senior Research Fellowship(No.F.17-40/2008(SA-1)dated 31.07.2014),respectively.Vivek Sharma thanks Himachal Pradesh University for Senior Research Fellowship(F.No.1-3/2013-HPU(DS)5111).Financial support from UGC-SAP(DRS-I)(No.F.540/3/DRS/2010(SAP-1))to Department of Chemistry,HPU is also acknowledged.
Supplementary Material
Apparent molar volume(Vφ)(m3∙mol-1),apparent molar adiabatic compression κS,φ(m3·mol-1·TPa-1)relative viscosity(ηr),viscous relaxation time(τ),molarvolume(Vm)and molar cohesive energy(MCE)of NaC and NaDC in aqueous solutions of galactose and lactose(0.5,1.0,1.5)%w/vat different temperatures(T/K).Supplementary data associated with this article can be found in the online version,at doi:https://doi.org/10.1016/j.cjche.2017.10.025.
[1]J.M.Valderrama,P.Wilde,A.Macierzanka,A.Mackie,The role of bile salts in digestion,Adv.Colloid Interf.Sci.165(2011)36-46.
[2]B.De Castro,P.G.Ameiro,C.Guimaraes,J.Lima,S.Reis,Study of partition of nitrazepam in bile salt micelles and the role of lecithin,J.Pharm.Biomed.Anal.24(2001)595-602.
[3]T.S.Wiedmann,L.Kamel,Examination of the solubilization of drugs by bile salt micelles,J.Pharm.Sci.9(2002)1743-1764.
[4]R.Ninomiya,K.Matsuoka,Y.Moroi,Micelle formation of sodium chenodeoxycholate and solubilization into the micelles:comparison with other unconjugated bile salts,Biochem.Biophys.Acta1634(2003)116-125.
[5]C.L.Bowe,L.Mokhtarzadeh,P.N.Venkatesen,S.Babu,H.Axelrod,M.J.Sofia,R.Kakarla,T.Y.Chan,J.S.Kim,H.J.Lee,G.L.Amidon,S.Y.Choe,S.Walker,D.Kahne,Design of compounds that increase the absorption of polar molecules,Proc.Natl.Acad.Sci.U.S.A.94(1997)12218-12223.
[6]G.S.Gordon,A.C.Moses,R.D.Silver,J.R.Flier,M.C.Carey,Nasal absorption of insulin:enhancement by hydrophobic bile salts,Proc.Natl.Acad.Sci.U.S.A.82(1985)7419-7423.
[7]D.Madenci,S.U.Egelhaaf,Self-assembly in aqueous bile salt solutions,Curr.Opin.Colloid Interface Sci.15(2010)109-115.
[8]Y.S.Elnaggar,Multifaceted applications of bile salts in pharmacy:an emphasis on nanomedicine,Int.J.Nanomedicine10(2015)3955-3971.
[9]J.Li,X.Wang,T.Zhang,C.Wang,Z.Huang,X.Luo,Y.Deng,A review on phospholipids and their main applications in drug delivery systems,Asian J.Pharm.Sci.10(2015)81-98.
[10]B.C.Herold,R.Kirkpatrick,D.Marcellino,A.Travelstead,V.Pilipenko,H.Krasa,J.Bremer,L.J.Dong,M.D.Cooper,Bile salts:natural detergents for the prevon of sexually transmitted diseases,Antimicrob.Agents Chemother.43(1999)745-751.
[11]P.K.Banipal,N.Aggarwal,T.S.Banipal,Study on interactions of saccharides and their derivatives with potassium phosphate monobasic(1:1 electrolyte)in aqueous solutions at different temperatures,J.Mol.Liq.196(2014)291-299.
[12]S.Nithiyanantham,L.Palaniappan,Ultrasonic study on some monosaccharides in aqueous media at 298.15 K,Arab.J.Chem.5(2012)25-30.
[13]T.C.Bai,G.B.Yan,Viscosity B-coefficients and activation parameters for viscous flow of a solution of heptanedioic acid in aqueous sucrose solution,Carbohydr.Res.338(2003)2921-2927.
[14]A.Ali,P.Bidhuri,N.A.Malik,S.Uzair,Density,viscosity,and refractive index of mono-,di-,and tri-saccharides in aqueous glycine solutions at different temperatures,Arab.J.Chem.(2014),https://doi.org/10.1016/j.arabjc.2014.08.027.
[15]D.M.Cirin,M.M.Posa,V.S.Krstonosic,Interactions between sodium cholate or sodium Deoxycholate and nonionic surfactant(tween 20 or tween 60)in aqueous solution,Ind.Eng.Chem.Res.51(2012)3670-3676.
[16]C.W.Njauw,C.Y.Cheng,V.A.Ivanov,A.R.Khokhlov,S.H.Tung,Molecular interactions between lecithin and bile salts/acids in oils and their effects on reverse micellization,Langmuir29(2013)3879-3888.
[17]D.Madenci,A.Salonen,P.Schurtenberger,J.S.Pedersen,S.U.Egelhaaf,Simple model for the growth behaviour of mixed lecithin-bile salt micelles,Phys.Chem.Chem.Phys.13(2011)3171-3178.
[18]N.Funasaki,M.Fukuba,T.Hattori,S.Ishikawa,T.Okunoa,S.Hirota,Micelle formation of bile salts and zwitterionic derivative as studied by two-dimensional NMR spectroscopy,Chem.Phys.Lipids142(2006)43-57.
[19]K.Kumar,S.Chauhan,Surface tension and UV-visible investigations of aggregation and adsorption behavior of NaC and NaDC in water-amino acid mixtures,Fluid Phase Equilib.394(2015)165-174.
[20]K.Manna,C.H.Chang,A.K.Panda,Physicochemical studies on the catanionics of alkyltrimethylammonium bromides and bile salts in aqueous media,Colloids Surf.A Physicochem.Eng.Asp.415(2012)10-21.
[21]G.G.Gaitano,A.Compostizo,L.S.Martin,G.Tardojas,Speed of sound,density,and molecular modeling studies on the inclusion complex between sodium cholate and β-cyclodextrin,Langmuir13(1997)2235-2241.
[22]K.Kumar,B.S.Patial,S.Chauhan,Conductivity and fluorescence studies on the micellization properties of sodium cholate and sodium deoxycholate in aqueous medium at different temperatures:effect of selected amino acids,J.Chem.Thermodyn.82(2016)25-33.
[23]A.P.Davis,R.S.Wareham,Carbohydrate recognition through noncovalent interactions:a challenge for biomimetic and supramolecular chemistry,Angew.Chem.Int.Ed.38(1999)2978-2996.
[24]P.Venkatesan,Y.Cheng,D.Kahne,Hydrogen bonding in micelle formation,J.Am.Chem.Soc.116(1994)6955-6956.
[25]S.Chauhan,V.Sharma,K.Singh,M.S.Chauhan,K.Singh,Influence of lactose on the micellar behaviour and surface activity of bile salts as revealed through fluorescence and surface tension studies at varying temperatures,J.Mol.Liq.222(2016)67-76.
[26]S.Chauhan,K.Singh,K.Kumar,S.C.Neelakantan,G.Kumar,Drug-amino acid interactions in aqueous medium:volumetric,compressibility,and viscometric studies,J.Chem.Eng.Data61(2016)788-796.
[27]S.Chauhan,K.Sharma,D.S.Rana,G.Kumar,A.Umar,Volumetric and conductance studies of cetyltrimethyl ammonium bromide in aqueous glycine,J.Solut.Chem.42(2013)634-656.
[28]V.Bhardwaj,P.Sharma,M.N.Noolvib,H.M.Patel,S.Chauhan,M.S.Chauhan,K.Sharma,Thermo-physical examination:synthesized2-furano-4(3H)-quinazolinone and open quinazolinone(diamide)anticancer analogs with sodium dodecyl sulphate,Thermochim.Acta573(2013)65-72.
[29]M.Das,S.Das,A.K.Pattanaik,Acoustical behaviour of sodium nitroprusside in aquoorganic solvent media at 308.15 K,J.Chem.(2013),https://doi.org/10.1155/2013/942430.
[30]S.Chauhan,M.Kaur,D.S.Rana,M.S.Chauhan,Volumetric analysis of structural changes of cationic micelles in the presence of quaternary ammonium salts,J.Chem.Eng.Data61(2016)3770-3778.
[31]I.Bahadur,N.Deenadayalu,Apparent molar volume and apparent molar isentropic compressibility for the binary systems{methyltrioctyl ammonium bis(trifluoromethylsulfonyl)imide+ethyl acetate or ethanol}at different temperatures under atmospheric pressure,Thermochim.Acta566(2013)77-83.
[32]T.S.Banipal,D.Kaur,P.K.Banipal,G.Singh,Thermodynamic and transportproperties of L-serine and L-threonine in aqueous sodium acetate and magnesium acetate solutions atT=298.15 K,J.Chem.Thermodyn.39(2007)371-384.
[33]J.Singh,T.Kaur,V.Ali,D.S.Gill,Ultrasonic velocities and isentropic compressibilities of some tetraalkylammonium and copper(I)salts in acetonitrile and benzonitrile,J.Chem.Soc.Faraday Trans.90(1994)579-582.
[34]T.Banerjee,N.Kishore,Interactions of some amino acids with aqueous tetraethylammonium bromide at 298.15 K:a volumetric approach,J.Solut.Chem.34(2005)137-153.
[35]R.Sadeghi,S.Shahabi,A comparison study between sodium dodecyl sulfate and sodium dodecyl sulfonate with respect to the thermodynamic properties,micellization,and interaction with poly(ethylene glycol)in aqueous solutions,J.Chem.Thermodyn.43(2011)1361-1370.
[36]T.Mehrian,A.De Keizer,A.Korteweg,J.Lyklema,Thermodynamics of micellization ofn-alkylpyridiniumchlorides,Colloids Surf.A Physicochem.Eng.Asp.71(1993)255-267.
[37]S.Chauhan,L.Pathania,K.Sharma,G.Kumar,Volumetric,acoustical and viscometric behavior of glycine and DL-alanine in aqueous furosemide solutions at different temperatures,J.Mol.Liq.212(2015)656-664.
[38]H.Kumar,K.Kaur,Investigation on molecular interaction of amino acids in antibacterial drug ampicillin solutions with reference to volumetric and compressibility measurements,J.Mol.Liq.173(2012)130-136.
[39]D.D.Miller,W.Lenhart,B.J.Williams,J.H.Hewitt,The use of NMR to study sodium dodecyl sulfate-gelatin interactions,Langmuir10(1994)68-71.
[40]K.Sharma,S.Chauhan,Apparentmolar volume,compressibility and viscometric studies of sodium dodecyl benzene sulfonate(SDBS)and dodecyltrimethylammonium bromide(DTAB)in aqueous amino acid solutions:a thermo-acoustic approach,Thermochim.Acta578(2014)15-27.
[41]D.Kaushal,D.S.Rana,S.Chauhan,Effect of furosemide on denaturation of lysozyme in the presence of ionic surfactant at different temperatures,Fluid Phase Equilib.360(2013)239-247.
[42]V.K.Syal,A.Chauhan,S.Chauhan,Ultrasonic velocity,viscosity and density studies of poly(ethylene glycols)(PEG-8,000,PEG-20,000)in acetonitrile(AN)and water(H2O)mixtures at 250C,J.Pure Appl.Ultrason.27(2005)61-69.
[43]S.Chauhan,P.Chaudhary,K.Sharma,K.Kumar Kiran,Temperature-dependent volumetric and viscometric properties of amino acids in aqueous solutions of an antibiotic drug,Chem.Pap.67(2013)1442-1452.
[44]R.Kameswari,G.Giridhar,M.Rangacharyulu,Density and ultrasonic studies on sunflower oil,IJESAT5(2015)465-473.
[45]S.Thirumaran,Deepesh George,Ultrasonic study of intermolecular association through hydrogen bonding in ternary liquid mixtures,ARPN J.Eng.Appl.Sci.4(2009)1-11.
[46]A.B.Naik,Densities,viscosities,speed of sound and some acoustical parameter studies of substituted pyrazoline compounds at different temperatures,Indian J.Pure Appl.Phys.53(2015)27-34.
[47]R.Kumar,R.Mahesh,B.Shanmugapriyan,V.Kannappan,Volumetric,viscometric,acoustic and refractometric studies of molecular interactions in certain binary systems ofo-chlorophenol at 303.15 K,Indian J.Pure Appl.Phys.50(2012)633-640.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
