Catalyst-free and solvent-free oxidation of cycloalkanes(C5-C8)with molecular oxygen:Determination of autoxidation temperature and product distribution☆
Haimin Shen,Yan Wang,Jinhui Deng,Long Zhang,Yuanbin She*
State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology,College of Chemical Engineering,Zhejiang University of Technology,Hangzhou 310014,China
1.Introduction
The oxidation of cycloalkanes to corresponding cycloalkanols,cycloalkanones and aliphatic diacids is an extremely important chemical transformation not only in chemical industry but also in academic research,especially for cyclohexane[1-9].In the oxidation of cyclohexane,the obtained cyclohexanol and cyclohexanone are very important intermediates in the production of caprolactam,and adipic acid which is the irreplaceable precursor in the production of nylon-66 and nylon-6 polymers[10-19].Glutaric acid,heptanedioic acid and octanedioic acid obtained from oxidation of the corresponding cycloalkane directly or indirectly are important precursors in the production of various macromolecule polymers too[20-24].In addition,cycloalkanols and cycloalkanones are important intermediates and solvents in the chemical industry and academic research[25-31].To realize this necessary transformation from cycloalkanes to their oxidized products,several oxidants can be employed,such as iodosylbenzene[32-38],iodosylbenzene diacetate[34,38-40],tert-butyl hydroperoxide[41-49],m-chloroperoxybenzoic acid[50-55],hydrogen peroxide[56-63],molecular oxygen[64-72],ozone[73,74],and so on.Among these oxidants,molecular oxygen is considered as the most promising choice from the perspective of Green Chemistry,which is inexpensive,readily available,and environmentally benign with harmless water as the only by-product.In current industrial process,the oxidation of cyclohexane is conducted at 150-170°C and 1.0-2.0 MPa pressure using homogeneous cobalt salt as catalyst and molecular oxygen as oxidant,and in order to improve the selectivity of desired products(cyclohexanol and cyclohexanone),the conversion of cyclohexane is usually kept at 3%-8%with an acceptable selectivity towards cyclohexanol and cyclohexanone,80%-85%[12,18,47,75-78].The main drawbacks of the current industrial process are the high temperature,low conversion,low selectivity and low yield,especially the high temperature which induces the oxidation of cyclohexane to process through uncatalyzed and unselective free radical autoxidation pathways,resulting in the low selectivity[75].In order to smooth the transformation from cycloalkanes to their oxidized products employing molecular oxygen as oxidant,several catalytic systems have been explored,such as transition metal complex catalysis[8-81],metal nanoparticle catalysis[68-72,82,83],metal oxide nanoparticle catalysis[3,16],molecular sieve catalysis[84-86],carbon material catalysis[69,87,88],photocatalysis[9,71,89],and so on.Although the conversion of cycloalkanes and the selectivity of desired oxidized products are improved obviously under the catalytic condition,the reaction temperature still stays at about150°C in most of the oxidation systems,and the obtained oxidized products still is a mixture of cycloalkanols and cycloalkanones,even some aliphatic diacids[8,70,72,80-82,86].At so high temperature,the oxidation of cycloalkanes at the presence of molecular oxygen as oxidant processes through both catalytic oxidation pathway and autoxidation pathway which is unselective and limits the improvement in the selectivity.How to eliminate the autoxidation in the catalytic oxidation of cycloalkanes is an enormous challenge in the precise adjustment of the product formation.
In our attempt to adjust the product formation precisely in the oxidation of cyclohexane using metalloporphyrin as catalyst and molecular oxygen as oxidant[90-95],it was found that,in most of the catalytic systems reported at present,the oxidation of cyclohexane processed through both catalytic oxidation pathway and autoxidation pathway because of the high reaction temperature,resulting in low selectivity[8,70,72,80-82,86].The high reaction temperature is the origin of the autoxidation.It may be a good choice to conduct the oxidation of cycloalkanes at lower temperature to avoid the unselective autoxidation and enhance the desired product selectivity through catalytic oxidation pathway.To the best of our knowledge,there is not a clear document at present involved in the temperature at which the autoxidation of cycloalkanes using molecular oxygen as oxidant would occur,no document distinguishing the autoxidation from the catalytic oxidation in the oxidation of cycloalkanes through reaction temperature control clearly.Thus in this work,we investigate the catalyst-free and solvent-free oxidation of cycloalkanes(C5-C8)with molecular oxygen systematically,focusing on the autoxidation temperature and product distribution,which will act as an important reference in screen of suitable reaction temperature and comparison of the performance of various catalysts in the catalytic oxidation of cycloalkanes.
2.Results and Discussion
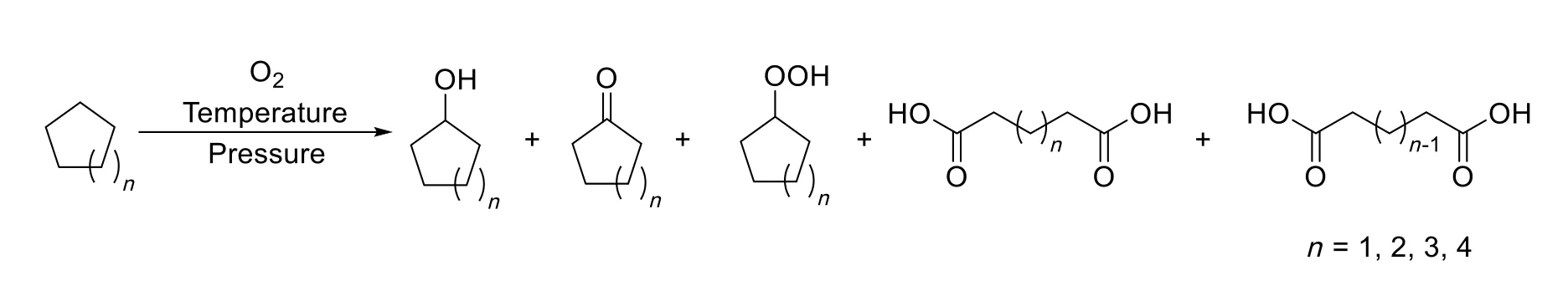
Scheme 1.Catalyst-free and solvent-free oxidation of cycloalkanes(C5-C8)with molecular oxygen.

Fig.1.Effect of reaction temperature on the autoxidation of cyclohexane.Reaction condition:cyclohexane(200 mmol,16.8320 g),O2(a.1.0 MPa,b.1.2 MPa,c.1.6 MPa,d.1.8 MPa),8.0 h.The yields of cyclohexanol and cyclohexanone were determined by GC after treatment with PPh3 employing toluene as internal standard,and the yield of cyclohexyl hydroperoxide was determined through the amount of O=PPh3 obtained from reduction of cyclohexyl hydroperoxide with PPh3.The yield of adipic acid and glutaric acid were determined by HPLC with benzoic acid as internal standard.
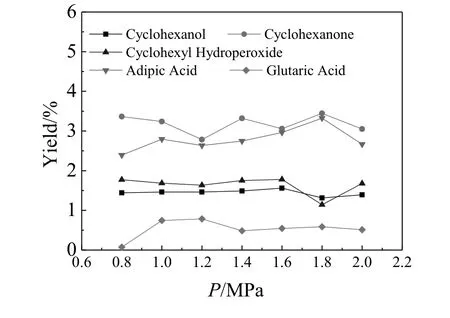
Fig.2.Effect of reaction pressure on the autoxidation of cyclohexane.Reaction condition:cyclohexane(200 mmol,16.8320 g),O2,140°C,8.0 h.The yields of cyclohexanol and cyclohexanone were determined by GC after treatment with PPh3 employing toluene as internal standard,and the yield of cyclohexyl hydroperoxide was determined through the amount of O=PPh3 obtained from reduction of cyclohexyl hydroperoxide with PPh3.The yield of adipic acid and glutaric acid were determined by HPLC with benzoic acid as internal standard.

Fig.3.Effect of reaction temperature on the autoxidation of cyclopentane(a),cycloheptane(b)and cyclooctane(c).Reaction condition:a.cyclopentane(200 mmol,14.0260 g),O2(1.6 MPa),8.0 h.b.cycloheptane(200 mmol,19.6380 g),O2(0.6 MPa),8.0 h.c.cyclooctane(200 mmol,22.4440 g),O2(0.6 MPa),8.0 h.The yields of cycloalkanol and cycloalkanone were determined by GC after treatment with PPh3 employing toluene as internal standard,and the yield of cycloalkyl hydroperoxide was determined through the amount of O=PPh3 obtained from reduction of cycloalkyl hydroperoxide with PPh3.The yield of aliphatic diacids was determined by HPLC with benzoic acid as internal standard.
In this work,the oxidation of cycloalkanes using molecular oxygen as oxidant under catalyst-free and solvent-free condition was conducted in a 100-ml autoclave reactor equipped with an inner Teflon liner and a magnetic stirrer.Cyclopentane,cyclohexane,cycloheptane and cyclooctane were selected as substrates as shown in Scheme 1 for their ready availability and the high values of their oxidized products.All the oxidized products which can be detected on GC and HPLC were analyzed quantitatively in order to investigate the autoxidation performance of various cycloalkanes in detail,including cycloalkanols,cycloalkanones,cycloalkyl hydroperoxide,aliphatic diacids with the same and one less carbon number compared with parent cycloalkanes.Firstly,catalyst-free and solvent-free oxidation of cyclohexane was carried out at the temperature ranging from 120 °C to 150 °C and the pressure ranging from 0.80 MPa to 2.0 MPa based on some exploratory experiments.The reaction time was selected as 8.0 h in order to achieve obvious oxidized products formation indicated in Fig.S4.As shown in Figs.1,2 and Figs.S1-S3,it is obvious that the reaction temperature plays the mostcriticalrole in the autoxidation ofcyclohexane compared to reaction pressure.When the reaction temperature was 120°C,no obvious oxidized product formed.As the reaction temperature increased,the autoxidation of cyclohexane occurred at about 130°C with obvious oxidized products formation.When the temperature reached 145°C,acceptable yields of oxidized products could be obtained,cyclohexanol(3.18%),cyclohexanone(4.59%),cyclohexyl hydroperoxide(0.15%),adipic acid(1.44%)and glutaric acid(0.39%)under1.0-MPa O2pressure,and no other obvious product was detected in the liquid and solid reaction mixture.The yields of the oxidized products at 145°C and 1.0 MPa increase as following:cyclohexyl hydroperoxide(0.15%)<glutaric acid(0.39%)<adipic acid(1.44%)<cyclohexanol(3.18%)<cyclohexanone(4.59%).The reaction temperature also affected the oxidized product distribution remarkably.For example,at 140°C and 1.0 MPa,the product distribution is very different from 145°C,and the yields of the products increase as following:glutaric acid<cyclohexanol<cyclohexyl hydroperoxide<adipic acid<cyclohexanone.The yield of glutaric acid becomes the lowestone instead of cyclohexyl hydroperoxide,and the yield of cyclohexanone still is the highest one.As for the reaction pressure,it does not seem to have significant effect on the autoxidation of cyclohexane as indicated in Fig.2 and Fig.S5,which just supplies enough oxygen to the oxidation system.
Then based on the results obtained from the autoxidation of cyclohexane,the substrates were extended to cyclopentane,cycloheptane and cyclooctane.The results were listed in Fig.3.It is demonstrated that the autoxidation temperatures are 120 °C,120 °C and 105 °C for cyclopentane,cycloheptane and cyclooctane respectively,at which obvious oxidized products could be detected.For cyclopentane,acceptable yields of oxidized products could be achieved at 140°C,and the yields of the oxidized products increase as following:cyclopentyl hydroperoxide(0.55%)<succinic acid(2.08%)<cyclopentanone(2.75%)<glutaric acid(3.88%),no obvious cyclopentanolbeing detected in the reaction mixture.Compared with cyclohexane,the yield of the aliphatic diacid possessing the same carbon number with parent cycloalkane became the highest one in the autoxidation of cyclopentane for its high strain energy.As for cycloheptane and cyclooctane,satisfying yields of oxidized products could be obtained at 130 °C and 125 °C respectively,and the products were mainly cycloalkyl hydroperoxides and cycloalkanones,no obvious cycloalkanoland diacid being detected.The yield of cycloalkyl hydroperoxide was higher than cycloalkanone in both autoxidation of cycloheptane and cyclooctane.The reason why the autoxidation of cycloheptane and cyclooctane were notconducted athigher reaction temperature is that the autoxidation process would become uncontrollable and the reaction temperature would be runway to above 170°C at higher initial reaction temperature.
Thus,it is clear that the autoxidation of cycloalkanes under catalystfree and solvent-free condition could be achieved at the temperature of 120 °C,130 °C,120 °C,and 105 °C for cyclopentane,cyclohexane,cycloheptane and cyclooctane respectively,with obvious oxidized products formation.Acceptable yields of the oxidized products could be obtained at140 °C,145 °C,130 °C and 125 °C for cyclopentane,cyclohexane,cycloheptane and cyclooctane respectively.For cyclopentane,the product distribution at 140°C increased as following:cyclopentyl hydroperoxide(0.55%)<succinic acid(2.08%)<cyclopentanone(2.75%)<glutaric acid(3.88%),no obvious cyclopentanol being detected.For cyclohexane,the product distribution at 145°C increased as following:cyclohexyl hydroperoxide(0.15%)<glutaric acid(0.39%)<adipic acid(1.44%)<cyclohexanol(3.18%)<cyclohexanone(4.59%),and for cycloheptane and cyclooctane,the products were cycloalkyl hydroperoxides and cycloalkanones mainly,no obvious cycloalkanol and diacid being detected.The yield of cycloalkyl hydroperoxide is higher than cycloalkanone.Itis also demonstrated that the autoxidation existed in mostof the currentcatalytic oxidation ofcycloalkanes indeed,especially in the oxidation of cyclohexane in which the reaction temperature is about 150°C[8,70,72,80-82,86].Therefore,it could be a good choice and a meaningful reference to conduct the catalytic oxidation of cycloalkanes below the autoxidation temperature,so the reaction can process smoothly and selectively.Taking into account the security,it is also suggested that(1)the autoxidation of cycloalkanes should be conducted in the autoclave reactor equipped with an inner Teflon liner to avoid the existence of any catalytic metal,which could induce the autoxidation process too severely to explode;(2)the autoxidation temperature should be below 150°C to avid the possible explosion;(3)the autoxidation pressure should be below 2.0 MPa to avid the possible explosion.
At last,the kinetics of the autoxidation of cycloalkanes were investigated employing cyclopentane and cyclohexane as model substrates based on the results obtained in the autoxidation of cycloalkanes above.It was found that the pseudo-first-order kinetic model fitted the autoxidation of cyclopentane and cyclohexane well,which is expressed as following:-ln(CA/CA0)=kt,whereCA0is the initial concentration of cycloalkane;CAis the concentration of cycloalkane at timet;kis the pseudo-first-order rate constant(h-1).The plots of-ln(CA/CA0)versus tat 130 °C,135 °C,140 °C are presented in Fig.4 and from the slope of the plots,the pseudo-first-order rate constantkfor the autoxidation of cyclopentane and cyclohexane at 130°C,135 °C,140 °C were obtained as shown in Table 1.Then the plots oflnkversus1/Twere listed in Fig.5 and the apparent activation energies(Ea)for the autoxidation of cyclopentane and cyclohexane could be obtained from the slope following equation lnk=-(Ea/R)×(1/T)+lnk0,which were 159.76 kJ·mol-1and 86.75 kJ·mol-1for cyclopentane and cyclohexane respectively.

Table 1 The pseudo-first-order kinetic parameters for the autoxidation of cycolpentane and cyclohexane at 130 °C,135 °C,140 °C

Fig.5.Plots of ln k versus 1/T for the autoxidation of cycolpentane and cyclohexane.

Fig.4.Pseudo-first-order fit for the autoxidation of cyclopentane(a)and cyclohexane(b)at130 °C,135 °C,140 °C.Reaction condition:cycloalkane(100 mmol),O2(1.6 MPa).The yields of cycloalkanol and cycloalkanone were determined by GC after treatment with PPh3employing toluene as internal standard,and the yield of cycloalkyl hydroperoxide was determined through the amount of O=PPh3obtained from reduction of cycloalkyl hydroperoxide with PPh3.The yields of aliphatic diacids were determined by HPLC with benzoic acid as internal standard.
3.Conclusions
In conclusion,catalyst-free and solvent-free oxidation of cycloalkanes(C5-C8)with molecular oxygen was conducted systematically for the first time,focusing on the determination of autoxidation temperature and product distribution.It is obvious that the reaction temperature plays the most critical role in the autoxidation of cycloalkanes compared to reaction pressure and the autoxidation occurs at 120 °C,130 °C,120 °C,and 105 °C for cyclopentane,cyclohexane,cycloheptane and cyclooctane respectively,with obvious oxidized products formation.Acceptable yields of the oxidized products could be obtained at 140 °C,145 °C,130 °C and 125 °C respectively for them,and the oxidized products distribution for cyclopentane increased as following:cyclopentyl hydroperoxide(0.55%)<succinic acid(2.08%)<cyclopentanone(2.75%)<glutaric acid(3.88%)at 140°C,no obvious cyclopentanol being detected;the yields of the oxidized products for cyclohexane increased as following:cyclohexyl hydroperoxide(0.15%)<glutaric acid(0.39%)<adipic acid(1.44%)<cyclohexanol(3.18%) < cyclohexanone(4.59%)at 145 °C;for cycloheptane and cyclooctane,the products were mainly cycloalkyl hydroperoxides and cycloalkanones,no obvious cycloalkanol and diacid being detected,cycloheptyl hydroperoxide(6.09%)> cycloheptanone(3.21%)at 130°C and cyclooctylhydroperoxide(10.51%)>cyclooctanone(4.78%)at125°C.At last,the autoxidation of cycloalkanes follow the pseudo- first-order kinetic model and the apparent activation energies(Ea)for the autoxidation of cyclopentane and cyclohexane are 159.76 kJ·mol-1and 86.75 kJ·mol-1respectively.To the best of our knowledge,this study is the first systematic document up to now which revealed the autoxidation temperature and product distribution of the catalyst-free and solvent-free oxidation of cycloalkanes(C5-C8)with molecular oxygen.This study will act as an important reference in screen of suitable reaction temperature and comparison of the performance of various catalysts in the catalytic oxidation of cycloalkanes in the attempt to enhance the oxidized product selectivity.
Supplementary Material
Supplementary materialto this article can be found online at https://doi.org/10.1016/j.cjche.2018.02.019.
[1]Y.Hitomi,K.Arakawa,T.Funabiki,M.Kodera,An iron(III)-monoamidate complex catalyst for selective hydroxylation of alkane C-H bonds with hydrogen peroxide,Angew.Chem.Int.Ed.Eng.51(14)(2012)3448-3452.
[2]S.Staudt,E.Burda,C.Giese,C.A.Muller,J.Marienhagen,U.Schwaneberg,W.Hummel,K.Drauz,H.Groger,Direct oxidation of cycloalkanes to cycloalkanones with oxygen in water,Angew.Chem.Int.Ed.Eng.52(8)(2013)2359-2363.
[3]J.H.Tong,L.L.Bo,X.D.Cai,H.Y.Wang,Q.P.Zhang,L.D.Su,Aerobic oxidation of cyclohexane effectively catalyzed by simply synthesized silica-supported cobalt ferrite magnetic nanocrystal,Ind.Eng.Chem.Res.53(25)(2014)10294-10300.
[4]P.F.Zhang,H.F.Lu,Y.Zhou,L.Zhang,Z.L.Wu,S.Z.Yang,H.L.Shi,Q.L.Zhu,Y.F.Chen,S.Dai,Mesoporous MnCeOx solid solutions for low temperature and selective oxidation of hydrocarbons,Nat.Commun.6(2015)8446,https://doi.org/10.1038/ncomms9446.
[5]A.P.Unnarkat,T.Sridhar,H.T.Wang,S.Mahajani,A.K.Suresh,Cobalt molybdenum oxide catalysts for selective oxidation of cyclohexane,AIChE J.62(12)(2016)4384-4402.
[6]D.N.J.Xiao,J.Oktawiec,P.J.Milner,J.R.Long,Pore environment effects on catalytic cyclohexane oxidation in expanded Fe-2(dobdc)analogues,J.Am.Chem.Soc.138(43)(2016)14371-14379.
[7]D.Mandal,S.Shaik,Interplay of tunneling,two-state reactivity,and Bell-Evans-Polanyi effects in C--H activation by nonheme Fe(IV)O oxidants,J.Am.Chem.Soc.138(7)(2016)2094-2097.
[8]G.Huang,W.L.Wang,X.X.Ning,Y.Liu,S.K.Zhao,Y.A.Guo,S.J.Wei,H.Zhou,Interesting green catalysis of cyclohexane oxidation over metal tetrakis(4-carboxyphenyl)porphyrins promoted by zinc sulfide,Ind.Eng.Chem.Res.55(11)(2016)2959-2969.
[9]Y.Shiraishi,S.Shiota,H.Hirakawa,S.Tanaka,S.Ichikawa,T.Hirai,Titanium dioxide/reduced graphene oxide hybrid photocatalysts for efficient and selective partial oxidation of cyclohexane,ACS Catal.7(1)(2017)293-300.
[10]A.Alshammari,A.Koeckritz,V.N.Kalevaru,A.Bagabas,A.Martin,Significant formation of adipic acid by direct oxidation of cyclohexane using supported nano-gold catalysts,ChemCatChem4(9)(2012)1330-1336.
[11]B.Sarkar,P.Prajapati,R.Tiwari,R.Tiwari,S.Ghosh,S.S.Acharyya,C.Pendem,R.K.Singha,L.N.S.Konathala,J.Kumar,T.Sasaki,R.Bal,Room temperature selective oxidation of cyclohexane over Cu-nanoclusters supported on nanocrystalline Cr2O3,Green Chem.14(9)(2012)2600-2606.
[12]A.R.Silva,T.Mourao,J.Rocha,Oxidation of cyclohexane by transition-metal complexes with biomimetic ligands,Catal.Today203(2013)81-86.
[13]Y.J.Xie,F.Y.Zhang,P.L.Liu,F.Hao,H.A.Luo,Zinc oxide supported trans-CoD(p-Cl)PPCltype Metalloporphyrins catalyst for cyclohexane oxidation to cyclohexanol and cyclohexanone with high yield,Ind.Eng.Chem.Res.54(9)(2015)2425-2430.
[14]Y.Ide,M.Iwata,Y.Yagenji,N.Tsunoji,M.Sohmiya,K.Komaguchi,T.Sano,Y.Sugahara,Fe oxide nanoparticles/Ti-modified mesoporous silica as a photo-catalyst for efficient and selective cyclohexane conversion with O2and solar light,J.Mater.Chem.A4(41)(2016)15829-15835.
[15]J.Jian,K.Y.You,Q.Luo,H.X.Gao,F.F.Zhao,P.L.Liu,Q.H.Ai,H.A.Luo,Supported Ni-Al-VPO/MCM-41 As efficient and stable catalysts for highly selective preparation of adipic acid from cyclohexane with NO2,Ind.Eng.Chem.Res.55(13)(2016)3729-3735.
[16]M.Z.Wu,W.C.Zhan,Y.L.Guo,Y.Guo,Y.S.Wang,L.Wang,G.Z.Lu,An effective Mn-Co mixed oxide catalyst for the solvent-free selective oxidation of cyclohexane with molecular oxygen,Appl.Catal.A Gen.523(2016)97-106.
[17]M.Rezaei,A.N.Chermahini,H.A.Dabbagh,Green and selective oxidation of cyclohexane over vanadium pyrophosphate supported on mesoporous KIT-6,Chem.Eng.J.314(2017)515-525.
[18]S.Rana,S.B.Jonnalagadda,CuO/graphene oxide nanocomposite as highly active and durable catalyst for selective oxidation of cyclohexane,ChemistrySelect2(7)(2017)2277-2281.
[19]X.F.Song,J.M.Hao,Y.J.Bai,L.M.Han,G.F.Yan,X.Lian,J.S.Liu,Solvent-free oxidation of cyclohexane by oxygen over Al-Cu-Co alloys:influence of the phase structure and electrical conductivity on catalytic activity,New J.Chem.41(10)(2017)4031-4039.
[20]V.T.Wyatt,M.Yadav,N.Latona,C.K.Liu,Thermal,mechanical,and absorbent properties of glycerol-based polymer films infused with plant cell wall polysaccharides,Abstr.Pap.Am.Chem.Soc.245(2013)(CELL-164).
[21]M.Vera,A.Almontassir,A.Rodriguez-Galan,J.Puiggali,Synthesis and characterization of a new degradable poly(ester amide)derived from 6-amino-1-hexanol and glutaric acid,Macromolecules36(26)(2003)9784-9796.
[22]K.Nemoto,T.Kubo,M.Nomachi,T.Sano,T.Matsumoto,K.Hosoya,T.Hattori,K.Kaya,Simple and effective 3D recognition of domoic acid using a molecularly imprinted polymer,J.Am.Chem.Soc.129(44)(2007)13626-13632.
[23]Y.Yu,Z.Y.Wei,C.Zhou,L.C.Zheng,X.F.Leng,Y.Li,Miscibility and competition of cocrystallization behavior of poly (hexamethylene dicarboxylate)s aliphatic copolyesters:effect of chain length of aliphatic diacids,Eur.Polym.J.92(2017)71-85.
[24]T.Baba,Y.Tachibana,S.Suda,K.Kasuya,Evaluation of environmental degradability based on the number of methylene units in poly(butylenen-alkylenedionate),Polym.Degrad.Stab.138(2017)18-26.
[25]H.Wu,Q.Wang,J.P.Zhu,Organocatalytic enantioselective acyloin rearrangement of alpha-hydroxy acetals to alpha-alkoxy ketones,Angew.Chem.Int.Ed.Eng.56(21)(2017)5858-5861.
[26]T.Tabanelli,E.Monti,F.Cavani,M.Selva,The design of efficient carbonate interchange reactions with catechol carbonate,Green Chem.19(6)(2017)1519-1528.
[27]Y.Ogawa,K.Yamamoto,C.Miura,S.Tamura,M.Saito,M.Mamada,D.Kumaki,S.Tokito,H.Katagiri,Asymmetric alkylthienyl thienoacenes derived from anthra[2,3-b]thieno[2,3-d]thiophene for solution-processable organic semiconductors,ACS Appl.Mater.Interfaces9(11)(2017)9902-9909.
[28]Q.Z.Hu,L.N.Yin,A.Ali,A.J.Cooke,J.Bennett,P.Ratcliffe,M.M.C.Lo,E.Metzger,S.Hoyt,R.W.Hartmann,Novel pyridyl substituted 4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines as potent and selective aldosterone synthase inhibitors with improved in vitro metabolic stability,J.Med.Chem.58(5)(2015)2530-2537.
[29]W.E.Noland,H.V.Kumar,G.C.Flick,C.L.Aspros,J.H.Yoon,A.C.Wilt,N.Dehkordi,S.Thao,A.K.Schneerer,S.Gao,K.J.Tritch,Hydrated ferric sulfate-catalyzed reactions of indole with aldehydes,ketones,cyclic ketones,and chromanones:synthesis of bisindoles and trisindoles,Tetrahedron73(27-28)(2017)3913-3922.
[30]J.Y.Shie,J.L.Zhu,Synthesis of bicyclic gamma-butyrolactone derivatives by rhodium catalyzed intramolecular C-H insertion of alpha-dizao alpha-phosphoryl cycloalkyl esters,Tetrahedron72(12)(2016)1590-1601.
[31]B.M.Trost,C.E.Stivala,D.R.Fandrick,K.L.Hull,A.Huang,C.Poock,R.Kalkofen,Total synthesis of(-)-lasonolide A,J.Am.Chem.Soc.138(36)(2016)11690-11701.
[32]G.S.Machado,O.J.de Lima,K.J.Ciuffi,F.Wypych,S.Nakagaki,Iron(III)porphyrin supported on metahalloysite:an efficient and reusable catalyst for oxidation reactions,Catal.Sci.Technol.3(4)(2013)1094-1101.
[33]G.M.Ucoski,F.S.Nunes,G.DeFreitas-Silva,Y.M.Idemori,S.Nakagaki,Metalloporphyrins immobilized on silica-coated Fe3O4nanoparticles:magnetically recoverable catalysts for the oxidation of organic substrates,Appl.Catal.A Gen.459(2013)121-130.
[34]V.S.da Silva,L.I.Teixeira,E.do Nascimento,Y.M.Idemori,G.DeFreitas-Silva,New manganese porphyrin as biomimetic catalyst of cyclohexane oxidation:effect of water or imidazole as additives,Appl.Catal.A Gen.469(2014)124-131.
[35]K.A.D.D.Castro,M.M.Q.Simoes,M.D.P.M.S.Neves,J.A.S.Cavaleiro,R.R.Ribeiro,F.Wypych,S.Nakagaki,Synthesis of new metalloporphyrin derivatives from[5,10,15,20-tetrakis(penta fluorophenyl)porphyrin]and 4-mercaptobenzoic acid for homogeneous and heterogeneous catalysis,Appl.Catal.A Gen.503(2015)9-19.
[36]L.D.Zanatta,I.A.Barbosa,F.B.Zanardi,P.C.de Sousa,L.B.Bolzon,A.P.Ramos,O.A.Serra,Y.Iamamoto,Hydrocarbon oxidation by iron-porphyrin immobilized on SBA-15 as biomimetic catalyst:role of silica surface,RSC Adv.6(106)(2016)104886-104896.
[37]V.H.A.Pinto,J.S.Reboucas,G.M.Ucoski,E.H.de Faria,B.F.Ferreira,R.A.S.S.Gil,S.Nakagaki,Mn porphyrins immobilized on non-modified and chloropropylfunctionalized mesoporous silica SBA-15 as catalysts for cyclohexane oxidation,Appl.Catal.A Gen.526(2016)9-20.
[38]V.S.da Silva,W.C.D.Vieira,A.M.Meireles,G.M.Ucoski,S.Nakagaki,Y.M.Idemori,G.DeFreitas-Silva,Biomimetic oxidation of cyclic and linear alkanes:high alcohol selectivity promoted by a novel manganese porphyrin catalyst,New J.Chem.41(3)(2017)997-1006.
[39]V.S.da Silva,A.M.Meireles,D.C.D.Martins,J.S.Reboucas,G.DeFreitas-Silva,Y.M.Idemori,Effect of imidazole on biomimetic cyclohexane oxidation by first-,second-,and third-generation manganese porphyrins using PhIO and PhI(OAc)2as oxidants,Appl.Catal.A Gen.491(2015)17-27.
[40]V.S.da Silva,Y.M.Idemori,G.DeFreitas-Silva,Biomimetic alkane oxidation by iodosylbenzene and iodobenzene diacetate catalyzed by a new manganese porphyrin:water effect,Appl.Catal.A Gen.498(2015)54-62.
[41]R.Maheswari,R.Anand,G.Imran,MnTUD-1:synthesis,characterization and catalytic behavior in liquid-phase oxidation of cyclohexane,J.Porous.Mater.19(3)(2012)283-288.
[42]D.W.Feng,H.L.Jiang,Y.P.Chen,Z.Y.Gu,Z.W.Wei,H.C.Zhou,Metal-organic frameworks based on previously unknown Zr-8/Hf-8 cubic clusters,Inorg.Chem.52(21)(2013)12661-12667.
[43]A.Bellifa,A.Choukchou-Braham,C.Kappenstein,L.Pirault-Roy,Preparation and characterization of MTiX for the catalytic oxidation of cyclohexane,RSC Adv.4(43)(2014)22374-22379.
[44]N.Pal,M.Pramanik,A.Bhaumik,M.Ali,Highly selective and direct oxidation of cyclohexane to cyclohexanone over vanadium exchanged NaY at room temperature under solvent-free conditions,J.Mol.Catal.A Chem.392(2014)299-307.
[45]Y.M.Yang,N.Y.Liu,S.Qiao,R.H.Liu,H.Huang,Y.Liu,Silver modified carbon quantum dots for solvent-free selective oxidation of cyclohexane,New J.Chem.39(4)(2015)2815-2821.
[46]T.Lopez-Ausens,M.Boronat,P.Concepcion,S.Chouzier,S.Mastroianni,A.Corma,A heterogeneous mechanism for the catalytic decomposition of hydroperoxides and oxidation of alkanes over CeO2nanoparticles:a combined theoretical and experimental study,J.Catal.344(2016)334-345.
[47]L.M.D.R.D.Martins,S.A.C.Carabineiro,J.W.Wang,B.G.M.Rocha,F.J.Maldonado-Hodar,A.J.L.D.Pombeiro,Supported gold nanoparticles as reusable catalysts for oxidation reactions of industrial significance,ChemCatChem9(7)(2017)1211-1221.
[48]M.V.Kirillova,C.I.M.Santos,W.Y.Wu,Y.Tang,A.M.Kirillov,Mild oxidative C-H functionalization of alkanes and alcohols using a magnetic core-shell Fe3O4@mSiO2@Cu4nanocatalyst,J.Mol.Catal.A Chem.426(2017)343-349.
[49]M.Saha,K.M.Vyas,L.M.D.R.S.Martins,N.M.R.Martins,A.J.L.Pombeiro,S.M.Mobin,D.Bhattacherjee,K.P.Bhabak,S.Mukhopadhyay,Copper(II)tetrazolato complexes:role in oxidation catalysis and protein binding,Polyhedron132(2017)53-63.
[50]S.Hikichi,K.Hanaue,T.Fujimura,H.Okuda,J.Nakazawa,Y.Ohzu,C.Kobayashi,M.Akita,Characterization of nickel(II)-acylperoxo species relevant to catalytic alkane hydroxylation by nickel complex with m-CPBA,Dalton Trans.42(10)(2013)3346-3356.
[51]J.Nakazawa,T.Hori,T.D.P.Stack,S.Hikichi,Alkane oxidation by an immobilized nickel complex catalyst:structural and reactivity differences induced by surface-ligand density on mesoporous silica,Chem.Asian.J.8(6)(2013)1191-1199.
[52]M.Sankaralingam,M.Palaniandavar,Diiron(III)complexes of tridentate 3N ligands as functional models for methane monooxygenases:effect of the capping ligand on hydroxylation of alkanes,Polyhedron67(2014)171-180.
[53]M.Sankaralingam,M.Balamurugan,M.Palaniandavar,P.Vadivelu,C.H.Suresh,Nickel(II)complexes of pentadentate N5 ligands as catalysts for alkane hydroxylation by using m-CPBA as oxidant:a combined experimental and computational study,Chem.Eur.J.20(36)(2014)11346-11361.
[54]R.R.Reinig,D.Mukherjee,Z.B.Weinstein,W.W.Xie,T.Albright,B.Baird,T.S.Gray,A.Ellern,G.J.Miller,A.H.Winter,S.L.Bud'ko,A.D.Sadow,Synthesis and oxidation catalysis of[tris(oxazolinyl)borato]cobalt(II)scorpionates,Eur.J.Inorg.Chem.(15-16)(2016)2486-2494.
[55]M.Balamurugan,E.Suresh,M.Palaniandavar,Non-heme μ-Oxo-and bis(μcarboxylato)-bridged diiron(III)complexes of a 3N ligand as catalysts for alkane hydroxylation:stereoelectronic factors of carboxylate bridges determine the catalytic efficiency,Dalton Trans.45(28)(2016)11422-11436.
[56]E.H.de Faria,G.P.Ricci,L.Marcal,E.J.Nassar,M.A.Vicente,R.Trujillano,A.Gil,S.A.Korili,K.J.Ciuffi,P.S.Calefi,Green and selective oxidation reactions catalyzed by kaolinite covalently grafted with Fe(III)pyridine-carboxylate complexes,Catal.Today187(1)(2012)135-149.
[57]S.Cheng,J.Li,X.X.Yu,C.C.Chen,H.W.Ji,W.H.Ma,J.C.Zhao,Selective activation of secondary C-H bonds by an iron catalyst:insights into possibilities created by the use of a carboxyl-containing bipyridine ligand,New J.Chem.37(10)(2013)3267-3273.
[58]I.Prat,A.Company,V.Postils,X.Ribas,L.Que,J.M.Luis,M.Costas,The mechanism of stereospecific CH oxidation by Fe(Pytacn)complexes:bioinspired non-Heme iron catalysts containing cis-labile exchangeable sites,Chem.Eur.J.19(21)(2013)6724-6738.
[59]M.Grau,A.Kyriacou,F.C.Martinez,I.M.de Wispelaere,A.J.P.White,G.J.P.Britovsek,Unraveling the origins of catalyst degradation in non-heme iron-based alkane oxidation,Dalton Trans.43(45)(2014)17108-17119.
[60]M.Chen,Y.Pan,H.K.Kwong,R.J.Zeng,K.C.Lau,T.C.Lau,Catalytic oxidation of alkanes by a(salen)osmium(VI)nitrido complex using H2O2as the terminal oxidant,Chem.Commun.51(71)(2015)13686-13689.
[61]S.Urus,H.Achguzel,M.Incesu,Synthesis of novel N4O4type bis(diazoimine)-metal complexes supported on mesoporous silica:microwave assisted catalytic oxidation of cyclohexane,cyclooctane,cyclohexene and styrene,Chem.Eng.J.296(2016)90-101.
[62]K.A.D.F.Castro,F.H.C.de Lima,M.M.Q.Simoes,M.G.P.M.S.Neves,F.A.A.Paz,R.F.Mendes,S.Nakagaki,J.A.S.Cavaleiro,Synthesis,characterization and catalytic activity under homogeneous conditions of ethylene glycol substituted porphyrin manganese(III)complexes,Inorg.Chim.Acta455(2017)575-583.
[63]A.P.C.Ribeiro,L.M.D.R.S.Martins,M.L.Kuznetsov,A.J.L.Pombeiro,Tuning cyclohexane oxidation:combination of microwave irradiation and ionic liquid with the CS corpionate[FeCl2(Tpm)]catalyst,Organometallics36(1)(2017)192-198.
[64]G.Huang,Z.C.Luo,Y.D.Hu,Y.A.Guo,Y.X.Jiang,S.J.Wei,Preparation and characterization of iron tetra(pentaflurophenyl)-porphyrin(TPFPP Fe)supported on boehmite(BM),Chem.Eng.J.195(2012)165-172.
[65]Y.Ide,N.Kawamoto,Y.Bando,H.Hattori,M.Sadakane,T.Sano,Ternary modified TiO2as a simple and efficient photocatalyst for green organic synthesis,Chem.Commun.49(35)(2013)3652-3654.
[66]K.Machado,J.Mishra,S.Suzuki,G.S.Mishra,Synthesis of superparamagnetic carbon nanotubes immobilized Pt and Pd pincer complexes:highly active and selective catalysts towards cyclohexane oxidation with dioxygen,Dalton Trans.43(46)(2014)17475-17482.
[67]Y.X.Jiang,T.M.Su,Z.Z.Qin,G.Huang,A zinc sulfide-supported iron tetrakis(4-carboxyl phenyl)porphyrin catalyst for aerobic oxidation of cyclohexane,RSC Adv.5(31)(2015)24788-24794.
[68]X.Liu,M.Conte,M.Sankar,Q.He,D.M.Murphy,D.Morgan,R.L.Jenkins,D.Knight,K.Whiston,C.J.Kiely,G.J.Hutchings,Liquid phase oxidation of cyclohexane using bimetallic Au-Pd/MgO catalysts,Appl.Catal.A Gen.504(2015)373-380.
[69]X.Wang,Y.W.Li,Nanoporous carbons derived from MOFs as metal-free catalysts for selective aerobic oxidations,J.Mater.Chem.A4(14)(2016)5247-5257.
[70]G.Huang,Y.Liu,J.L.Cai,X.F.Chen,S.K.Zhao,Y.A.Guo,S.J.Wei,X.Li,Heterogeneous biomimetic catalysis using iron porphyrin for cyclohexane oxidation promoted by chitosan,Appl.Surf.Sci.402(2017)436-443.
[71]D.X.Yang,T.B.Wu,C.J.Chen,W.W.Guo,H.Z.Liu,B.X.Han,The highly selective aerobic oxidation of cyclohexane to cyclohexanone and cyclohexanol over V2O5@TiO2under simulated solar light irradiation,Green Chem.19(1)(2017)311-318.
[72]L.F.Chen,Y.Y.Zhou,Z.Y.Gui,H.Y.Cheng,Z.W.Qi,Au nanoparticles con fined in hybrid shells of silica nanospheres for solvent-free aerobic cyclohexane oxidation,J.Mater.Sci.52(12)(2017)7186-7198.
[73]K.C.Hwang,A.Sagadevan,One-pot room-temperature conversion of cyclohexane to adipic acid by ozone and UV light,Science346(6216)(2014)1495-1498.
[74]A.P.C.Ribeiro,L.M.D.R.S.Martins,A.J.L.Pombeiro,N2O-free single-pot conversion of cyclohexane to adipic acid catalysed by an iron(II)scorpionate complex,Green Chem.19(6)(2017)1499-1501.
[75]X.Liu,M.Conte,Q.He,D.W.Knight,D.M.Murphy,S.H.Taylor,K.Whiston,C.J.Kiely,G.J.Hutchings,Catalytic partial oxidation of cyclohexane by bimetallic Ag/Pd nanoparticles on magnesium oxide,Chem.Eur.J.23(49)(2017)11834-11842.
[76]Y.P.Xiao,J.C.Liu,Y.T.Lin,W.J.Lin,Y.X.Fang,Novel graphene oxide-silver nanorod composites with enhanced photocatalytic performance under visible light irradiation,J.Alloys Compd.698(2017)170-177.
[77]L.M.D.R.S.Martins,M.P.de Almeida,S.A.C.Carabineiro,J.L.Figueiredo,A.J.L.Pombeiro,Heterogenisation of a C-Scorpionate Fe-II complex on carbon materials for cyclohexane oxidation with hydrogen peroxide,ChemCatChem5(12)(2013)3847-3856.
[78]A.Denicourt-Nowicki,A.Lebedeva,C.Bellini,A.Roucoux,Highly selective cycloalkane oxidation in water with ruthenium nanoparticles,ChemCatChem8(2)(2016)357-362.
[79]E.Tabor,J.Poltowicz,K.Pamin,S.Basag,W.Kubiak,Influence of substituents in mesoaryl groups of iron μ-oxo porphyrins on their catalytic activity in the oxidation of cycloalkanes,Polyhedron119(2016)342-349.
[80]Z.Feng,Y.J.Xie,F.Hao,P.L.Liu,H.A.Luo,Catalytic oxidation of cyclohexane to KA oil by zinc oxide supported manganese 5,10,15,20-tetrakis(4-nitrophenyl)porphyrin,J.Mol.Catal.A Chem.410(2015)221-225.
[81]K.Machado,S.Mukhopadhyay,G.S.Mishra,Nanoparticles silica anchored Cu-(II)and V-(IV)scorpionate complexes for selective catalysis of cyclohexane oxidation,J.Mol.Catal.A Chem.400(2015)139-146.
[82]S.Saxena,R.Singh,R.G.S.Pala,S.Sivakumar,Sinter-resistant gold nanoparticles encapsulated by zeolite nanoshell for oxidation of cyclohexane,RSC Adv.6(10)(2016)8015-8020.
[83]Z.Y.Gui,W.R.Cao,L.F.Chen,Z.W.Qi,Propene carbonate intensified cyclohexane oxidation over Au/SiO2catalyst,Catal.Commun.64(2015)58-61.
[84]W.Z.Zhong,T.Qiao,J.Dai,L.Q.Mao,Q.Xu,G.Q.Zou,X.X.Liu,D.L.Yin,F.P.Zhao,Visible light-responsive sulfated vanadium-doped TS-1 with hollow structure:enhanced photocatalytic activity in selective oxidation of cyclohexane,J.Catal.330(2015)208-221.
[85]Y.Fu,W.C.Zhan,Y.L.Guo,Y.Q.Wang,X.H.Liu,Y.Guo,Y.S.Wang,G.Z.Lu,Effect of surface functionalization of cerium-doped MCM-48 on its catalytic performance for liquidphase free-solvent oxidation of cyclohexane with molecular oxygen,Microporous Mesoporous Mater.214(2015)101-107.
[86]X.G.Duan,W.M.Liu,L.M.Yue,W.Fu,M.N.Ha,J.Li,G.Z.Lu,Selective oxidation of cyclohexane on a novel catalyst Mg-Cu/SBA-15 by molecular oxygen,Dalton Trans.44(39)(2015)17381-17388.
[87]K.Chen,Z.G.Chai,C.Li,L.R.Shi,M.X.Liu,Q.Xie,Y.F.Zhang,D.S.Xu,A.Manivannan,Z.F.Liu,Catalyst-free growth of three-dimensional graphene flakes and graphene/g-C3N4composite for hydrocarbon oxidation,ACS Nano10(3)(2016)3665-3673.
[88]X.X.Yang,H.J.Wang,J.Li,W.X.Zheng,R.Xiang,Z.K.Tang,H.Yu,F.Peng,Mechanistic insight into the catalytic oxidation of cyclohexane over carbon nanotubes:kinetic and in situ spectroscopic evidence,Chem.Eur.J.19(30)(2013)9818-9824.
[89]H.Wang,Y.Zhang,Y.Y.Guo,L.M.Zhang,Y.Han,X.X.Zhao,Visible-light-driven oxidation of cyclohexane using Cr-supported mesoporous catalysts prepared via phenyl functionalized mesoporous silica,RSC Adv.6(44)(2016)38176-38182.
[90]Y.Yuan,H.B.Ji,Y.X.Chen,Y.Han,X.F.Song,Y.B.She,R.G.Zhong,Oxidation of cyclohexane to adipic acid using Fe-porphyrin as a biomimetic catalyst,Org.Process.Res.Dev.8(3)(2004)418-420.
[91]Y.Chen,Y.She,J.Xu,Y.Li,Studies on QSAR of metalloporphyrin catalysts in the oxidation of cyclohexane to adipic acid,Front.Chem.Eng.China1(2)(2007)155-161.
[92]H.Li,Y.She,T.Wang,Advances and perspectives in catalysts for liquid-phase oxidation of cyclohexane,Front.Chem.Sci.Eng.6(3)(2012)356-368.
[93]T.Wang,Y.B.She,H.Y.Fu,H.Li,Selective cyclohexane oxidation catalyzed by manganese porphyrins and co-catalysts,Catal.Today264(2016)185-190.
[94]H.Li,Y.B.She,H.Y.Fu,M.J.Cao,J.Wang,T.Wang,Synergistic effect of co-reactant promotes one-step oxidation of cyclohexane into adipic acid catalyzed by manganese porphyrins,Can.J.Chem.93(7)(2015)696-701.
[95]M.J.Cao,Y.B.She,H.Y.Fu,Y.M.Yu,H.Li,T.Wang,Rate-limiting step of the iron porphyrin-catalysed oxidation of cyclohexane to adipic acid by DFT method,Mol.Simul.41(4)(2015)262-270.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
