Effect of copper precursors on the catalytic performance of Cu-ZSM-5 catalysts in N2O decomposition☆
Tao Meng ,Nan Ren *,Zhen Ma *
1 School of Chemical and Environmental Engineering,Shanghai Institute of Technology,Shanghai 201418,China
2 Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention(LAP3),Department of Environmental Science and Engineering,Fudan University,Shanghai 200433,China
3 Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials,Department of Chemistry,Fudan University,Shanghai 200433,China
4 Shanghai Institute of Pollution Control and Ecological Security,Shanghai 200092,China
1.Introduction
Nitrous oxide(N2O),one of NOxpollutants,contributes to global warming and the ozone-layer depletion[1,2].To eliminate N2O coming from industrial production and vehicle exhausts,one may subject the formed N2O to catalytic decomposition,i.e.,N2O is decomposed into N2and O2over a heterogeneous catalyst.
Initially,Iwamoto and co-workers[3]found that Cu-ZSM-5(Cu-exchanged ZSM-5 zeolite)is active for catalytic decomposition of NO(another NOxpollutant).Since then,many researchers have explored the application of a number of metal cation-exchanged zeolites in catalytic decomposition ofN2O[4-11].It was found that Cu-ZSM-5 shows higher activity than other transition metal cation-exchanged ZSM-5 zeolite(M-ZSM-5,where M=Ni,Mn,Pd,Ce,Zn,Cd)in N2O decomposition[5,12].Cu-ZSM-5 is also more active than some other Cu-exchanged zeolites(e.g.,Cu-FER,Cu-MOR,Cu-BEA,Cu-Y)in N2O decomposition[4].These observations demonstrate that Cu-ZSM-5 is a good candidate for catalytic decomposition of N2O.Moreover,Wanget al.[13]found that the catalytic activity of Cu-ZSM-5 for N2O decomposition increased with the Cu ion-exchange level,and the Cu+content on Cu-ZSM-5 is much higher than that on Cu-Y.Thus,Cu+has been regarded as the active center for catalytic decomposition of N2O,and the catalytic activity should be correlated to the number of Cu+species in Cu-exchanged zeolites.
The catalytic activity of Cu-ZSM-5 in N2O decomposition was found to be enhanced when decreasing the Si/Al ratio of Cu-ZSM-5,as explained by the increased amount of active Cu+species and the weakened reducibility of active sites[11].In addition,the catalytic performance of Cu-ZSM-5 can be tuned by adjusting its structures[14]or particle sizes[15].The structures of ZSM-5 samples can influence the dispersion of framework Al species,thus influencing the dispersion of active Cu species[14].Zouetal.[16]found that Cu-ZSM-5 nanosheets showed higher activity in N2O decomposition than conventional Cu-ZSM-5 particles,probably due to the better accessibility of Cu+active sites,better reducibility of Cu+species,and the weakened interaction between oxygen and Cu+for Cu-ZSM-5 nanosheets.
The catalytic activity and physicochemical properties of Cu-containing catalysts can be greatly influenced by Cu precursors used in the preparation process.For example,Sunet al.[17]prepared CuO/CeO2catalysts by impregnation using different Cu precursors(acetate,nitrate,chloride,and sulfate).Among these catalysts,CuO/CeO2prepared by using copper acetate as a Cu precursor was found to be the most active in CO oxidation,due to the presence of more finely dispersed CuO.Buláneket al.[18]prepared Cu-ZSM-5 by ion-exchange using Cu(CH3COO)2,Cu(NO3)2,or CuCl2as the Cu precursor.The authors found that Cu-ZSM-5 prepared using Cu(CH3COO)2exhibited high activity and high selectivity in total oxidation of propane.Yashniket al.[19]found that the Cu/Al ratio in Cu-ZSM-5 prepared from Cu(CH3COO)2was 2-3 times higher than those of the samples prepared from CuCl2,Cu(NO3)2,or CuSO4solutions,and the former Cu-ZSM-5 catalyst was more active than the latter catalysts(even with the same Cu/Al ratio)in selective catalytic reduction of NO by C3H8.As the amount of supported Cu species may influence catalytic activity,alternative Cu precursors for making Cu-ZSM-5 catalysts have been sought.It was found that more Cu species can be exchanged onto ZSM-5 when using copper-ammonia solution as the precursor[19,20].Cu-ZSM-5 catalysts prepared that way were found to be active for DeNOx[20].However,the influence of different Cu precursors on the performance of Cu-ZSM-5 in catalytic decomposition of N2O has been rarely studied systematically.
We previously studied the decomposition ofN2O on Silicalite-1@Cu-ZSM-5 core-shell structure[14]and Cu-ZSM-5 with different particle sizes[15].In both studies,Cu(CH3COO)2was used as the Cu precursor.In the current work,Cu-ZSM-5 catalysts were prepared using five different Cu precursors(CuCl2,Cu(NO3)2,CuSO4,Cu(CH3COO)2,and ammoniacal copper(II)complex ion),and their catalytic performance in N2O decomposition was compared.The effect of Cu precursors on the active Cu+species and catalytic performance are discussed based on the characterization results.
2.Experimental
2.1.Sample preparation
A commercial NH4-ZSM-5(Alfa-Aesar,Si/Al ratio=15)was used as the starting material for ion exchange.The commercial zeolite was first exchanged with NaNO3followed by Cu2+exchange[4,21].First,10 g NH4-ZSM-5 was dispersed in 1 L of0.1 mg·L-1aqueous NaNO3solution and the slurry was magnatically stirred at 80°C for 8 h.Then,the mixture was filtrated and washed by de-ionized water.This Na-exchange procedure was repeated three times,and the sample collected was dried at 100°C for 8 h.
A defined amount of Na-ZSM-5 sample was added into an appropriate amount of 0.1 mg·L-1aqueous solution of a copper salt(with the liquid to solid ratio of150 mlsolution per gram of Na-ZSM-5)under vigorous stirring.The copper salts used were Cu(CH3COO)2,Cu(NO3)2,CuSO4,CuCl2,and ammoniacal copper(II)complex ion(prepared by adding concentrated aqueous ammonia to CuSO4solution till a stable dark blue color appeared).
The mixture was then stirred continuously at room temperature for 24 h,and it was then washed three times with de-ionized water(200 ml water for each time)except when using ammoniacal copper(II)complex ion,the sample was washed with 0.1 mg·L-1aqueous ammonia solution.The ion exchange procedure was repeated for three times.
The Cu-ZSM-5 samples prepared from Cu(NO3)2,CuSO4,CuCl2,Cu(CH3COO)2,or ammoniacal copper(II)complex ion precursors are denoted as CZM-CN,CZM-CS,CZM-CC,CZM-CA,and CZM-AC(II),respectively.
2.2.Catalyst characterization
X-ray diffraction(XRD)experiments were conducted on a PANalytical X'Pert diffractometer(operated at 40 kV and 40 mA)with Ni β- filtered Cu Kαradiation.The scanning speed was 6(°)·min-1.Inductively coupled plasma atomic emission spectrometer(ICP-AES,Electron IRIS Intrepid II XSP,Thermo)was used to determine the Cu and Al contents of catalysts.Transmission electron microscopy(TEM)and EDX-mapping experiments were conducted on a FEI Tecani G2F20 S-Twin instrument.N2adsorption-desorption experiments at 77 K were conducted using a Micrometrics ASAP 2020 M+C adsorption apparatus.
In situDRIFTS spectra of adsorbed CO were recorded on a FTIR spectrometer(Nicolet 6700)equipped with an MCT detector cooled by liquid N2.Cu-ZSM-5,placed in an IR cell,was pretreated in He(50 ml/min)at room temperature,250,or 400°C for 2 h.The sample was then cooled down to room temperature,and exposed to flowing 0.2 vol%CO-He mixture for 1 h.The sample was then swept by flowing He flow at room temperature for 1 h,and the CO-adsorbed IR spectra(with a resolution of 4 cm-1and accumulation of 100 scans)were recorded subsequently.
2.3.Catalytic evaluation
Catalytic decomposition of N2O was carried out in fixed-bed microreactor under atmospheric pressure.Prior to catalytic reaction testing,0.2 g catalyst(40-60 mesh)was fixed in a U-shape quartz tube,and pretreated in the microreactor by He(50 ml·min-1)at 500°C for 2 h.
After undergoing pretreatment in He,the catalyst was cooled down to room temperature,and the reactant gas mixture(0.5%N2O,with balance He)with a total flow rate of 60 ml·min-1was flowed through the catalyst bed for 1 h.The reaction temperature was subsequently ramped to 100,150,200,250,275,300,325,350,375,400,425,450,475,and 500°C,and maintained at each temperature step for 0.5 h.The effluent gas was analyzed online with a gas chromatograph(7890A,Agilent)equipped with a TCD detector[14].
3.Results
3.1.Catalytic activity in N2O decomposition

Fig.1.N2O conversion over different Cu-ZSM-5 zeolites.
Fig.1 shows N2O conversions over Cu-ZSM-5 catalysts,as tested as a function of reaction temperature.CZM-CS,CZM-CN,and CZM-CC show similar catalytic activity for N2O decomposition.These three samples are virtually inactive below 350°C,and then the N2O conversion increases as the reaction temperature rises above 350°C and reaches about 90%at 500°C.CZM-CA is more active than these three catalysts mentioned above,initially being active above 325°C and showing almost complete N2O conversion at 425°C.The most active catalyst among these five Cu-ZSM-5 samples is CZM-AC(II).The N2O conversion on CZM-AC(II)increases above 250 °C,and it is close to 100%at 400 °C.The T50(temperature required for 50%conversion)values of CZM-CS,CZM-CN,CZM-CC,CZM-CA,and CZM-AC(II)are 430,437,430,375,and 358°C,respectively.The data indicate that the influence of different Cu precursors is significant.In addition,CZM-AC(II)is more active than Fe-ZSM-5(Fe content=3.83 wt%)and a commercial Co3O4(Fig.S1).
3.2.Basic physicochemical properties of Cu-ZSM-5 samples
Table 1 shows the Cu contents of five Cu-ZSM-5 samples,as determined by ICP analysis.The Cu contents of these samples follow the sequence of CZM-AC(II)(9.4 wt%)>CZM-CA(2.8 wt%)>CZM-CS(1.7 wt%)≈CZM-CN(1.7 wt%)≈CZM-CC(1.6 wt%).The Cu/Al molar ratios of these catalysts follow the same sequence.The data mean that the Cu contents of the samples prepared using different Cu precursors can differ significantly,and such a difference may influence the catalytic activity.Because CZM-AC(II)is the most active catalyst,CZM-CA is the second most active catalyst,whereas CZM-CS,CZM-CN,and CZM-CC show similar activities and Cu contents,we picked CZM-AC(II),CZMCA,and CZM-CS for further characterization.
Table 2 shows the surface areas and pore volumes of representative Cu-ZSM-5 samples.The surface areas of CZM-AC(II),CZM-CA,and CZM-CS are 194,231,and 365 m2·g-1,respectively,indicating that the existence of more Cu species can decrease the surface area significantly.As seen in Table 2,the existence of more Cu species can also decrease the pore volume obviously.
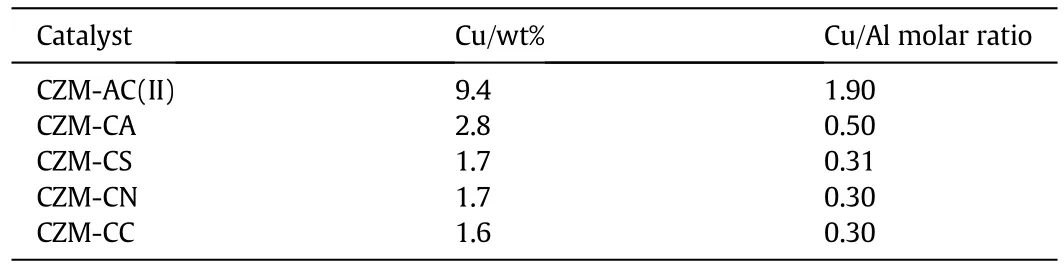
Table 1 Cu contents and Cu/Al molar ratios of Cu-ZSM-5 samples

Table 2 Surface areas and pore volumes of Cu-ZSM-5 samples
Fig.2 shows the XRD patterns of three representative Cu-ZSM-5 samples collected after being treated in He at500°C for2 h.Such a treatment is adopted in our catalytic studies prior to reaction testing.As shown in Fig.2,the MFI zeolite structure is preserved after ion-exchange.No obvious peaks other than those of zeolites can be observed in CZM-CA and CZM-CS,but some small peaks(2θ=35.5°,38.8°)corresponding to CuO are observed in CZM-AC(II).The observation is consistent with the low Cu contents(2.8 wt%and 1.7 wt%,respectively)of CZM-CA and CZM-CS as well as high Cu content(9.4 wt%)of CZM-AC(II).
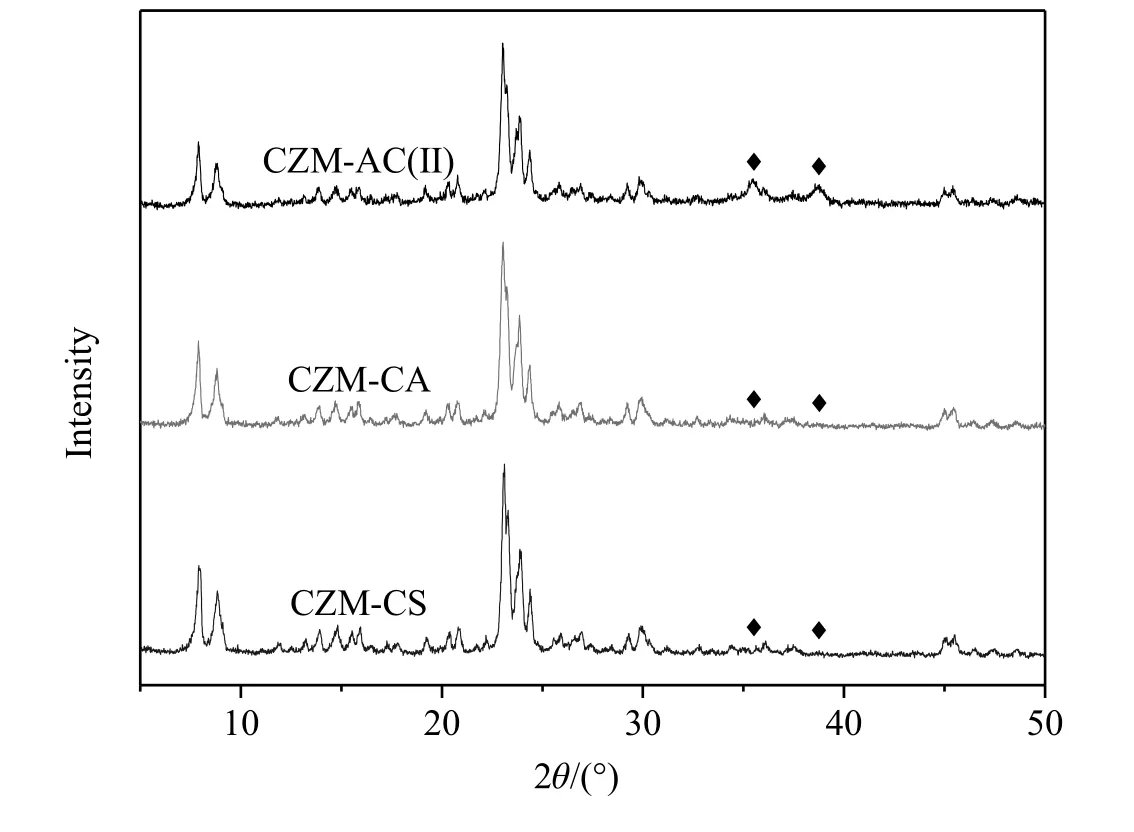
Fig.2.XRD patterns of different Cu-ZSM-5 zeolites after pretreatment.
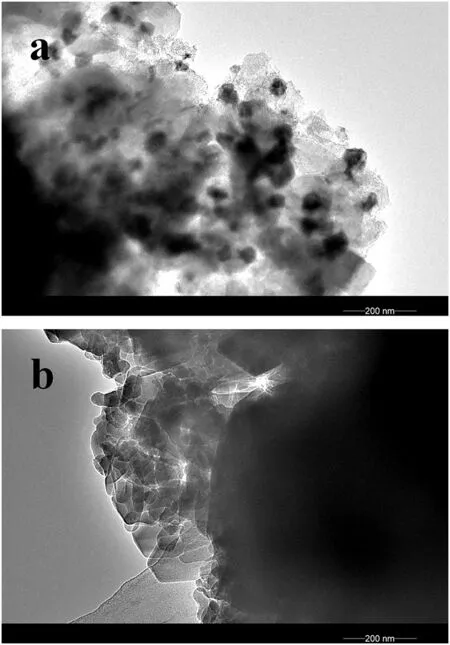
Fig.3.TEM images of fresh CZM-AC(II)(a)and CZM-CS(b)after pretreatment.
Fig.3 displays the TEM images of CZM-AC(II)and CZM-CS.There are lots of nanoparticles dispersed on CZM-AC(II)after being pretreated in He at 500°C,and most of the nanoparticles are as large as 50 nm(Fig.3a).Combined with XRD diffraction peaks,these nanoparticles should be CuO.In contrast,no particles are seen on pretreated CZM-CS(Fig.3b),indicating that Cu species disperse highly on the surface.
TEM-mapping analysis was further used to investigate the existing form of the Cu species.For pretreated CZM-AC(II)(Fig.4),besides the well dispersed Cu species,there are still aggregated Cu species,most likely CuO nanoparticles.However,Cu species are well dispersed on He-pretreated CZM-CS(Fig.5).
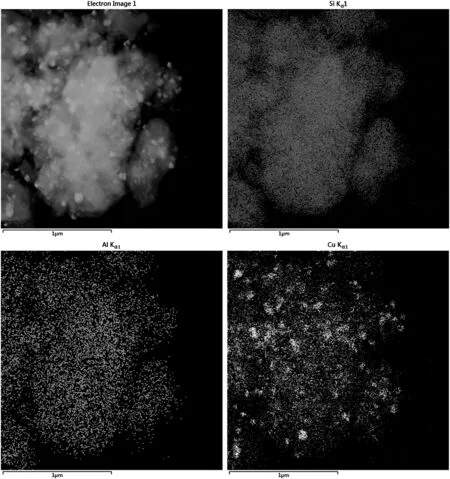
Fig.4.TEM-Mapping images of CZM-AC(II)after pretreatment.
3.3.CO-IR
Cu-ZSM-5 samples prepared by different Cu precursors show different catalytic activities in N2O decomposition(Fig.1).It is obvious that the catalytic activities(in view of the N2O conversions)of Cu-ZSM-5 samples increase with the Cu contents(Table 1).However,not all of the Cu species are catalytically active.Therefore,in situFT-IR experiments using COas a probe were conducted to clarify the nature of active sites.The oxidation state,the coordination state,and the concentration ofCu species can be determined by the positions and intensities of the IR peaks ascribed to adsorbed CO.
Fig.6 presents the DRIFTS spectra of CO adsorbed on CZM-AC(II).These experiments are individual experiments using CZM-AC(II)from the same batch.In each experiment,CZM-AC(II)was pretreated in the IR cell with flowing He at a certain temperature,cooled down,allowed for CO adsorption,and the IR spectra were recorded at room temperature.The fresh CZM-AC(II)(without being heated in He)showed no CO peaks at 2000-2300 cm-1.The Cu species of fresh CZM-AC(II)may exist in the form of Cu2+and CO does not interact with Cu2+at room temperature[22,23].CZM-AC(II)exhibited two peaks at 2138 cm-1(assigned to CO adsorbed on dimeric Cu+(Cu+-CO-Cu+)sites[11,24-26])and 2157 cm-1(assigned to isolated Cu+(Cu+-CO)sites[11,24-28])when the pretreatment temperature was 250°C and above.The intensities of both peaks increased significantly with the pretreatment temperature was increased from 250 to 400°C,indicating that the pretreatment of Cu-ZSM-5 in He at elevated temperatures can lead to the reduction of some framework Cu2+to Cu+(including dimeric and isolated Cu+)and the amount of Cu+increases with the pretreatment temperature.
Fig.7 and Table 3 compare the CO-IR spectra ofCZM-AC(II),CZM-CA,and CZM-CS pretreated in He at 400°C.CZM-CA and CZM-CS also contain dimeric Cu+(Cu+-CO-Cu+)at 2138 cm-1and isolated Cu+(Cu+-CO)at 2157 cm-1,indicating that reduction of some framework Cu2+to Cu+during thermal treatment in He is common for these samples.Nevertheless,both the peak area of dimeric Cu+and the total peaks area follow the sequence of CZM-AC(II)>CZM-CA>CZM-CS(Table 3).The data correlated nicely with the catalytic activity,as will be discussed later.

Fig.5.TEM-mapping images of CZM-CS after pretreatment.
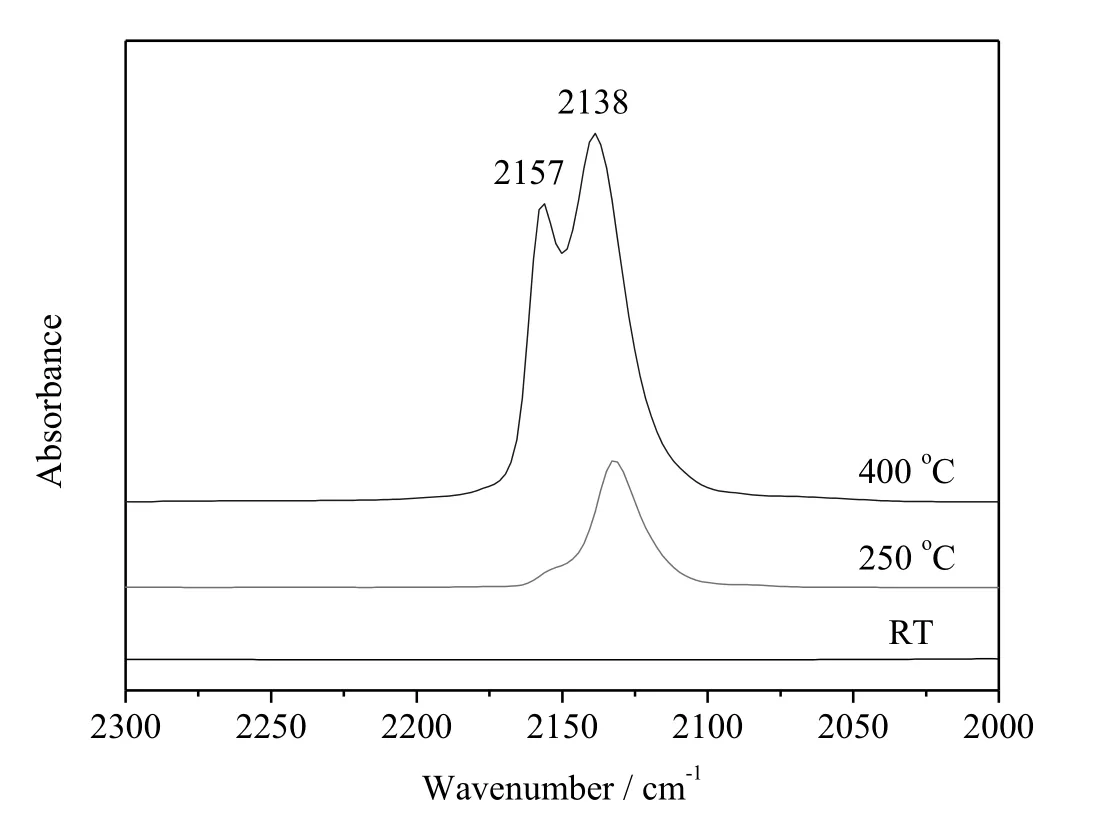
Fig.6.CO-IR spectra for room-temperature CO adsorption on of CZM-AC(II)pretreated in He at different temperatures.
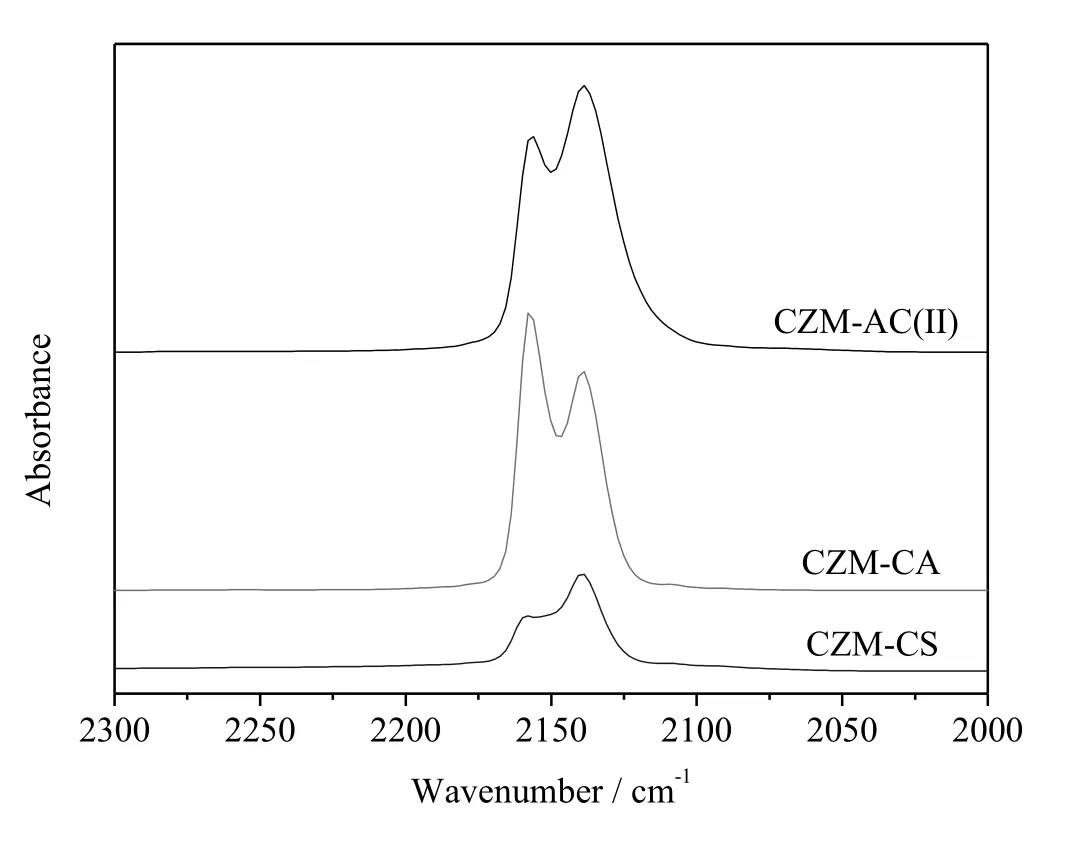
Fig.7.CO-IR spectra for room-temperature CO adsorption on of Cu-ZSM-5 samples pretreated in He at 400°C.
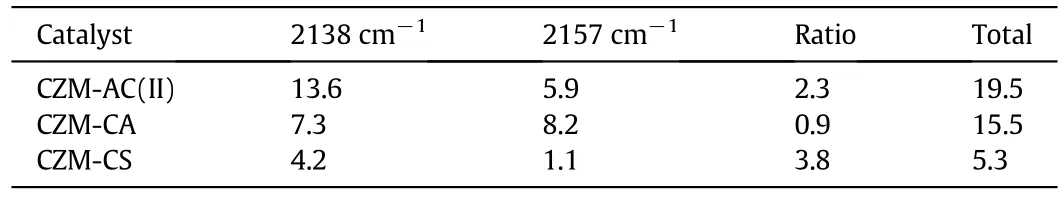
Table 3 In situ CO-IR data of pretreated Cu-ZSM-5 zeolites
3.4.Effect of co-fed O2 or H2O on catalytic performance
As the presence of impurity gases(e.g.,O2,H2O)in the actual environment is unavoidable and may influence the catalytic activity,we then investigated the influence of these gases.The influence of co-fed 5%O2or 2%H2O on the catalytic performance of CZM-AC(II),CZM-CA,and CZM-CS was investigated at a fixed reaction temperature of 390°C.O2(5%)or H2O(2%)was switched on and off periodically during the reaction at 390°C.As shown in Figs.8,S2,and S3,in the first step(0-2 h)when no O2or H2O was added,the N2O conversions for each catalyst at 390°C were consistent with the values seen in Fig.1.However,the N2O conversion decreased dramatically when O2or H2O was added into the reaction mixture(2-4 h).By removing O2or H2O in the third step(4-6 h),the N2O conversion was partially recovered.A similar trend was observed in an additional cycle.After two cycles of periodic addition of O2or H2O,the N2O conversions(8-10 h)of CZM-AC(II),CZM-CA,and CZM-CS decreased.

Fig.8.N2O Conversion over CZM-AC(II)in the presence of O2 or H2O.
3.5.Catalytic stability
Fig.S4 shows N2O conversion over different Cu-ZSM-5 samples in 2-cycle experiments.In each set of experiment,the catalyst was tested in the first cycle as described in Section 2.3,cooled down to room temperature,and tested again.The T50values of these catalysts in the second cycle clearly show the sequence of CZM-AC(II)>CZM-CA>CZM-CS,consistent with the trend seen in the first cycle.A marginal decrease in the N2O conversion was observed for all catalysts.
The catalytic stability was also compared by testing the N2O conversion as a function of reaction time at390°C.As shown in Fig.9,the initial N2O conversions of CZM-AC(II),CZM-CA,and CZM-CS are 91%,75%,and 24%,respectively.The N2O conversion over CZM-AC(II),CZM-CA,and CZM-CS decreased gradually with the reaction time.However,their activities still follow the sequence ofCZM-AC(II)>CZM-CA>CZM-CS during 50 h testing.
4.Discussion
Here we prepared Cu-ZSM-5 catalysts using different Cu precursors(CuCl2,Cu(NO3)2,CuSO4,Cu(CH3COO)2,or ammoniacal copper(II)complex ion)by ion-exchange.The activities of these catalysts in N2O decomposition follow the sequence of CZM-AC(II)>CZM-CA>CZMCS≈CZM-CN≈CZM-CC(Fig.1).
CRP电子文件是指高校从事教学、科研、管理等活动形成的文字、图表、图像、音频、视频、数据库等不同形式的电子信息记录,以及履行公共服务等职能活动在网上办事大厅通过申请、受理、办理、办结等办理事项过程中形成、获取的电子文件。高校CRP平台实现了审批事项集中进驻、网上服务集中提供、数据资源集中共享。但是,CRP平台毕竟只是一个集成的业务系统,它只关注于业务的形成和办理,对于办理完毕的电子文件的凭证价值、归档保存和安全利用等问题并未做重点关注,随着高校CRP平台建设的全面推进,产生的电子文件越来越多,电子文件的归档问题越来越凸显。
Both the Cu contents(≈1.7 wt%)and Cu/Al molar ratio(≈0.30)of CZM-CS,CZM-CN,and CZM-CC are fairly similar,whereas they are much lower than those of CZM-AC(II)and CZM-CA(Table 1).This can be explained by the fact that in 0.1 mol·L-1CuSO4,Cu(NO3)2,or CuCl2solutions used for ion-exchange,Cu2+exists as hydrated mononuclear ions[Cu(H2O)6]2+and[Cu(OH)(H2O)5]+[19].For comparison,a significant portion of Cu2+species in 0.1 mol·L-1Cu(CH3COO)2solution exist as binuclear hydroxocomplexes[Cu2(OH)2(H2O)8]2+[19,20,29],thus leading to the increase of the Cu content(2.8 wt%)and Cu/Al molar ratio(0.50)of CZM-CA.
As the pH value of CuSO4solution is raised when adding aqueous ammonia,dinuclear and polynuclear hydroxocopper cations are formed by partialhydrolysis and polycondensation of hydrated copper ions[19,20,30].Thus,the highest Cu content(9.4 wt%)is obtained when Cu-ZSM-5 is prepared from ammoniacal copper(II)complex ion solution(CZM-AC(II)).An excess of ion exchange level over the theoretically stoichiometric value(Cu/Al=1.90 instead of 0.5)is clearly observed in Table 1.As shown in Fig.S5,there are some nanoparticles(about 5-10 nm)on the surface of fresh CZM-AC(II)(without being heated in He),con firming the formation of large copper polyhydroxocomplexes.These nanoparticles on fresh CZM-AC(II)aggregate to form big CuO particles upon pretreating in He at 500°C(Figs.2-4).In contrast,no CuO particles is found on CZM-CS with low Cu content(Cu/Al=0.3)(Figs.2,3,5).
Not allCu species are involved in catalytic N2O decomposition.Maet al.found that mesoporous CuO(composed of CuO nanoparticles)and commercial bulk CuO are not active at all for this reaction[31].On the other hand,Cu+has been widely recognized as the active center for N2O decomposition[22,23,32-36].In Cu-ZSM-5,Cu2+is reduced to Cu+(including isolated and dimeric Cu+)gradually by thermal treatment in He flow at 250°C and above(Fig.6).The contents of dimeric Cu+and total Cu+,as derived from the integrated areas of the IR band at ca.2157 cm-1and 2138 cm-1,follow the sequence of CZM-AC(II)>CZM-CA>CZM-CS(Fig.7,Table 2),consistent with the activity trend(Fig.1).Note that the specific rates(the moles of the N2O molecules decomposed per gramof Cu per hour)of catalysts at375°C follow the sequence of CZM-CA>CZM-AC(II)>CZM-CS≈CZM-CN≈CZM-CC(Table S1).The fact that CZM-AC(II)shows a lower specific rate than CZM-CA is due to the presence of a number of inactive CuO nanoparticles on CZM-AC(II).
N2O decomposition entails the adsorption of N2O on active sites followed by decomposition,resulting in the formation of N2and surface oxygen.The surface oxygen can be desorbed by combining with another oxygen atom or by directly reacting with another N2O[37-40].Cu+in Cu-exchanged zeolite catalysts has been widely recognized as the active center for N2O decomposition,and Cu+includes isolated Cu+and dimeric Cu+.The distance between dimeric Cu+sites is shorter that the distance between isolated Cu+sites,so oxygen atoms can combine on dimeric Cu+sites more easily,thus favoring N2O decomposition[4,34,41].Therefore,CZM-AC(II)shows the highest activity in N2O decomposition because of its high dimeric Cu+content.
A negative effect of O2or H2O on the N2O decomposition is observed for all Cu-ZSM-5 catalysts(Fig.8 and Figs.S2-S3),in agreement with our previous results[14,15].In our experiment,the release of O2may be restricted by co-fed O2.The periodical exposure to O2results in a partial deactivation of all Cu-ZSM-5,which may be caused by the oxidation of a portion of Cu+[16].The obvious inhibiting effect of H2O is consistent with the literature[41-43].The catalytic activity of all Cu-ZSM-5 decrease in the presence of H2O,most likely due to competitive adsorption of H2O on the active Cu sites[41].Of course,the dealumination of Cu-ZSM-5 or the sintering of Cu ions may also lead to deactivation in the long run[43-45].
The activity of each Cu-ZSM-5 in the second cycle of N2O decomposition is similar to that in the first cycle(Fig.S4),probably because the reaction period(especially the period during which the catalyst is exposed to the reaction ambient at high temperatures)is relatively short.In contrast,after 50 h reaction at 390°C,the N2O conversions on CZM-AC(II),CZM-CA,and CZM-CS decrease more obviously(Fig.9).The oxidation of a portion of Cu+by O2produced from N2O decomposition at 390°C may be responsible for the loss of catalytic activity[14].
5.Conclusions
Supplementary Material
Supplementary data to this article can be found online athttps://doi.org/10.1016/j.cjche.2018.02.015.
[1]S.Parres-Esclapez,I.Such-Basañez,M.J.Illán-Gómez,C.Salinas-Martínez de Lecea,A.Bueno-López,Study by isotopic gases and in situ spectroscopies(DRIFTS,XPS and Raman)of the N2O decomposition mechanism on Rh/CeO2and Rh/γ-Al2O3catalysts,J.Catal.276(2010)390-401.
[2]Y.Lin,T.Meng,Z.Ma,Catalytic decomposition of N2O over RhOxsupported on metal phosphates,J.Ind.Eng.Chem.28(2015)138-146.
[3]M.Iwamoto,H.Furukawa,Y.Mine,F.Uemura,S.Mikuriya,S.Kagawa,Copper(II)ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide,J.Chem.Soc.Chem.Commun.(1986)1272-1273.
[4]P.J.Smeets,M.H.Groothaert,R.M.van Teeffelen,H.Leeman,E.J.M.Hensen,R.A.Schoonheydt,Direct NO and N2O decomposition and NO-assisted N2O decomposition over Cu-zeolites:Elucidating the influence of the Cu-Cu distance on oxygen migration,J.Catal.245(2007)358-368.
[5]B.M.Abu-Zied,W.Schwieger,A.Unger,Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion-exchange method,Appl.Catal.B84(2008)277-288.
[6]J.K.Lee,Y.J.Kim,H.J.Lee,S.H.Kim,S.J.Cho,I.S.Nam,S.B.Hong,Iron-substituted TNU-9,TNU-10,and IM-5 zeolites and their steam-activated analogs as catalysts for direct N2O decomposition,J.Catal.284(2011)23-33.
[7]X.Y.Zhang,Q.Shen,C.He,Y.F.Wang,J.Cheng,Z.P.Hao,CoMOR zeolite catalyst prepared by buffered ion exchange for effective decomposition of nitrous oxide,J.Hazard.Mater.192(2011)1756-1765.
[8]X.Y.Zhang,Q.Shen,C.He,C.Y.Ma,J.Cheng,Z.M.Liu,Z.P.Hao,Decomposition of nitrous oxide over co-zeolite catalysts:role of zeolite structure and active site,Catal.Sci.Technol.2(2012)1249-1258.
[9]N.Liu,R.D.Zhang,B.H.Chen,Y.P.Li,Y.X.Li,Comparative study on the direct decomposition of nitrous oxide over M(Fe,Co,Cu)-BEA zeolites,J.Catal.294(2012)99-112.
[10]Q.Xiao,F.F.Yang,J.Zhuang,G.P.Qiu,Y.J.Zhong,W.D.Zhu,Facile synthesis of uniform FeZSM-5 crystals with controlled size and their application to N2O decomposition,Microporous Mesoporous Mater.167(2013)38-43.
[11]P.F.Xie,Z.Ma,H.B.Zhou,C.Y.Huang,Y.H.Yue,W.Shen,H.L.Xu,W.M.Hua,Z.Gao,Catalytic decomposition of N2O over Cu-ZSM-11 catalysts,Microporous Mesoporous Mater.191(2014)112-117.
[12]Y.Li,J.N.Armor,Catalytic decomposition of nitrous oxide on metal exchanged zeolites,Appl.Catal.B1(1992)L21-L29.
[13]J.Wang,N.Mizuno,M.Misono,Comparison of catalytic decomposition of dinitrogen oxide and nitrogen monoxide over Cu/ZSM-5 and Cu/Y zeolites,Bull.Chem.Soc.Jpn.71(1998)947-954.
[14]T.Meng,N.Ren,Z.Ma,Silicalite-1@Cu-ZSM-5 core-shell catalyst for N2O decomposition,J.Mol.Catal.A404-405(2015)233-239.
[15]T.Meng,Y.Lin,Z.Ma,Effect of the crystal size of Cu-ZSM-5 on the catalytic performance in N2O decomposition,Mater.Chem.Phys.163(2015)293-300.
[16]W.Zou,P.F.Xie,W.M.Hua,Y.D.Wang,D.J.Kong,Y.H.Yue,Z.Ma,W.M.Yang,Z.Gao,Catalytic decomposition of N2O over Cu-ZSM-5 nanosheets,J.Mol.Catal.A394(2014)83-88.
[17]S.S.Sun,D.S.Mao,J.Yu,Z.Q.Yang,G.Z.Lu,Z.Ma,Low-temperature CO oxidation on CuO/CeO2catalysts:The significant effect of copper precursor and calcination temperature,Catal.Sci.Technol.5(2015)3166-3181.
[18]R.Bulánek,B.Wichterlová,Z.Sobalík,J.Tichý,Reducibility and oxidation activity of Cu ions in zeolites:Effect of Cu ion coordination and zeolite framework composition,Appl.Catal.B31(2001)13-25.
[19]S.A.Yashnik,Z.R.Ismagilov,V.F.Anufrienko,Catalytic properties and electronic structure of copper ions in Cu-ZSM-5,Catal.Today110(2005)310-322.
[20]S.Yashnik,Z.Ismagilov,Cu-substituted ZSM-5 catalyst:Controlling of DeNOxreactivity via ion-exchange mode with copper-ammonia solution,Appl.Catal.B170-171(2015)241-254.
[21]M.H.Groothaert,P.J.Smeets,B.F.Sels,P.A.Jacobs,R.A.Schoonheydt,Selective oxidation of methane by the bis(μ-oxo)dicopper core stabilized on ZSM-5 and mordenite zeolites,J.Am.Chem.Soc.127(2005)1394-1395.
[22]Z.R.Ismagilov,R.A.Shkrabina,L.T.Tsikoza,S.A.Yashnik,V.A.Sazonov,V.V.Kuznetsov,M.V.Luzgin,A.V.Kalinkin,H.Veringa,The stability of monolith CuZSM-5 catalysts for the selective reduction of nitrogen oxides with hydrocarbons:I.Synthesis and characterization of bulk CuZSM-5 catalysts,Kinet.Catal.42(2001)847-853.
[23]H.B.Zhou,Z.Huang,C.Sun,F.Qin,D.S.Xiong,W.Shen,H.L.Xu,Catalytic decomposition of N2O over CuxCe1-xOymixed oxides,Appl.Catal.B125(2012)492-498.
[24]K.I.Hadjiivanov,M.M.Kantcheva,D.G.Klissurski,IR study of CO adsorption on Cu-ZSM-5 and CuO/SiO2catalysts:σ and π components of the Cu+-CO bond,J.Chem.Soc.Faraday Trans.92(1996)4595-4600.
[25]T.T.H.Dang,H.L.Zubowa,U.Bentrup,M.Richter,A.Martin,Microwave-assisted synthesis and characterization of Cu-containing AlPO4-5 and SAPO-5,Microporous Mesoporous Mater.123(2009)209-220.
[26]L.Wang,W.Li,G.S.Qi,D.Weng,Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3,J.Catal.289(2012)21-29.
[27]J.Y.Yan,G.D.Lei,W.M.H.Sachtler,H.H.Kung,Deactivation of Cu/ZSM-5 catalysts for lean NOxreduction:Characterization of changes of Cu state and zeolite support,J.Catal.161(1996)43-54.
[28]Y.Kuroda,Y.Yoshikawa,R.Kumashiro,M.Nagao,Analysis of active sites on copper ion-exchanged ZSM-5 for CO adsorption through IR and adsorption-heat measurements,J.Phys.Chem.B101(1997)6497-6503.
[29]C.F.Baes Jr.,R.F.Mesmer,The hydrolysis of cations,Wiley/Interscience,New York,1976.
[30]L.T.Tsikoza,E.V.Matus,Z.R.Ismagilov,V.A.Sazonov,V.V.Kuznetsov,Correlation between the Cu/Al value and activity of Cu-ZSM-5 catalysts and the chemical nature of the starting copper salt,Kinet.Catal.46(2005)613-617.
[31]Z.Ma,Y.Ren,Y.B.Lu,P.G.Bruce,Catalytic decomposition of N2O on ordered crystalline metal oxides,J.Nanosci.Nanotechnol.13(2013)5093-5103.
[32]M.V.Konduru,S.S.C.Chuang,Dynamics of NO and N2O decomposition over Cu-ZSM-5 under transient reducing and oxidizing conditions,J.Catal.196(2000)271-286.
[33]P.E.Fanning,M.A.Vannice,A DRIFTS study of Cu-ZSM-5 prior to and during its use for N2O decomposition,J.Catal.207(2002)166-182.
[34]X.Liu,Z.Y.Yang,Y.P.Li,F.Z.Zhang,Theoretical study of N2O decomposition mechanism over binuclear Cu-ZSM-5 zeolites,J.Mol.Catal.A396(2015)181-187.
[35]L.Chen,H.Y.Chen,J.Lin,K.L.Tan,FTIR,XPS and TPR studies of N2O decomposition over Cu-ZSM-5,Surf.Interface Anal.28(1999)115-118.
[36]E.S.Shpiro,W.Grunert,R.W.Joyner,G.N.Baeva,Nature,distribution and reactivity of copper species in over-exchanged Cu-ZSM-5 catalysts:An XPS/XAES study,Catal.Lett.24(1994)159-169.
[37]F.Kapteijn,G.Marbán,J.Rodriguez-Mirasol,J.A.Moulijn,Kinetic analysis of the decomposition of nitrous oxide over ZSM-5 catalysts,J.Catal.167(1997)256-265.
[38]G.Mul,J.Pérez-Ramírez,F.Kapteijn,J.A.Moulijn,NO-assisted N2O decomposition over ex-framework FeZSM-5:mechanistic aspects,Catal.Lett.77(2001)7-13.
[39]E.V.Kondratenko,J.Pérez-Ramírez,Mechanism and kinetics of direct N2O decomposition over Fe-MFI zeolites with different iron speciation from temporal analysis of products,J.Phys.Chem.B110(2006)22586-22595.
[40]C.M.Fu,V.N.Korchak,W.K.Hall,Decomposition of nitrous oxide on FeY zeolite,J.Catal.68(1981)166-171.
[41]P.J.Smeets,B.F.Sels,R.M.van Teeffelen,H.Leeman,E.J.M.Hensen,R.A.Schoonheydt,The catalytic performance of Cu-containing zeolites in N2O decomposition and the influence of O2,NO and H2O on recombination of oxygen,J.Catal.256(2008)183-191.
[42]M.Kögel,V.H.Sandoval,W.Schwieger,A.Tissler,T.Turek,Catalytic activity and hydrothermal stability of Cu-ZSM-5 used for the decomposition of N2O,Chem.Eng.Technol.21(1998)655-658.
[43]B.I.Palella,M.Cadoni,A.Frache,H.O.Pastore,R.Pirone,G.Russo,S.Coluccia,L.Marchese,On the hydrothermal stability of CuAPSO-34 microporous catalysts for N2O decomposition:A comparison with CuZSM-5,J.Catal.217(2003)100-106.
[44]A.Frache,B.I.Palella,M.Cadoni,R.Pirone,H.O.Pastore,L.Marchese,CuAPSO-34 catalysts for N2O decomposition in the presence of H2O.A study of zeolitic structure stability in comparison to Cu-SAPO-34 and Cu-ZSM-5,Top.Catal.22(2003)53-57.
[45]B.I.Palella,R.Pirone,G.Russo,A.Albuquerque,H.O.Pastore,M.Cadoni,A.Frache,L.Marchese,On the activity and hydrothermal stability of CuMCM-22 in the decomposition of nitrogen oxides:A comparison with CuZSM-5,Catal.Commun.5(2004)191-194.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
