Kinetic contribution of CO2/O2 additive in methane conversion activated by non-equilibrium plasmas☆
Qi Chen*,Jintao Sun,Xiaojun Zhang
School of Mechanical and Electronic Control Engineering,Beijing Jiaotong University,Beijing 100044,China
1.Introduction
During the past decades,there has been a growing interest on developing technologies taking advantage of clean energy sources to reduce the atmospheric pollution and the emission of greenhouse gases.Among the new technologies for greenhouse gas reduction,plasmachemical methods of hydrocarbon fuel reforming are considered one of the most promising,and have the ability to efficiently convert methane[1,2],carbon dioxide and other greenhouse species into hydrogen-rich syngas and useful hydrocarbon products[3-6].A number of works have accordingly appeared on the experimental and prediction study of plasma reforming of hydrocarbons by means of gas discharges of various types such as radio frequency discharge(RF)[7,8],arc and gliding arc discharges[9-11],microwave discharge[12-15],nano-second pulsed discharge(NSD)[16-18],and so on[19,20].
The hydrocarbon reforming is currently an oxidation reaction in which oxygen,carbon dioxide or water plays the role of the oxidant.Three main reforming reactions are possible:partial oxidation,steam conversion,and dry reforming[2,4].Partial oxidation of methane is described by the global reaction 2CH4+O2→2CO+4H2with energy release 71 kJ·mol-1.The dry reforming process is defined by the global reaction CH4+CO2→2CO+2H2,which requires the highest input energy 247 kJ·mol-1.And the process of steam conversion,CH4+H2O→CO+3H2,is also highly endothermic and requires large input energy 206 kJ·mol-1[21,22].Traditional CH4conversion depends on catalytic processes,which are normally carried out under high reaction temperatures or high-pressure conditions[23,24].Comparatively,nonequilibrium plasma assisted low temperature CH4reforming can take the advantage of higher electron energy with low neutral gas temperature in plasmas,and the chemical reactions for fuel reforming can therefore proceed at much lower temperatures than that in the conventional catalytic reforming processes[1,3,18,19].On the other hand,plasmaassisted reforming processes for hydrocarbon fuels have advantages over catalytic processes because they often successfully stimulate the chemical reactions impossible in conventional chemistry and permit essential increases of system efficiency.Many experimental and simulation studies have been carried out to understand the role of plasma generated composition on fuel reforming chemistry.A.M.Stariket al.[25]numerically studied the kinetic processes responsible for methane or methane-steam partial oxidation stimulated by non-equilibrium oxygen discharge plasma comprising highly reactive species as O2(a1Δg),O2(b1)molecules and O atoms.The results suggested the
minor induction zone length during partial oxidation of methane in a flow reactor atE/nof 10 Td.Lefkowitzet al.[18]measured the plasma generated intermediate species by mid-IR laser absorption spectroscopy of C2H4/Ar and C2H4/O2/Ar mixtures activated by a nanosecond repetitively pulsed plasma in a low temperature flow reactor.HP-mechanism was assembled and three fuel consumption pathways for plasma activated low temperature ethylene oxidation were validated.Major fuel consumption pathway for plasma activated hydrocarbon dissociation and further oxidation at low temperature were proposed in Refs[16-18].The results showed that the active composition(charged particles,excited atoms and molecules,active atoms and radicals)generated by various plasmas selectively depended on electron energy,electron density,gas temperature,gas pressure,properties of gases and so on.Kinetically changing electron parameters as well as gas properties often permitted optimization of chemical-plasma processes[26].Unfortunately,in spite of rigorous experimental investigations,the kinetic contribution from the plasma/methane reforming interaction has not been fully characterized.Physical kinetic studies that demonstrate the roles electron-induced chemistry plays are therefore needed for a full understanding of the fundamental mechanisms of plasma-assisted reformation.
Accordingly,we consider the kinetic processes of partial oxidation and dry reforming of methane in a laminar flow reactor by experiments as well as modeling prediction,aiming to analyze the different kinetic contributions of CO2and O2gases in methane conversion activated by a radio frequency discharge(RF)plasma.In order to characterize the formation of key species in plasmas,the concentrations of reactants and products were measured with on-line Gas Chromatography(GC-TCD)and used to validate the kinetic models and further examine the different roles of CO2and O2in a plasma-chemistry system.Path flux analysis for two plasma-chemistry systems of He/CH4/CO2and He/CH4/O2was conducted.Key contributions ofO2and CO2additives in methane conversion were kinetically identified.
2.Methods and Kinetic Modeling
2.1.Methane reforming system with RF discharge
A schematic of the experimental system is shown in Fig.1.The experimental setup consists of a wire-cylinder dielectric barrier discharge(DBD)reactor,an RF power supply,gas lines and a GC-TCD measurement system.The DBD flow reactor is a cylindrical glass vessel with 10 mm I.D.and 12 mm O.D.The high-voltage electrode was packaged into a quartz tube with 3 mm I.D.and 4 mm O.D.to avoid the catalytic effect of copper,and then located in the center of the DBD flow reactor.The 19 mm length outer copper electrode was wrapped around the DBD reactor and then grounded.The electrode edges were smoothed to avoid the local concentration of electric field and dielectric breakdown[16].Helium was used as a dilution gas for methane reforming in order to improve the uniformity of the RF discharge.The experimental pressure and temperature were fixed at 100 Torr and 373 K respectively.Experiments were conducted in a nearly isothermal flow reactor to minimize the temperature changes from the reaction zone in the DBD reactor.A Type-K thermocouple probe(±1 K)of 1-mm-diameter was located at the end of the reaction zone after plasma discharge to measure the mixture temperature.The composition of the discharge mixtures(He/CH4/CO2and He/CH4/O2)was fixed to the same ratio 0.55:0.36:0.09,in order to compare the contributions of CO2and O2additives in methane conversion.An RF power supply(Cesar RF Power Generator),which could supply sine-wave voltage and current up to 4 kV and 1.5A,was used to generate plasma at a fixed frequency of 13.56 MHz.The applied voltage and discharge current were measured by a digital oscilloscope(Tektronix DPO 7410C)together with a Lecroy high voltage probe(PPE20KV)and a Pearson Coil current probe(Model 6585),respectively.Voltage and current curvesversustime are shown in Fig.2(a).The reduced electric field(E/n)of 77-100 Td in the continuous RF discharge at experimental conditions was calculated by the voltages applied to the high-voltage electrode,providing boundary conditions for simulation prediction.The behavior of the plasma equivalent resistance as well as gas composition in a discharge mixture at each experiment was found to be sensitive to the RF power absorption as well as theE/nvalues applied to the DBD electrodes.RF power and the corresponding reduced electric field applied to the DBD electrodes are therefore nonlinearly related,as shown in Fig.2(b).
The collected gas samples were then analyzed by an online micro GC system(INFICON 3000)coupled with thermal conductivity detection(GC-TCD)techniques.GC column temperature was fixed at 363 K to achieve favorable chromatographic separation of the sampled species.Species identification was made by comparing the retention time of the sampled species against the known calibration standard mixtures for the same chromatographic conditions.The major products,CH4,O2,CO,CO2,CH2O,H2O,H2,C2and C3hydrocarbon species from the plasma assisted methane conversion were simultaneously analyzed using the GC-TCD in the same manner as the calibration sampling.Helium was used as a carrier gas.H2O species can be measured in an INFICON 3000 micro GC system,by keeping H2O in a sampling mixture at gas state along the gas lines from the exhaust of reactor to the GC inlet.Additionally,the overall efficiency in the reforming system in this study calculated from Eq.(1)was below 10%due to a large heat consumption.
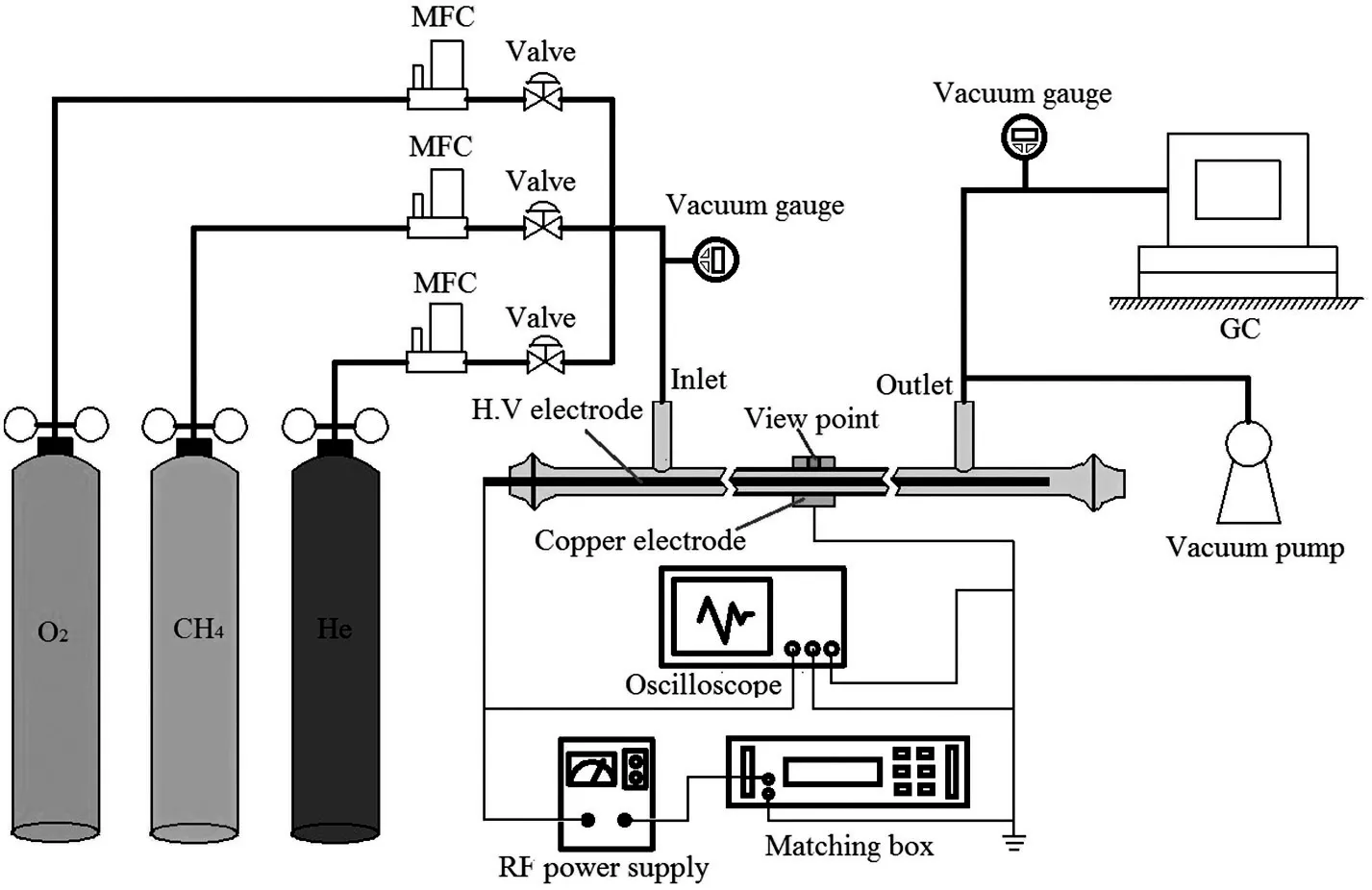
Fig.1.Schematic of experimental system.
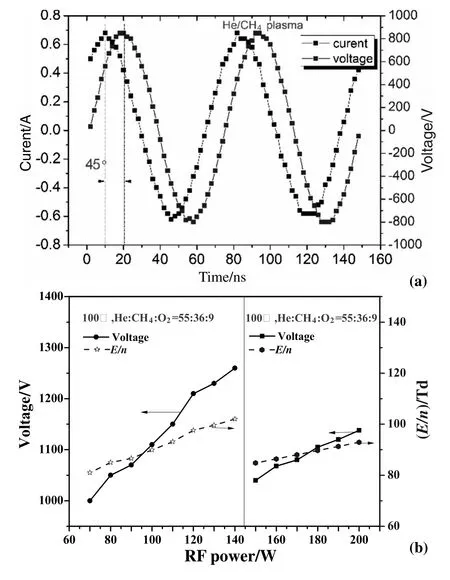
Fig.2.(a)Voltage and current curves;(b)peak voltage and E/n varying with RF power for He/CH4/O2 plasmas.
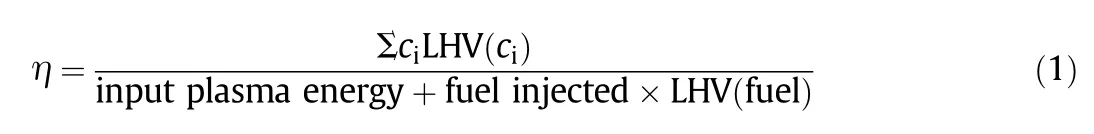
where,ciis the key compositions of the reformed products,including C2and C3hydrocarbon species,syngas,CO2,etc.LHV is the lower heating value of the key species.
2.2.Computational approach with plasma-reforming kinetic model
To simulate the partial oxidation and dry reforming of methane in a non-equilibrium plasma,kinetic modeling was carried out using Bolsig+and Chemkin PRO.The plasma assisted methane conversion models incorporated a set of species conservation equations for number densities of neutral,charged,and excited species produced in the plasma.In order to consider species dissociation by excited He,additional elementary reactions involving the dilution gas excitation were added.Simultaneously,the energy conservation equation was solved as well to consider the energy transfer in the plasma-chemistry system.These above equations were coupled with the steady state,two-term expansion Boltzmann equation solving for the electron energy distribution function(EEDF)using experimentally measured cross sections for electron impact electronic excitation,dissociation,ionization,and dissociative attachment processes,to calculate the rate coefficients of the electron impact elementary reactions.The cross sections of electron elastic impact,electron excitation and dissociation used in this study came from the online LXCAT(Plasma Data Exchange Project)database[27]and related literatures[28,29]and then were integrated over the electron energy distribution function(EEDF)to get the rate coefficient of electron involving reactions.
During the calculation process,all the electrons with global mean electron energy were used to substitute for the partial energetic electrons.HP mechanism[30-32]with some update of elementary reaction rates from NIST(http://kinetics.nist.gov/chemistry)chemistry data was integrated to decide rate coefficients of radical/ion reactions at low temperature.The kinetic model also incorporated chemical reactions of excited electronic species,electron-ion recombination and ion-ion neutralization processes,ion-molecule reactions,and electron attachment and detachment processes.Note that the present models can't solve the Poisson equation for the electric field and therefore do not take into account charge separation and sheath formation near the electrode.The reduced electric fieldE/nis determined by the measured average applied voltage,the discharge channel distance,the quartz layer thickness,and its dielectric constant[17].
Rotational-rotational(RR)and rotational-translational(RT)energy transfer processes are usually non-adiabatic and fast[33].So,rotational excited molecules were treated as inactive species for the non-thermal acceleration of chemical reactions in the simulation herein.In contrast to the fast process of RR and RT relaxation,the quenching of the vibrationally excited molecules is relatively slow[33].Nonetheless,for the high vibrational levels,their concentrations are very low and reached only for a very limited time in the order of tens of nanoseconds.For the low vibrational levels,the energy is not sufficiently high to promote significant dissociation,though it seems to be close to the threshold[34,35].As such,the effects of vibrational molecules on the kinetic process of plasma assisted methane conversion were also neglected in this calculation,in order to make the reaction pathway more efficient.All species included in the model are summarized in Table 1.
3.Results and Discussion
3.1.Species selectivity and methane reforming
The main purpose of this experimental study is to compare the kinetic contributions of CO2and O2additives in methane conversion activated by RF plasmas,and to provide experimental data for model validation.In order to provide a comparative reference to the methane conversion with O2or CO2additive,pure methane pyrolysis experiments in a He/CH464/36 mixture were also conducted.As shown in Fig.3,the dry reforming experiments with CO2are carried out at higher RF energy input than the partial oxidation experiments with O2.This is mainly attributed to the different dissociation and ionization energy of the two additive gases,as well as the thus produced electron energy distribution function(EEDF)in the plasma-chemistry system.
In a complex plasma-chemistry system,energy branching allocated among the electron involving reactions is a function of gas composition in a discharge mixture,as well as the reduced electric fieldE/nvalue.For the methane conversion experiments in Figs.3 and 4,pressure,temperature and gas ratio in the discharge mixture remained unchanged,the reactant species as well as theE/nvalue therefore became the decisive parameters controlling the energy deposition and further the product selectivity.Fig.3 clearly shows the dependence of the measured selectivity of H2,CO,C2and C3hydrocarbon species on the RF energy input for pure methane pyrolysis,partial oxidation and dry methane conversion.In contrast to the pure methane pyrolysis(He/CH464/36 mixture),a large amount of syngas(H2and CO)as well as oxygenates(CH2O and CH3OH)are generated at the presence of oxidants including O2and CO2gases.Furthermore,the chemical processes strongly depend on the oxidant used.For the partial oxidation with O2,the selectivity ofH2,C2andC3hydrocarbon species increases significantly with the increase of RF energy input.On the contrary,the selectivity of CO,CH2O and CH3OH decreases.At the same time,H2O and CO2are also formed by the slow oxidation process.Correspondingly,the selectivity of H2and C2hydrocarbon species is extremely high(up to 90%for H2and 50%for C2)in the dry reforming of CH4with CO2,however,oxygenates including CH2O,CH3OH relatively low(less than 2%).All the above suggests the much higher chemical reactivity of O2additive than CO2,which will be discussed later.Dry reforming of methane with CO2is by far the easier to produce the syngas(CO and H2)as well C2hydrocarbon species.
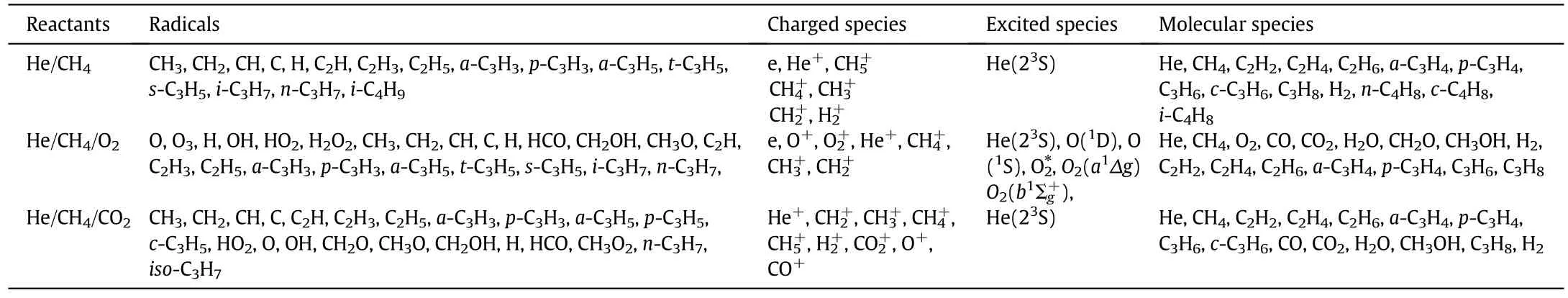
Table 1 Summary of all species included in the model
As for the pure methane pyrolysis and the dry reforming of methane,no significant effect of carbon deposition(less than 0.05%)was observed in the experiments.Correspondingly,carbon black deposited on the electrodes and reactor walls was observed during the partial oxidation experiments conversion of methane,which was less than 5%and primarily formed in the plasma discharge.
As discussed above,the presence of different oxidants(CO2and O2)dramatically altered the reaction pathway for methane pyrolysis.The individual species selectivity of C2and C3hydrocarbons with respect to RF energy input is further plotted in Fig.4.C2and C3hydrocarbons are all beneficial by-products for synthetic fuels and industrial chemicals.It can be seen that C2H6and C3H8selectivity generally decreases with the increase of RF energy input for the experiments of pure methane pyrolysis.However,almost all the species selectivity increases with respect to RF power increasing in the partial oxidation as well as dry reforming processes.This is mainly because the majority of the electron energy goes to the ionization and dissociation of methane with the increase of energy input,as oxidant is added.Fig.4 also shows that the dry reforming with CO2additive tends to generate C2species in contrast to partial oxidation conversion with O2additive.This is primarily because CH4as well as CH3,CH2and CH radicals generated in a He/CH4/CO2discharge mixture slowly react with O and O(1D).Instead,C2species generated in a He/CH4/O2discharge mixture is easier to react with O,O(1D),O2(a1Δg),O2(b1)and O+to generate oxygenates including CO,CO2,CH2O,CH3OH,and so on.

Fig.3.Selectivity of H2,CO,C2 and C3 hydrocarbon species for pure methane pyrolysis in a He/CH4 64/36 mixture(left),partial oxidation(middle)and dry reforming(right)of methane in He/CH4/CO2(O2)55/36/9 mixtures at 373 K and 100 Torr as a function of energy input.Solid and dotted lines are used for experimental data fitting,in order to clearly show the product change tendency with the RF power increasing.

Fig.4.Selectivity of(a)C2 and(b)C3 hydrocarbon species for pure methane pyrolysis(left),partial oxidation conversion(middle)and dry reforming(right)of CH4.Solid and dotted lines are used for experimental data fitting,in order to clearly show the product change tendency with the RF power increasing.
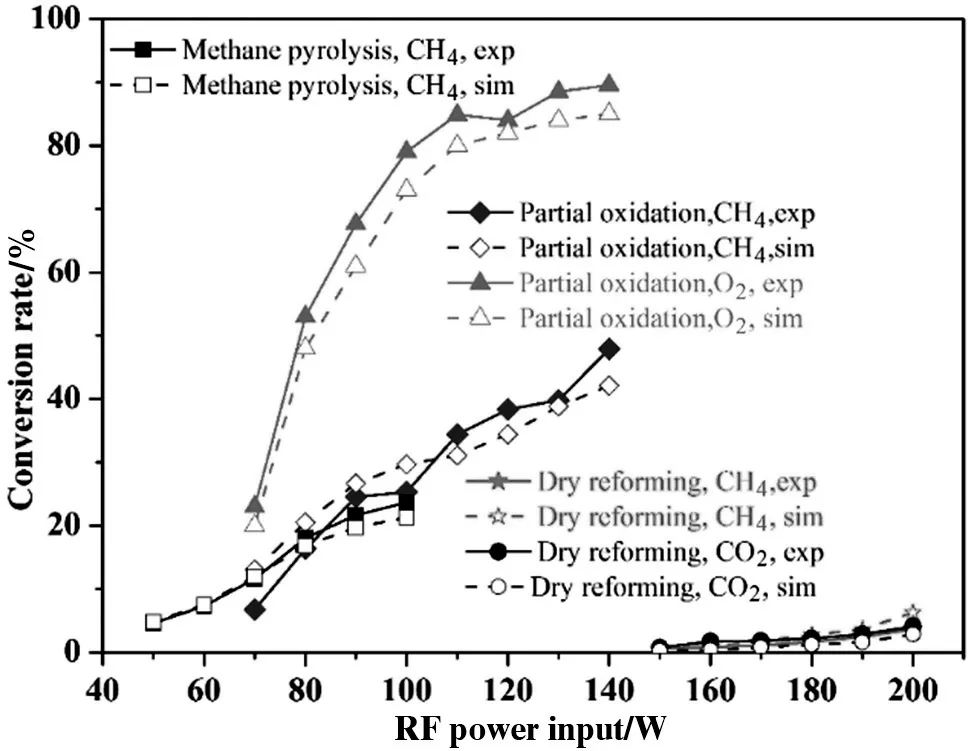
Fig.5.Comparison between experimental and modeling results in different conversion reactions:methane pyrolysis(left),partial oxidation conversion(middle)and dry reforming(right).
Fig.5 presents the measured and predicted conversion rates of CH4,CO2and O2as a function of RF energy input.The results show the highest methane conversion rate in the partial oxidation conversion process.This is mainly because of the relatively lower values of the dissociation and ionization energy of O2additive.Fuel oxidation by plasma generated O and O(1D),O2(a1Δg),O2(b1)and O+is also essential for methane consumption.The results show that in the measured range of the energy input,the fuel and oxygen conversion for pure methane pyrolysis and partial oxidation conversion are predicted to be within 5%discrepancy from the experimental results,which is within the experimental uncertainty.As discussed above,the initial electron density is treated as an adjustable parameter to match the methane conversion rate in the experimental results.The resulting CH4conversion is thus in good agreement with the experimental values.Accordingly,the relative agreement of O2conversion in the comparison between the modeling and experimental results indicates that the related electron collision reaction rates are well modeled in this study.On the other hand,for the dry reforming of methane,the CH4and CO2conversion rates are measured to be at very low percentage(less than 6%)even at the higher energy input compared to the partial oxidation conversion process.In spite of this,it can be found the model accurately predicted the change tendency of the conversion rate.

Fig.6.Comparison of simulation and experimental results of C2,C3,CO and H2.
Fig.6 shows the comparison of the model prediction and the GC measured selectivity for C2,C3hydrocarbon species as well as syngas with respect to RF power input for partial oxidation conversion and dry reforming respectively.The models capture the correct changing trends,but under-predicted or over-predicted their absolute concentrations.From the measured and predicted results for the partial oxidation of methane,C2agreement in the comparison between the modeling and experimental results is within 35%,C3within 30%(except for the case of RF 70 W input),CO within 32%.This model over-predicted H2(up to 41%),which is probably due to the overall underestimation of higher hydrocarbons.On the other hand,for the dry reforming prediction,the production of C2and CO is predicted to be within 13%of the measured values,which is in excellent agreement.However,C3species has very small percentage in their concentrations in the experiment,but is over-predicted by the model.H2in this model is 33%under predicted of the measured one,mainly because H atoms in C3hydrocarbons were over-predicted.In summary,the major trends of reactant consumption and major product species production are well captured by the models,again indicating that the electron collision rates and dominant reaction pathways are well modeled.
3.2.Physical kinetics of methane conversion with O2/CO2 additive
As we know,the elementary reaction rates for all plasma-chemistry processes are usually determined by the micro-kinetic characteristics of individual reactive collisions(reaction cross sections or elementary reaction probabilities)as well as the relevant kinetic distribution functions,i.e.,the electron energy distribution function(EEDF)[3].Fig.7 shows sets of electron cross-section for CO2and O2with respect to the electron energy.It can be seen clearly in Fig.7 that O2molecule has much larger cross-section at relatively lower electron energy,compared to CO2molecule.This indicates O2molecule has larger probabilities to collide with highly energized electrons and by far is the easier to begin the plasma-chemistry processes including electron impact electronic excitation,dissociation,ionization,and dissociative attachment.
A non-equilibrium electron energy distribution function(EEDF)can be determined by solving the Boltzmann equation with inputdata of the experimentally measured cross sections(in Fig.6)for electron impact electronic excitation,dissociation,ionization,and dissociative attachment processes.The EEDF describes the population of electrons at specific energy levels.Overall,since electron processes strongly govern the kinetics in plasma assisted methane pyrolysis systems,understanding the optimized reduced electric fieldE/nas well as the electron energy distribution function(EEDF)is therefore vital.The electron energy distribution strongly depends on gas composition.So,adding CO2or O2to CH4gas changes the electron energy distribution significantly.Fig.8 shows that the electron energy distribution function(EEDF)in a He/CH4/O2discharge mixture has a relatively higher head,in contrast to the EEDF in a He/CH4/O2discharge mixture.This indicates that relatively larger populations of energized electrons at efficient energy levels(<10 eV)in the He/CH4/O2discharge mixture can be generated,which would result in an intensification of ionization,excitation,dissociation,and other plasma-chemistry processes.
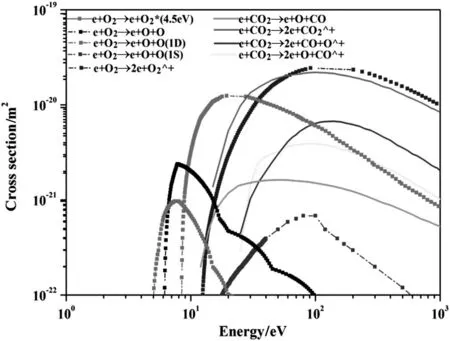
Fig.7.Electron collision cross sections for O2 and CO2 as a function of electron energy.
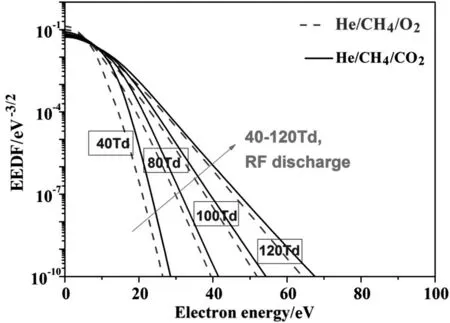
Fig.8.Comparison of the electron energy distribution function(EEDFs)in He/CH4/O2 55/36/9 and He/CH4/CO2 55/36/9 discharge mixtures.
The rate constants for elementary reactions resulted from the integration of the reaction cross section(in Fig.7)or probability over the relevant distribution function.Fig.9 shows the variation of rate constants as a function of the reduced electric field for electron impact reactions involved by O2and CO2respectively.It can be seen that the rate constants of electron-oxygen molecule impact reactions for dissociation are greater than those of the electron-carbon dioxide molecule impact reactions by at least a factor of two or three.This presents the strong influence of electron collision cross-section and further the EEDF on the rate constants of electron impact reactions.Rate constants herein significantly represent the impact probabilities of reactants and highly energized electrons,determining the dissociation and ionization processes in the plasma chemistry system.
As discussed above,for a plasma-chemistry system,theE/nvalue as well as the gas composition control the electron energy deposition direction in a plasma.In the simplest case,E/nis a function of the gas density,the applied voltage and the geometry of the reactor.Temperature and pressure in discharge mixtures were fixed in this study,as such,the applied voltage and gas composition in each experiment became the decisive parameters for species selectivity and reactant conversion at the discharge termination.Concentrations of radical species in Figs.10 and 11 therefore have their unique changing tendency over the propagating time of a discharge mixture passing through the DBD discharge channel.
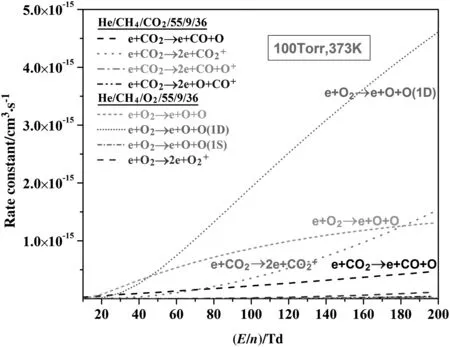
Fig.9.Rate constants of electron impact reactions involved by O2 and CO2 as a function of the reduced electric field.
The calculation results in Figs.10 and 11 show that most of the active species are produced and then increase rapidly at the discharge stage(ns-μs time scale)due to the electron impact dissociation,excitation and ionization,however,decrease after discharge termination to produce higher hydrocarbon,syngas and oxygenates.From the EEDFs in Fig.8,more efficient plasma energy(<10 eV)is coupled into the He/CH4/O2mixture for electron impact dissociation,excitation and ionization.It is therefore clear that active radicals generated in a He/CH4/O2discharge mixture are easier to be accumulated and always several orders higher than those in a He/CH4/CO2mixture,which would lead to an efficient decomposition of reactants and higher conversion rates in partial oxidation of methane with O2.
3.3.Effects of O2/CO2 additives on reaction pathways of methane conversion
Good agreement between the measured and calculated products observed in most cases indicates that the given kinetic model adequately describes the different processes of methane conversion.As discussed above,due to the different dissociation and ionization energy and further the different features of cross-section for CO2and O2gases,the dry reforming experiments of methane conversion with CO2additive were carried out at higher RF energy input than the partial oxidation experiments.Reaction pathways for dry reforming of CH4with CO2(He/CH4/CO255/36/9)are analyzed atE/n=90 Td,however,partial oxidation of CH4with O2(He/CH4/O255/36/9)is analyzed atE/nvalue 70 Td,as shown in Fig.12(a)and(b).The similar oxidation and pyrolysis pathways for methane conversion processes with different oxidants can be found.Reaction pathways for partial oxidation of CH4with O2have been analyzed in detail in another paper[36].So,we focus on the kinetic effects of oxidants including O2and CO2on the species selectivity here.
It is seen in Fig.12(a)and(b)that CH4oxidation and pyrolysis in a RF plasma volume begin with the formation of the active species including CH3,CH2,CH,H,O,and O(1D),due to the energetic electron impact reactions,such as e+CH4→e+CH3+H,e+O2→e+O+O(1D),and e+CO2→e+CO+O.As for the partial oxidation conversion of methane with O2at the reduced electric field of 70 Td,most of the electron energy goes to the dissociative excitation and dissociation of oxygen additive.However,only 4%of electron energy goes to the ionization of CO2in a dry reforming of mixture.As such,fuel oxidation process becomes dominant while O and O(1D)are significantly generated in a He/CH4/O2mixture discharge.Furthermore,the observation of the reaction pathways in Fig.12(a)and(b)suggests that the role of plasma dissociation is not only to break down the fuel molecules but also to build up larger hydrocarbon species[18].The recombination of plasma generated radical-radical or radical-molecule leads to the formation of new larger hydrocarbons.
Sensitivity analysis reveals the reactions governing predictions of species generation in plasma assisted methane conversion.Normalized sensitivitysimeasures the impact of a reaction rate or set of reaction rates towards influencing key species production under a specified set of conditions and is given by Eq.(1).

whereciis the concentration for theith species,kjis the rate constant for thejth element reaction.

Fig.10.Time evolution of radicals at E/n=70 Td in He/CH4/O2 plasma.
Fig.13 plots the reactions to which the mechanism is sensitive for key species generation including CO,H2,C2H4and C3H6in dry reforming of methane at 90 Td.Syngas formation is primarily important in methane conversion,so,analyzed herein by the normalized sensitivity results.It can be seen in Fig.13(a)that the sensitivity coefficients of the electron impact reaction e+CO2→e+CO+O and the promotion reaction CH+CO2=CO+HCO characterize the CO chemistry in dry reforming.On the other hand,CO formation is also sensitive to electron-CH4impact reactions including e+CH4→2e++H,e+CH4→e+CH2+H2and so on.As can be seen in the path flux analysis in Fig.12(b),there are two major CO generation pathways in the He/CH4/CO2discharge mixture.The first pathway is direct collisional dissociation of CO2molecule by highly energized electrons to form CO,e+CO2→e+CO+O.The second pathway is the recombination of methane fragments(CH3,CH2,CH,H)with HCO and O radicals to form CO.It can be therefore found that the direct electron collisional dissociations of reactants(CO2and CH4)to CO and also the recombination of methane fragments with HCO and O are dominant in methane conversion.Accordingly,it is clear that the dominant formation of H2in dry reforming is sensitive to the decomposition reactions of CH4and its fragments by electron impact reactions,e+CH4→2H2+CH2.e+CH4→e+H2+CH+H,etc.Furthermore,hydrogen abstraction reactions of CH2,CH,CH2O,CHO radicals also make great contributions to H2formation.From the above,syngas formation in dry reforming of methane is strongly sensitive to the collisional dissociation of CO2and CH4molecules by electrons.The total electron collision rates are therefore considered primarily important to be well performed in this model.
As discussed above,C2and C3hydrocarbons are all beneficial byproducts for synthetic fuels and industrial chemicals.We especially focus on the C2H4and C3H6formation mechanism due to its important industrial interest.The pathway flux analysis in Fig.12(a)suggests the dominant formation path of C2H4is primarilyviarecombination of radical-radical and radical-molecule,in particular,CH radical with CH4and C2H6molecules.Electron impact dissociation reactions of CH4have great effects on radical generation of CH3,CH2,CH,H and further C2H4formation.It is clearly apparent in Fig.13(c)that the radical recombination reaction,CH+CH4=H+C2H4,as well as the main electron-CH4impact reactions are primarily sensitive to C2H4formation.On the other hand,some other recombination reactions,such as H+C2H4(+M)=C2H5(+M)and CH2+C2H4=C3H6,contribute to the C2H4consumption,which build blocks for C2H4formation in its large concentration.Also,C3H6species is primarilyviarecombination of CH2with C2H6.Therefore,as shown in Fig.13(d),the mechanism sensitive for C3H6generation included all the reactions related to C2H4formation.
In contrast to the sensitivity analysis of key species formation in dry reforming of methane,similar sensitivity coefficients are worked out for H2,C2H4and C3H6formation in partial oxidation conversion,as shown in Fig.14(b)and(d).This reveals the similar formation mechanisms for these key species in both dry and partial oxidation conversion processes.However,due to the different dissociation and ionization characteristics of CO2and O2additives,the numerical ranges of sensitivity coefficients for H2,C2H4and C3H6formation in dry and partial oxidation conversion are different.
It is worth noting that different reactions in Figs.13(a)and 14(a)can be observed sensitive to CO formation in dry and partial oxidation conversion of methane.For dry reforming,CO is primarily from electron impact dissociation of CO2.Only a small fraction of CO comes from hydrogen abstraction reactions as well as radical oxidation reactions.However,for partial oxidation conversion,CO formation primarily comes from hydrogen abstraction and radical oxidation reactions.
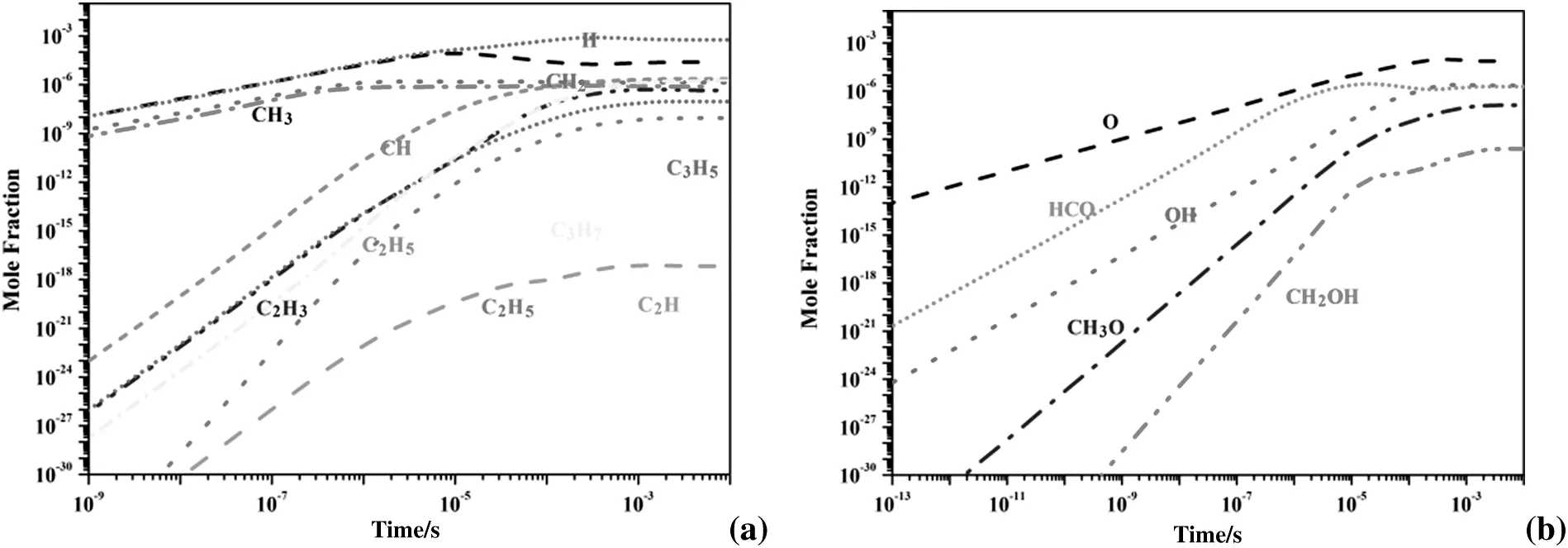
Fig.11.Time evolution of radicals at E/n=90 Td in He/CH4/CO2 plasma.

Fig.12.Reaction pathways for(a)dry reforming of CH4 with CO2(He/CH4/O2 55/36/9)at E/n=90 Td,and(b)partial oxidation of CH4 with O2(He/CH4/CO2 55/36/9)at E/n=70 Td.
4.Conclusions
Species measurement both in dry reforming and partial oxidation conversion of CH4in RF discharges has been performed by on-line Gas Chromatography(GC-TCD).A modeling tool for prediction of homogenous plasma discharge and subsequent chemistry reactions has been assembled to predict the present measurements.
It was experimentally and numerically observed that the dry reforming experiments were carried out at relatively higher RF energy input than the partial oxidation experiments.This was mainly attributed to the different dissociation and ionization energy of the two additive gases,and the thus produced electron energy distribution function(EEDF)in the corresponding plasma-chemistry system.The results also showed the highest oxygen conversion rate in the partial oxidation conversion process due to the effective discharge in He/CH4/O2mixture at lower electron energy.On the other hand,fuel oxidation by plasma generated O,O(1D),O2(a1Δg),O2(b1)and O+in partial oxidation conversion of methane was essential for methane consumption,which resulted in an increase in methane conversion rate,compared to pure methane pyrolysis and dry methane conversion with CO2additive.
It was found that dry reforming of methane with CO2was by far the easier to produce the syngas(CO and H2)as well as C2hydrocarbon species,due to the weak oxidation of CO2additive.On the other hand,C2species generated in a partial oxidation conversion discharge with O2additive would be more easier to react with atom oxygen O and O(1D),O2(a1g),O2(b1Σ+g)and O+to generate oxygenates including CO,CO2,CH2O,CH3OH,and so on.
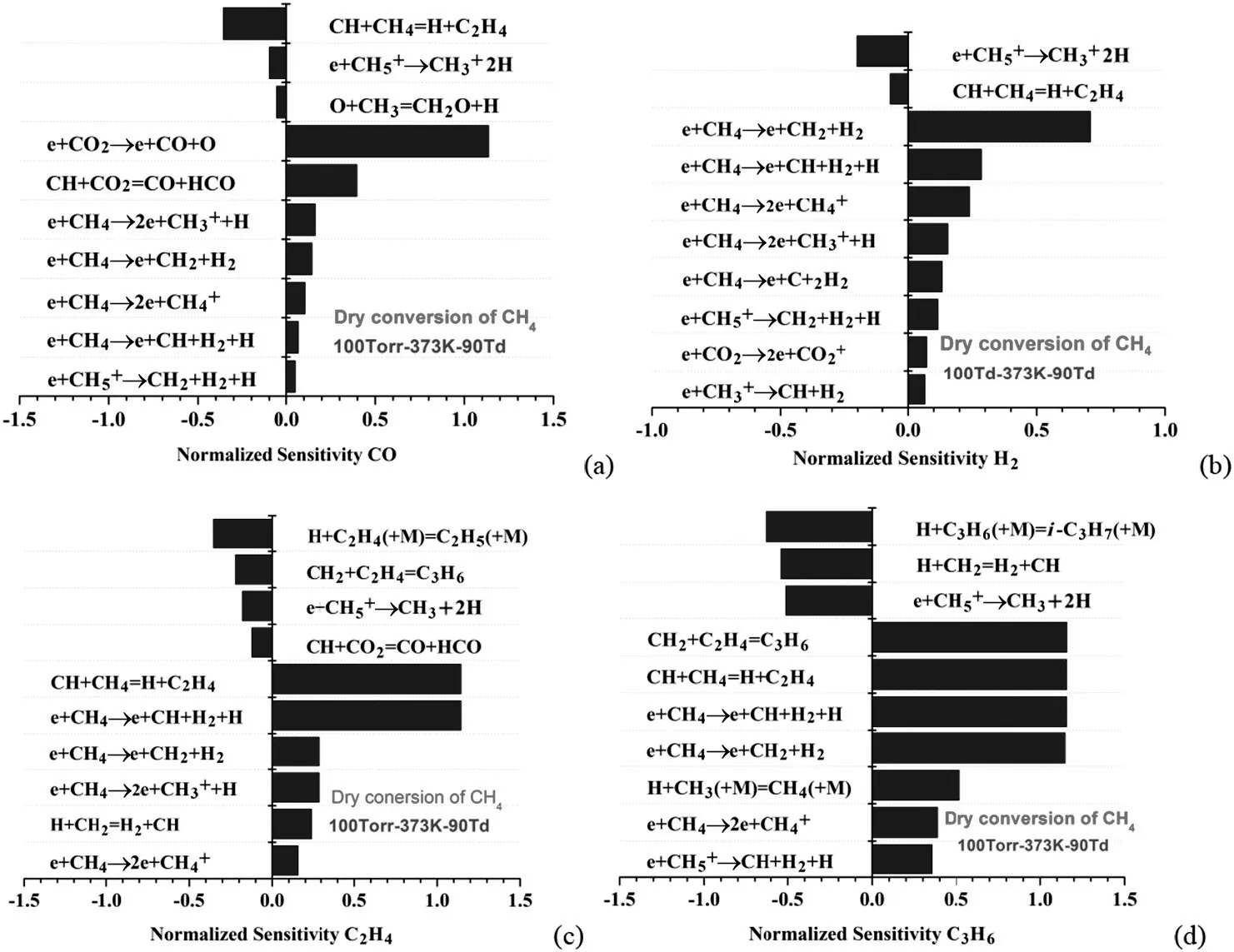
Fig.13.Normalized sensitivity of(a)CO,(b)H2,(c)C2H4 and(d)C3H6 for dry reforming of CH4 with CO2.

Fig.14.Normalized sensitivity of(a)CO,(b)H2,(c)C2H4 and(d)C3H6 formation for partial oxidation conversion of CH4 with O2.
To clarify the kinetic modeling,comparison of the measured and numerical predicted species concentrations in RF plasma assisted methane conversion was performed.Comparison of the GC sampling measurements with the model predictions showed that the major trends of reactant consumption and major product species production were well captured by the models.This indicated that the electron collision rates and dominant reaction pathways were well modeled.
Acknowledgments
The authors thank Prof.Yiguang Ju at Princeton University for the interesting suggestions and discussions.
[1]Y.G.Ju,W.T.Sun,Plasma assisted combustion:Dynamics and chemistry,Prog.Energy Combust.48(2015)21-83.
[2]Y.Jamal,M.L.Wyszynski,On-board generation of hydrogen-rich gaseous fuels—A review,Int.J.Hydrog.Energy19(1994)557-572.
[3]S.M.Starikovskaia,Plasma assisted ignition and combustion,J.Phys.D.Appl.Phys.39(2006)R265-R299.
[4]G.Petitpasa,J.-D.Rollier,A.Darmon,J.Gonzalez-Aguilar,R.Metkemeijer,L.Fulcheri,A comparative study of non-thermalplasma assisted reforming technologies—A review,Int.J.Hydrog.Energy32(2007)2848-2867.
[5]X.M.Zhang,M.S.Cha,Electron-induced dry reforming of methane in a temperature controlled dielectric barrier discharge react,J.Phys.D.Appl.Phys.46(2013)415205(10 pp.).
[6]B.Wang,G.Xu,H.Sun,Kinetics of conversion of methane with electric field enhanced plasma,Chin.J.Chem.Eng.12(1)(2004)131-133.
[7]Kazunari Katayama,Satoshi Fukada,Masabumi Nishikawa,Direct decomposition of methane using helium RF plasma,Fusion Eng.Des.85(2010)1381-1385.
[8]Pedro Patino,Yasnahir Perez,Manuel Caetano,Coupling and reforming of methane by means of low pressure radio-frequency plasmas,Fuel84(2005)2008-2014.
[9]B.Wang,Q.Sun,L.U.Yijun,M.Yang,W.Yan,Steam reforming of dimethyl ether by gliding arc gas discharge plasma for hydrogen production,Chin.J.Chem.Eng.22(1)(2014)104-112.
[10]C.S.Kalra,A.F.Gutsol,A.A.Fridman,Gliding arc discharges as a source of intermediate plasma for methane partial oxidation,IEEE Trans.Plasma Sci.33(1)(2005)32-41.
[11]A.Czernikowski,Glidarc assisted preparation of the synthesis gas from natural gas and waste hydrocarbons gases,Oil Gas Sci.Technol.Rev.IFP2(56)(2001)181-198.
[12]H.Sekiguchi,Y.Mori,Steam plasma reforming using microwave discharge,Thin Solid Films435(2003)44-50.
[13]Changsheng Shen,Dekun Sun,Hongsheng Yang,Methane coupling in microwave plasma under atmospheric pressure,J.Nat.Gas Chem.20(2011)449-456.
[14]V.D.Rusanov,A.I.Babaritskii,E.N.Geramisov,M.A.Deminskii,S.A.Demkin,V.K.Zhivotov,et al.,Stimulation of the partial oxidation of methane in a microwave discharge,Dokl.Phys.48(3)(2003)119-122.
[15]I.Babararistkii,I.R.Baranov,M.B.Bibikov,S.A.Demkin,V.K.Zhivotov,G.M.Konovalov,et al.,Partial hydrocarbon oxidation processes induced by atmospheric-pressure microwave-discharge plasma,High Energy Chem.38(6)(2004)407-410.
[16]W.T.Sun,Mruthunjaya Uddi,Sang Hee Won,Timothy Ombrello,Campbell Carter,Y.G.Ju,Kinetic effects of non-equilibrium plasma-assisted methane oxidation on diffusion flame extinction limits,Combust.Flame159(2012)221-229.
[17]J.K.Lefkowitz,M.Uddi,B.C.Windom,G.F.Lou,Y.G.Ju,In situ species diagnostics and kinetic study of plasma activated ethylene dissociation and oxidation in a low temperature flow reactor,Proc.Combust.Inst.35(3)(2015)3505-3512.
[18]J.K.Lefkowitz,P.Guo,A.Rousso,Y.G.Ju,Species and temperature measurements of methane oxidation in a nanosecond repetitively pulsed discharge,Philos.Trans.R.Soc.A373(2015),20140333.
[19]B.Zhu,X.S.Li,J.L.Liu,X.B.Zhu,A.M.Zhu,Kinetics study on carbon dioxide reforming of methane in kilohertz spark-discharge plasma,Chem.Eng.J.264(2015)445-452.
[20]B.Wang,E.Yang,G.Xu,J.Hao,Theoretical study of reaction paths and transition states on conversion methane into C2hydrocarbons through plasma,Chin.J.Chem.Eng.15(1)(2007)44-50.
[21]V.I.Zyn,Kinetic identification of a mechanism of complex plasma chemical reactions,Plasma Chem.Plasma Process.18(3)(1998)395-408.
[22]J.R.Fincke,R.P.Anderson,T.Hyde,B.A.Detering,R.Wright,R.L.Bewley,D.C.Haggard,W.D.Swank,Plasma thermal conversion of methane to acetylene,Plasma Chem.Plasma Process.22(1)(2002)105-136.
[23]Xiaoguang Guo,Guangzong Fang,Gang Li,Direct,nonoxidative conversion of methane to ethylene,aromatics,and hydrogen,Science344(2014)616-619.
[24]AI-Fatesh Ahmed,Naeem Muhammad,Anis,Role of La2O3as promoter and support in Ni/γ-Al2O3catalysts for dry reforming of methane,Chin.J.Chem.Eng.22(1)(2014)28-37.
[25]A.M.Starik,P.S.Kuleshov,B.I.Loukhovitski,N.S.Titova,Theoretical study of partial oxidation of methane by non-equilibrium oxygen plasma to produce hydrogen rich syngas,Chin.J.Chem.Eng.40(32)(2015)9872-9884.
[26]Alexander Fridman,Plasma Chemistry,Cambridge University Press,2008.
[27]LAPLACE database,www.lxcat.net(retrieved on September 29,2016).
[28]H.C.Straub,D.Lin,B.G.Lindsay,K.A.Smith,R.F.Stebbings,Absolute partial cross sections for electron-impact ionization of CH4from threshold to 1000 eV,J.Chem.Phys.106(11)(1997)4430-4435.
[29]R.K.Janev,D.Reiter,Collision processes of CHy and CHy hydrocarbons with plasma electrons and protons,Phys.Plasmas9(9)(2002)4071-4081.
[30]X.Shen,X.Yang,J.Santner,J.Sun,Y.Ju,Experimental and kinetic studies of acetylene flames at elevated pressures,Proc.Combust.Inst.35(2015)721-728.
[31]H.Zhao,J.Fu,F.M.Haas,Y.Ju,Effect of prompt dissociation of formyl radical on 1,3,5-trioxane and CH2O laminar flame speeds with CO2dilution at elevated pressure,Combust.Flame183(2017)253-260.
[32]H.Zhao,X.Yang,Y.Ju,Kinetic studies of ozone assisted low temperature oxidation of dimethyl ether in a flow reactor using molecular-beam mass spectrometry,Combust.Flame173(2016)187-194.
[33]Tomas Kozak,Annemie Bogaerts,Splitting of CO2by vibrational excitation in nonequilibrium plasmas:A reaction kinetics model,Plasma Sources Sci.Technol.23(2014)1-17.
[34]Tomas Kozak,Annemie Bogaerts,Evaluation of the energy efficiency of CO2conversion in microwave discharges using a reaction kinetics model,Plasma Sources Sci.Technol.24(2015),015024.(17 pp.).
[35]Tomohiro Nozaki,Nahoko Muto,Shigeru Kado,Ken Okazaki,Dissociation of vibrationally excited methane on Ni catalyst:Part 1.Application to methane steam reforming,Catal.Today89(1-2)(2004)57-65.
[36]Q.Chen,X.F.Yang,J.T.Sun,X.J.Zhang,X.G.Mao,Y.G.Ju,B.E.Koel,Pyrolysis and oxidation of methane in a RF plasma reactor,Plasma Chem.Plasma Process.37(2017)1551-1571.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
